Abstract
The effects upon memory of normal aging and two age-related neurodegenerative diseases, Alzheimer disease (AD) and Parkinson disease, are analyzed in terms of memory systems, specific neural networks that mediate specific mnemonic processes. An occipital memory system mediating implicit visual-perceptual memory appears to be unaffected by aging or AD. A frontal system that may mediate implicit conceptual memory is affected by AD but not by normal aging. Another frontal system that mediates aspects of working and strategic memory is affected by Parkinson disease and, to a lesser extent, by aging. The aging effect appears to occur during all ages of the adult life-span. Finally, a medial-temporal system that mediates declarative memory is affected by the late onset of AD. Studies of intact and impaired memory in age-related diseases suggest that normal aging has markedly different effects upon different memory systems.
A major goal of cognitive neuroscience is to identify and characterize memory systems of the human brain, which are specific neural networks that mediate specific mnemonic processes. One purpose is to provide an accurate and comprehensive understanding of the functional neural architecture of human memory. A second purpose is to understand how acute or degenerative injuries to particular systems result in particular memory disabilities. These two purposes are intertwined in research because, historically, identification and characterization of human memory systems has occurred through clinical and experimental evaluations of patients with focal brain lesions. More recently, functional neuroimaging has provided new views of the memory systems that record, retain, and retrieve various forms of experience.
The modern study of memory systems began in the 1950s with the appreciation of the brain–behavior relations underlying global amnesia, a neurological syndrome characterized by a deficit in learning new information that does not appear to be secondary to any other behavioral incapacity (1, 2). Amnesic patients perform poorly on explicit tests of memory in which they are asked to recall or recognize recent events or the material (stimuli) encountered in those events (3). Such patients have bilateral lesions of the limbic system, specifically medial temporal-lobe structures (including the hippocampus, amygdala, and entorhinal and parahippocampal regions) (1, 4), diencephalic structures (including the mediodorsal nucleus of the thalamus and mammillary bodies) (5), or basal forebrain structures (6). Thus, amnesic patients provide evidence for medial-temporal and diencephalic components of a system that mediates declarative memory for events (7) and facts (8).
A more restricted memory deficit is seen in patients with dorsolateral frontal-lobe lesions. Such patients often perform well on tests of memory but may fail on tests of strategic memory that require a person to work with or reason about memories. Thus, frontal-lobe patients often perform well on recognition tests of word lists and other materials, in which they are presented with recently encountered stimuli and foils and asked to select which stimuli had been encountered. Little reasoning is usually required to know whether a presented stimulus has been encountered recently. In contrast, these patients often perform poorly on memory tasks where they must devise an internally generated strategy to guide memory performance, such as free recall (9, 10, 11), recency or temporal order judgments (12, 13), temporal dating of public events (13), frequency judgments (14), self-ordered-pointing (15), conditional associative learning (16), and recollection of the source of information (17). Thus, these patients provide evidence for a dorsolateral prefrontal component of a strategic memory system.
The vast majority of people with memory difficulties, however, do not have focal lesions. Rather, their memory difficulties are due to age-related neurodegenerative diseases. There are an estimated 4 million Americans with Alzheimer disease (AD), and a profound deficit in declarative memory is often the first and most severe manifestation of the disease. There are also an estimated 1 million Americans with Parkinson disease (PD). Although PD is noted for its motor abnormalities, there is now clear evidence that PD patients have strategic memory deficits (18, 19). Further, declarative memory is affected not only by age-related diseases but also by aging in healthy individuals over 65 years of age, who constitute an eighth of the population (20).
Understanding age-related mnemonic difficulties in terms of memory systems is important for clinical and basic-science purposes. For clinical purposes, biological analyses of memory dysfunction are needed in the rational search for treatments that could ameliorate or even cure age-related memory disturbances. For purposes of basic neuroscience research, theoretically motivated analyses of age-related memory dysfunctions may reveal new knowledge about the identity and character of memory systems that are not revealed by focal-lesion patients. For example, experiments have revealed that amnesic patients can show intact performance on implicit memory tests of skill learning, repetition priming, and conditioning (21). These findings indicate that some forms of memory do not depend upon the medial-temporal or diencephalic structures injured in amnesia, but the findings fail to indicate what neural networks do subserve such implicit memories.
This paper focuses on the status of two forms of memory in age-related diseases. First, the status of repetition priming is examined in AD. Second, the status of strategic memory is reviewed in PD. Finally, some implications are considered for normal age-related changes in memory performance.
Alzheimer Disease
AD is a progressive neurodegenerative disease typically characterized by an initial, profound amnesia for recently experienced events (see, for example, ref. 22). Subsequently, a dementia develops with multiple impairments in higher cognitive functions, including linguistic, visuospatial, attentional, and reasoning incapacitates (23). The pattern of neuropathology in AD may be described as multifocal and hierarchical. It is multifocal in that several, but not all, brain regions are affected. Early and severe degenerative changes in the same medial-temporal structures (24, 25) that are acutely injured in global amnesia probably account for the early and severe declarative memory deficit in AD. Indeed, AD patients in the earliest stage of the disease may differ from age-matched control subjects only by reduced hippocampal volume (L. deToledo-Morrell, M. Sullivan, F. Morrell, R. S. Wilson and D. A. Bennett, unpublished data). Unlike amnesic patients, AD patients have additional damage to frontal, parietal, and temporal association neocortices that result in multiple cognitive deficits in attention, language, reasoning, and other domains (24, 25, 27).
Other brain regions, however, are less affected in AD. Primary motor cortex, the cerebellum, and the basal ganglia are minimally affected. The sparing of these brain regions is thought to underlie the preservation of motor skill learning in early-stage AD patients (28, 29, 30). In vivo metabolic imaging studies with early-stage AD patients (31) and postmortem studies of late-stage AD patients (24, 25, 27) show that there is relatively little compromise of primary somatosensory, auditory, and visual cortices. In the visual pathway, there appears to be a hierarchical pattern of cortical vulnerability, with successive stages of cortical processing being increasingly affected by AD (24, 27). The sparing of primary visual cortex is thought to underlie the preservation of long-term visual aftereffects in AD (32).
Comparison of memory deficits in AD and amnesia can help to identify association neocortical memory systems. Similar poor performance on declarative memory tests by AD and amnesic patients may reflect similar damage to the medial-temporal memory system. Dissimilar performance on other memory measures could reflect the additional neocortical damage that occurs in AD but not in amnesia.
Repetition Priming in AD
Memory can be measured in many ways, but most experimental studies have used a three-stage procedure: (i) a study phase in which people are exposed to a set of target materials, such as a list of words; (ii) a retention interval that can vary in duration and in whether it involves the performance of an unrelated task; and (iii) a test phase in which memory for the target materials is measured. Traditional explicit or declarative measures of recall and recognition memory, respectively, ask people to produce the target items, or to discriminate target items from other items that were not presented during the study phase.
Studies of repetition priming memory use study phases and retention intervals identical to standard recall and recognition tests. In the test phase, however, no reference is made to the prior study phase, and people are not told that they are performing a memory test. Instead, they are simply instructed to perform a new task. Typically, half the items in the test phase are repetitions of target items or related to target items. The other items are unrelated to the study-phase targets and provide a baseline measure of performance. Priming is measured as the difference in performance, usually gains in accuracy or speed, with target items relative to baseline items, a difference that is due to study-phase exposure to the target items. Thus, priming constitutes an implicit or indirect measure of long-term memory for a stimulus.
There are multiple kinds of priming. A fundamental distinction is that between perceptual priming, which is linked to stimulus form, and conceptual priming, which is linked to stimulus meaning (33). Perceptual priming is maximal when corresponding study-phase and test-phase items are perceived in the same modality, and reduced when they are experienced in different modalities. Conceptual priming is enhanced by semantic relative to nonsemantic study and unaffected by modality manipulations.
A theoretically important and remarkable finding is that despite their severe declarative memory deficits, amnesic patients show normal priming on many perceptual and conceptual tasks (21, 34). The long-term memory that manifests in priming, therefore, must not depend upon the medial-temporal or diencephalic structures critical for declarative memory. We hypothesized that AD patients could be useful in delineating the memory systems that mediate perceptual and conceptual priming. If perceptual (in this case visual) priming is mediated by relatively lower-order, modality-specific cortices, then such priming could be intact in AD. Further, if conceptual priming is mediated by polymodal association cortices, such priming could be impaired in AD.
To examine perceptual priming in AD, we used a word-identification priming task (35). In one study, AD patients and age-matched control subjects were exposed to study-phase words in two different ways (36). For half the words, subjects saw each word (e.g., truck) and read it aloud (read trials). For the other words, subjects saw a definition and a single letter (e.g., a vehicle for moving the injured—A) and were asked to generate the word that fit the definition and began with that letter (e.g., ambulance; generate trials). After one such list of read and generate trials, subjects were given an explicit test of recognition memory in which they had to discriminate words they had read or generated from new, foil words. There were two main results (Fig. 1). First, subjects had superior memory for words they had generated. This is thought to reflect the greater conceptual effort needed to generate a word versus reading a word. Second, AD patients had impaired memory.
Figure 1.
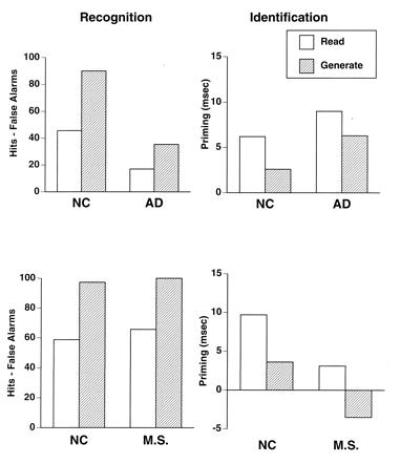
Performance of patients with AD and age-matched normal control (NC) subjects (Upper), and patient M. S. and age-matched normal control subjects (Lower) on tests of explicit word recognition (Left) and implicit word-identification priming (Right) after reading words (read) or generating words to definitions (generate).
After another list of read and generate trials, subjects performed a word-identification test. On each trial, subjects were asked to identify a word presented very briefly (17 msec) and with a mask. If the subject identified a word correctly, the next word was shown. If the subjects failed to identify a word correctly, the word was presented for longer durations, in 17 msec increments, until the word was identified. The measure of priming was how much more accurately (i.e., how much less time was required) subjects identified words read or generated than new, baseline words. There were three main results (Fig. 1). First, subjects showed priming by identifying studied words more accurately than baseline words. Second, opposite to the explicit recognition results, subjects showed more priming for read trials than generate trials. This is thought to reflect the perceptual nature of this priming task: actually perceiving a word in a read trial yields a superior visual memory trace than producing a word in a generate trial. Thus, greater priming in the read condition validated the perceptual nature of the priming. Third, despite their impaired recognition memory for study-phase words, the AD patients showed normal magnitudes of priming.
To examine conceptual priming, we used a category-exemplar task with AD patients, age-matched control subjects (mean age of 70 years), and younger subjects (mean age of 21 years) (37). In the study phase, subjects saw atypical exemplars (e.g., cucumber or tricycle) of various conceptual categories (e.g., vegetables or vehicles). For half the words, subjects judged whether the words named natural or manufactured objects. This judgment requires consideration of the meaning of the word and was called the deep condition. For the other words, subjects judged whether the words appeared in upper-case or lower-case letters. This judgment does not require any consideration of meaning and was called the shallow condition. After one such list, subjects were given the categories as cues (e.g., vegetables) and asked to explicitly recall what exemplars from those categories were shown in the study list. Subjects had superior memory for words studied under the deep condition (Fig. 2). This is thought to reflect the greater conceptual effort needed to judge the meaning rather than the letter-case of a word. Younger subjects recalled more words than older subjects, who, in turn, recalled more words than AD patients. Notably, the effect of deep versus shallow study was quite similar in younger and older healthy subjects but absent in AD patients.
Figure 2.
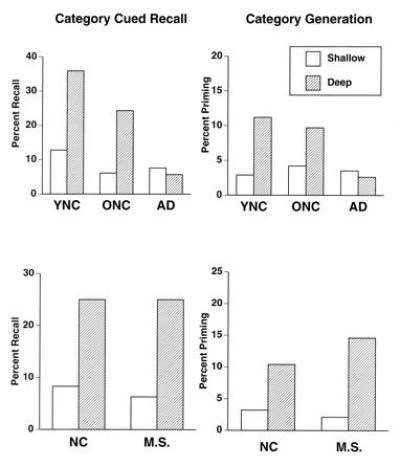
Performance of patients with AD, age-matched older normal control (ONC) subjects, and younger normal control (YNC) subjects (Upper), and patient M. S. and age-matched normal control (NC) subjects (Lower) on tests of explicit category-cued recall (Left) and implicit category-cued priming (Right) after judging the meaning (deep) or appearance (shallow) of words.
After another such list, subjects performed an exemplar generation task in which they were provided category names (e.g., vehicles or instruments) and asked to produce the first eight exemplars they thought of belonging to each category. The measure of priming was how much more likely than chance subjects were to provide the specific exemplars shown in the study list. Subjects showed priming, and priming was greater for exemplars studied in deep than shallow conditions (Fig. 2). The enhanced memory due to conceptual analysis validated the conceptual nature of the priming. Older and younger subjects had equal priming, a result unlike that seen in the cued-recall condition. This demonstrates a dissociation in normal aging between sustained implicit and reduced explicit memory for exemplars (38, 39). Finally, AD patients had reduced priming and failed to show any benefit of conceptual processing.
These results indicate a dissociation between intact perceptual and impaired conceptual priming in AD. The preserved perceptual priming may reflect relatively spared modality-specific occipital cortex, and the impaired conceptual priming compromised polymodal association cortices. Two additional lines of evidence support this possibility. First, two patients with occipital lesions have shown the reverse dissociation with impaired visual-perceptual priming and intact conceptual priming (40, 41, 42). One patient (M. S.) had most of his right occipital lobe removed for the treatment of otherwise intractable epilepsy. The unilateral excision included all of areas 17 and 18 and a portion of area 19 of Brodmann. He was given the same tests of memory described above, and his performance was compared with age-matched control subjects. M. S. had the exact opposite pattern of performance from the AD patients: intact explicit memory (word recognition and cued recall), intact conceptual priming, and impaired perceptual priming (Figs. 1 and 2). Second, functional neuroimaging studies provide convergent evidence. Positron emission tomography and functional MRI studies have indicated the involvement of occipital regions in visual-perceptual priming (43, 44, 45) and left frontal regions in conceptual priming (46, 47, 48).
PD and Strategic Memory
PD is a neurodegenerative motor disorder characterized by resting tremor, cogwheel rigidity, bradykinesia, and postural reflex impairment. PD’s signature lesion is loss of neurons in the pars compacta of the substantia nigra and consequent dopamine depletion in the striatum (49). In addition, there is damage to the mesocortical dopaminergic projection from the ventral tegmental area to the frontal lobes (50, 51). PD patients in even the earliest stages of the disease exhibit a pattern of intact and impaired cognition and memory that is strikingly similar to that shown by patients with frontal-lobe lesions (reviewed in ref. 18). In particular, PD and frontal-lobe patients exhibit strategic memory impairments.
We hypothesized that many of the strategic memory impairments in PD are the consequence of reduced working memory capacity (19). Working memory is a multicomponent psychological system that supports the temporary storage, manipulation, and transformation of information needed to perform cognitive tasks (52, 53). We measured working memory capacity with a Listening Span test (54, 55). Subjects were instructed to listen to a sentence (e.g., “The boy ran with the dog.”), to immediately select from among three choices the correct answer to a question about the sentence (e.g., Who ran, a boy, man, or girl?), and to remember the last word from each sentence for later recall. The number of sentences presented on each trial increased successively from one to seven, with three trials presented at each series length. After selecting the answers for the questions about each sentence in a trial, subjects were asked to write down the last word of each sentence in the order that they had been presented. The span score was the maximum number of sentences for which a subject recalled all final words in the correct order for at least two of three trials. Thus, this measure operationalizes working memory directly by making subjects simultaneously (i) process sentences one after another to answer a question about each sentence, and (ii) hold in memory the final words of each sentence for later recall.
PD patients had only half the working memory capacity of age-matched control subjects. The same PD patients were unimpaired on a two-alternative forced-choice recognition memory test, a nonstrategic declarative memory test (Fig. 3). They were, however, impaired on three strategic memory tests: free recall, temporal ordering, and self-ordered pointing (Fig. 3). There was a strong correlation in the PD patients between working memory capacity and strategic memory performance. There was also a strong correlation in the PD patients between working memory capacity and psycho-motor speed. Psycho-motor speed was measured with the Symbol Digit Modality Test. Subjects were presented with a “key” consisting of nine simple geometric designs (e.g., a plus sign, a circle, and an inverted T); each design was matched with a unique digit from one to nine. The test form consisted of a series of the nine designs, and the subject’s task was to write down next to each design the corresponding digit from the key. Their score was the number of correctly completed targets in a 90-sec trial. Working memory capacity did not correlate with more pure measures of motor performance in the PD patients, suggesting that working memory was related to psycho-motor rather than motor speed.
Figure 3.
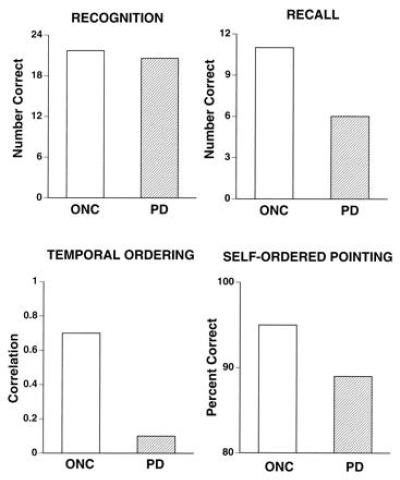
Performance of patients with PD and age-matched older normal control (ONC) subjects on four tests of memory for words.
We have hypothesized that PD may serve as a model for normal aging because many postmortem and in vivo imaging studies find striking, decade-by-decade linear decreases in dopaminergic function across the adult life-span (reviewed in ref. 56). We tested this hypothesis directly by examining the performance of PD patients, age-matched healthy subjects in their 60s and 70s, and younger healthy subjects in their 20s. Two tests examined psycho-motor processing speed (Symbol Digit Modality Test and Numeric Comparison). Two tests examined working memory capacity (Listening Span and Digit Ordering). One test examined reasoning and problem-solving (Raven’s Progressive Matrices). The pattern of performance was identical on all tests: younger normal control subjects outperformed older normal control subjects, and older normal control subjects outperformed PD patients (Fig. 4). The three groups performed similarly on a test of vocabulary knowledge, a test of semantic memory that has minimal working-memory and strategic-memory demands. Of interest was the finding that if psycho-motor processing speed was factored out by analysis of covariance, the age-related and PD-related deficits in working memory and reasoning were eliminated.
Figure 4.
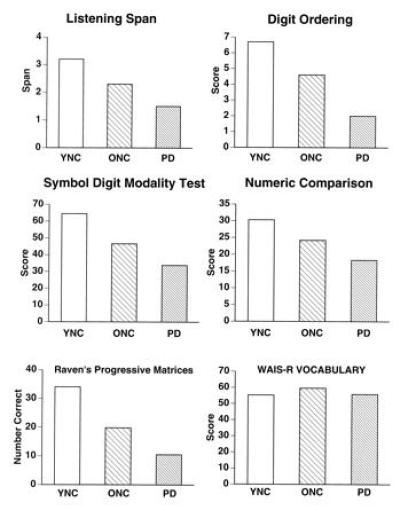
Performance of patients with PD, age-matched older normal control (ONC) subjects, and younger normal control (YNC) subjects on two tests of working memory capacity (Top), two tests of psycho-motor processing speed (Middle), a test of reasoning (Bottom left), and a test of semantic knowledge (Bottom right). WAIS-R, Wechsler Adult Intelligence Scale-Revised.
The pattern of relatively intact nonstrategic and sharply impaired strategic declarative memory is seen not only in PD and in patients with focal dorsolateral prefrontal lesions but also in healthy older relative to healthy younger individuals (reviewed in ref. 18). The similarity between PD patients and focal frontal-lesion patients supports the idea that the PD deficit reflects mesocortical rather than nigral dopaminergic dysfunction. The close relation between prefrontal cortex, dopamine, and working memory is well established in animal models (53). It may be asked whether the PD or focal frontal-lesion patients provide a more suitable model for the neurobiological basis of age-related declines in working and strategic memory. We hypothesize that the PD model may be more applicable for three reasons. First, the degenerative nature of PD seems more applicable to aging than does an acute focal lesion. Second, dopaminergic function is selectively vulnerable in aging and in PD. Third, reasoning deficits in patients with prefrontal lesions are not associated with slowed cognitive processing, as in aging and PD, but are associated with normal or even pathologically fast but inaccurate cognitive processing (57, 26).
Correlational analyses have multiple possible interpretations, but the above results lend support to a hypothesis about the consequences of moderately reduced dopaminergic function across the healthy adult life-span and severely reduced dopaminergic function in PD. Dopaminergic dysfunction may lead to a slowing of psycho-motor processing speed, which in turn, reduces working-memory capacity. Reduced working-memory capacity impairs reasoning ability, which in turn, impairs strategic memory even when the necessary information is available in recent (declarative) or long-term (semantic) memory stores.
Memory Systems and Normal Aging
The above studies reveal multiple relations between normal aging, age-related diseases, and memory systems. Perceptual memory systems appear minimally, if at all, affected by either aging or AD. Conceptual memory systems appear to be unaffected by aging but injured in AD. Strategic memory systems appear to undergo a continuous decline across the adult life-span. Nonstrategic memory may be minimally unaffected for much of the adult life-span. A decline in nonstrategic memory may be an early marker for the initial, preclinical medial-temporal neurodegeneration that ultimately becomes expressed as AD. Because age is a major risk factor for AD, one would expect to see an increasing number of individuals exhibiting recognition-memory deficits in older age.
Thus, studies of intact and impaired memory in age-related diseases suggest that normal aging has markedly different effects upon different memory systems. Aging may have little or no effect upon neocortical memory systems that mediate perceptual and conceptual priming. Aging has a continuous, life-long effect upon a frontal-lobe system that mediates critical aspects of working and strategic memory. Degeneration in this system may account for much of the age-related decline in declarative memory seen in healthy people in their 60s and 70s. Finally, the insidious, late onset of AD may account for the further declarative memory deficits in old age.
Acknowledgments
I thank Marion Zabinski for help with preparation of the manuscript. This work was supported by grants from the Office of Naval Research (N00014-92-J-184), the National Institute of Neurological Disorders and Stroke (1P50NS26985), the National Institute on Aging (AG11121), and the National Institute of Mental Health (MH53673).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: AD, Alzheimer disease; PD, Parkinson disease.
References
- 1.Scoville W B, Milner B. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milner B, Teuber H-L. Analysis of Behavioral Changes. New York: Harper & Row; 1968. [Google Scholar]
- 3.Graf P, Schacter D L. J Exp Psychol Learn Mem Cognit. 1985;11:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- 4.Zola-Morgan S, Squire L R. Behav Neurosci. 1986;100:155–160. doi: 10.1037//0735-7044.100.2.155. [DOI] [PubMed] [Google Scholar]
- 5.Victor M R, Adams R D, Collins G H. The Wernicke-Korsakoff Syndrome. Philadelphia: Davis; 1971. [PubMed] [Google Scholar]
- 6.Damasio A R, Graff-Radford N R, Eslinger P J, Damasio H, Kassell N. Arch Neurol. 1985;42:263–271. doi: 10.1001/archneur.1985.04060030081013. [DOI] [PubMed] [Google Scholar]
- 7.Cohen N J, Squire L R. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 8.Gabrieli J D E, Cohen N J, Corkin S. Brain Cognit. 1988;7:525–539. [Google Scholar]
- 9.Della Rocchetta A I. Cortex. 1986;22:189–211. doi: 10.1016/s0010-9452(86)80045-4. [DOI] [PubMed] [Google Scholar]
- 10.Janowsky J S, Shimamura A P, Kritchevsky M, Squire L R. Behav Neurosci. 1989;103:548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- 11.Jetter W, Poser U, Freeman R B J, Markowitsch H J. Cortex. 1986;22:229–242. doi: 10.1016/s0010-9452(86)80047-8. [DOI] [PubMed] [Google Scholar]
- 12.Milner B. Brit Med J. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 13.Shimamura A P, Janowsky J S, Squire L R. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- 14.Smith M L, Milner B. Neuropsychologia. 1988;26:297–306. doi: 10.1016/0028-3932(88)90082-6. [DOI] [PubMed] [Google Scholar]
- 15.Petrides M, Milner B. Neuropsychologia. 1982;20:601–614. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 16.Petrides M. Neuropsychologia. 1985;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 17.Janowsky J S, Shimamura A P, Squire L R. Neuropsychologia. 1989;8:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- 18.Gabrieli J D E. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Amsterdam: Elsevier; 1991. pp. 149–166. [Google Scholar]
- 19.Gabrieli J D E, Singh J, Stebbins G T, Goetz C G. Neuropsychology. 1996;10:322–332. [Google Scholar]
- 20.Salthouse T A. Adult Cognition: An Experimental Psychology of Human Aging. New York: Springer; 1982. [Google Scholar]
- 21.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Corkin S. In: Alzheimer’s Disease: A Report of Progress in Research. Corkin S, Davis K L, Growdon J H, Usdin E, editors. New York: Raven; 1982. pp. 149–164. [Google Scholar]
- 23.Nebes R D. Psychol Bull. 1989;106:377–394. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- 24.Arnold S E, Hyman B T, Flory J, Damasio A R, Van Hoesen G W. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 25.Brun A, Englund E. Histopathol. 1981;5:549–564. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 26.Owen A M, Downes J J, Sahakian B J, Polkey C E, Robbins T W. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 27.Lewis D A, Campbell M J, Terry R D, Morrison J H. J Neurosci. 1987;7:1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eslinger P J, Damasio A R. J Neurosci. 1986;6:3006–3009. doi: 10.1523/JNEUROSCI.06-10-03006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrieli J D E, Corkin S, Mickel S F, Growdon J H. Behav Neurosci. 1993;107:899–910. doi: 10.1037//0735-7044.107.6.899. [DOI] [PubMed] [Google Scholar]
- 30.Heindel W C, Butters N, Salmon D P. Behav Neurosci. 1988;102:141–147. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- 31.Frackowiak R S J, Pozzilli C, Legg N J, Du Boulay G H, Marshall J, Lenzi G L, Jones T. Brain. 1981;104:753–778. doi: 10.1093/brain/104.4.753. [DOI] [PubMed] [Google Scholar]
- 32.Savoy R L, Gabrieli J D E. Percept Psychophys. 1991;49:448–455. doi: 10.3758/bf03212178. [DOI] [PubMed] [Google Scholar]
- 33.Blaxton T A. J Exp Psychol Learn Mem Cognit. 1989;15:657–668. [Google Scholar]
- 34.Vaidya C J, Gabrieli J D E, Keane M M, Monti L A. Neuropsychology. 1995;9:580–591. [Google Scholar]
- 35.Keane M M, Gabrieli J D E, Fennema A C, Growdon J H, Corkin S. Behav Neurosci. 1991;105:326–342. doi: 10.1037//0735-7044.105.2.326. [DOI] [PubMed] [Google Scholar]
- 36.Fleischman D A, Gabrieli J D E, Reminger S, Rinaldi J, Morrell F, Wilson R. Neuropsychology. 1995;9:187–197. [Google Scholar]
- 37.Monti L A, Gabrieli J D E, Reminger S L, Rinaldi J A, Wilson R S, Fleischman D A. Neuropsychology. 1996;10:101–112. [Google Scholar]
- 38.Light L L, Singh A, Capps J L. J Clin Exp Neuropsychology. 1986;8:62–74. doi: 10.1080/01688638608401297. [DOI] [PubMed] [Google Scholar]
- 39.Light L L, Albertson S A. Psychol Aging. 1989;4:487–492. doi: 10.1037//0882-7974.4.4.487. [DOI] [PubMed] [Google Scholar]
- 40.Fleischman D A, Gabrieli J D E, Reminger S, Rinaldi J, Morrell F, Wilson R. Neuropsychology. 1995;9:187–197. [Google Scholar]
- 41.Gabrieli J D E, Fleischman D A, Keane M M, Reminger S L, Morrell F. Psychol Sci. 1995;6:76–82. [Google Scholar]
- 42.Keane M M, Gabrieli J D E, Mapstone H, Johnson K A, Corkin S. Brain. 1995;118:1129–1148. doi: 10.1093/brain/118.5.1129. [DOI] [PubMed] [Google Scholar]
- 43.Squire L R, Ojemann J G, Miezin F M, Petersen S E, Videen T O, Raichle M E. Proc Natl Acad Sci USA. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;12:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schacter D L, Reiman E, Uecker A, Polster M R, Yun L S, Cooper L A. Nature (London) 1995;376:587–590. doi: 10.1038/376587a0. [DOI] [PubMed] [Google Scholar]
- 46.Gabrieli J D E, Desmond J E, Demb J B, Wagner A D, Stone M V, Vaidya C J, Keane M M, Glover G H. Psychol Sci. 1996;7:278–283. [Google Scholar]
- 47.Demb J B, Desmond J E, Wagner A D, Vaidya C J, Glover G H, Gabrieli J D E. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raichle M E, Fiez J A, Videen T O, MacLeod A K, Pardo J V, Fox P E, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 49.Hornykiewicz O. Pharmacol Rev. 1966;18:925–964. [PubMed] [Google Scholar]
- 50.Javoy-Agid F, Agid Y. Neurology. 1980;30:1326–1330. doi: 10.1212/wnl.30.12.1326. [DOI] [PubMed] [Google Scholar]
- 51.Scatton B, Rouquier L, Javoy-Agid F, Agid Y. Neurology. 1982;32:1039–1040. doi: 10.1212/wnl.32.9.1039. [DOI] [PubMed] [Google Scholar]
- 52.Baddeley A. Working Memory. Oxford: Oxford Univ. Press; 1986. [Google Scholar]
- 53.Goldman-Rakic P S. In: Handbook of Physiology. Plum F, Mountcastle V, editors. Vol. 5. Bethesda: Am. Physiol. Soc.; 1987. pp. 373–417. [Google Scholar]
- 54.Daneman M, Carpenter P. J Verbal Learn Verbal Behav. 1980;19:450–466. [Google Scholar]
- 55.Salthouse T A, Babcock R L. Dev Psychol. 1991;27:763–776. [Google Scholar]
- 56.Gabrieli J D E. In: Models of Information Processing in the Basal Ganglia. Houk J C, Davis J L, Beiser D G, editors. Boston: MIT Press; 1994. pp. 277–294. [Google Scholar]
- 57.Duncan J, Burgess P, Emslie H. Neuropsychologia. 1995;33:261–268. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]


