Abstract
The DNA recombination and repair machineries of Mycoplasma genitalium and Mycoplasma pneumoniae differ considerably from those of gram-positive and gram-negative bacteria. Most notably, M. pneumoniae is unable to express a functional RecU Holliday junction (HJ) resolvase. In addition, the RuvB homologues from both M. pneumoniae and M. genitalium only exhibit DNA helicase activity but not HJ branch migration activity in vitro. To identify a putative role of the RuvA homologues of these mycoplasmas in DNA recombination, both proteins (RuvAMpn and RuvAMge, respectively) were studied for their ability to bind DNA and to interact with RuvB and RecU. In spite of a high level of sequence conservation between RuvAMpn and RuvAMge (68.8% identity), substantial differences were found between these proteins in their activities. First, RuvAMge was found to preferentially bind to HJs, whereas RuvAMpn displayed similar affinities for both HJs and single-stranded DNA. Second, while RuvAMpn is able to form two distinct complexes with HJs, RuvAMge only produced a single HJ complex. Third, RuvAMge stimulated the DNA helicase and ATPase activities of RuvBMge, whereas RuvAMpn did not augment RuvB activity. Finally, while both RuvAMge and RecUMge efficiently bind to HJs, they did not compete with each other for HJ binding, but formed stable complexes with HJs over a wide protein concentration range. This interaction, however, resulted in inhibition of the HJ resolution activity of RecUMge.
Introduction
A significant proportion of the genomes of Mycoplasma pneumoniae and Mycoplasma genitalium (approximately 8% and 4%, respectively) is composed of repeated DNA elements. These elements are referred to as RepMP elements in M. pneumoniae [1], [2], [3] and MgPa repeats (MgPars) in M. genitalium [4], [5], [6]. Although the different variants of these elements show a high level of sequence homology, they are not identical. Moreover, one or more of these variants are contained within open reading frames (ORFs) that encode antigenic surface proteins. Among these proteins are P1, P40 and P90 of M. pneumoniae and MgPa and P110 of M. genitalium. As these proteins can display amino acid sequence variation within the regions encoded by the RepMP and MgPar sequences, it has been proposed that this variation originates from recombination between different variants of RepMP or MgPar [7], [8],[9],[10],[11],[12],[13]. Consequently, homologous recombination between the repeated DNA elements in both Mollicutes species may play a crucial role in immune evasion [14].
It has previously been suggested that the mechanism of recombination between repeated DNA elements in M. pneumoniae and M. genitalium is similar to that of general homologous DNA recombination in these species [15], [16]. As a consequence, these processes may utilize the same enzymatic machinery. Recent studies that were aimed at elucidation of the mechanism of recombination between repeated DNA elements therefore focused on the characterization of Mycoplasma proteins predicted to be involved in homologous DNA recombination, such as RecA [15], single-stranded DNA-binding protein (SSB) [16], RuvA [17], RuvB [18] and RecU [19], [20]. The RecA proteins from M. pneumoniae and M. genitalium (RecAMpn and RecAMge, respectively) and the SSB protein from M. pneumoniae (SSBMpn) were reported to possess similar activities as their counterparts from Escherichia coli [15], [16]. Both RecAMpn and RecAMge were found to catalyze the exchange of homologous DNA strands in an ATP- and Mg2+-dependent fashion [15]. This activity was stimulated strongly by SSBMpn, which is a tetrameric protein that selectively binds to single-stranded DNA (ssDNA) [16].
In contrast to the SSB and RecA proteins, the RecU, RuvA and RuvB proteins from M. pneumoniae and M. genitalium displayed in vitro activities that differed considerably from those of their counterparts from other bacterial classes. Specifically, the RecU protein from M. genitalium (RecUMge) was found to diverge from other Holliday junction (HJ) resolving enzymes in four major aspects [19]. First and foremost, RecUMge only displayed HJ resolvase activity in the presence of Mn2+ and not in the presence of Mg2+. In contrast, the RecU homologue from Bacillus subtilis (RecUBsu) and the RuvCEco and RusAEco resolvases from E. coli possess Mg2+-dependent resolvase activity. Second, RecUMge has a unique target DNA sequence, cleaving HJ substrates at the sequence 5′-G/TC↓PyTPuG-3′. This cleavage site differs from the cleavage sites of RecUBsu, RuvCEco and RusAEco (5′-G/TG↓CA/C-3′, 5′-A/TTT↓G/C-3′ and 5′-↓CC-3, respectively) [21], [22], [23], [24], [25]. Third, unlike the RecUBsu protein [21], RecUMge is unable to anneal circular ssDNA to homologous, linear double-stranded DNA (dsDNA). Fourth, RecUMge does not stably bind to long ssDNA substrates, in contrast to the RecUBsu protein [21].
Another crucial finding regarding the RecU orthologues from M. pneumoniae and M. genitalium was the inability of M. pneumoniae to produce a functional RecU protein [19], [20]. While a subset of M. pneumoniae strains (so-called subtype 2 strains) is able to express a RecU homologue (RecUMpn), this protein was found to be inactive in HJ-binding and -cleavage in vitro. Moreover, the other major subset of M. pneumoniae strains (subtype 1 strains) was reported to be incapable of producing a full-length RecU homologue, due to the presence of a nonsense codon in the RecU gene [19]. The inability of M. pneumoniae to produce a functional RecU protein was suggested to be (one of) the causative factor(s) of the relatively low level of homologous DNA recombination in this bacterium [19].
Unique properties were recently also attributed to the RuvB homologues from M. genitalium and M. pneumoniae (RuvBMge and RuvBFH, respectively). In contrast to the E. coli DNA branch migration motor protein RuvBEco, both RuvBMge and RuvBFH were found to have RuvA-independent DNA helicase activity [18]. The activity of RuvBMge, however, was significantly lower than that of RuvBFH. Interestingly, RuvBFH is exclusively expressed by subtype 2 strains of M. pneumoniae. The RuvB protein expressed by subtype 1 strains (RuvBM129) displays only marginal levels of DNA helicase activity, due to a single amino acid substitution with respect to RuvBFH [18]. Although RuvBFH did not appear to be stimulated at all by M. pneumoniae RuvA (RuvAMpn), the helicase activity of the RuvBMge protein was found to be promoted by M. genitalium RuvA (RuvAMge) under specific reaction conditions [18].
The apparent inability of RuvAMpn to stimulate RuvBFH activity can be caused by specific, aberrant features of the RuvBFH protein in comparison with RuvBEco. Alternatively, RuvAMpn itself may be unable to interact with, and/or activate, RuvBFH. In this regard, it is interesting to note that RuvAMpn did not stimulate the branch migration activity of RuvBEco in vitro, and could not functionally substitute for RuvAEco in vivo (in E. coli) [17]. Thus, while the E. coli RuvA protein has a vital role in the interaction with both RuvB and the HJ resolving enzyme RuvC (within the RuvABC resolvasome), the function of RuvAMpn within a putative branch migration and resolution complex remains enigmatic.
In this study, the activities of RuvAMpn and RuvAMge are characterized and compared. We show that these proteins differ considerably in (i) their affinities for branched and non-branched DNA substrates, (ii) complex formation with HJs, and (ii) their interaction with other proteins from the DNA recombination machinery.
Results
M. pneumoniae ORF MPN535 and M. genitalium ORF MG358 encode RuvA homologues
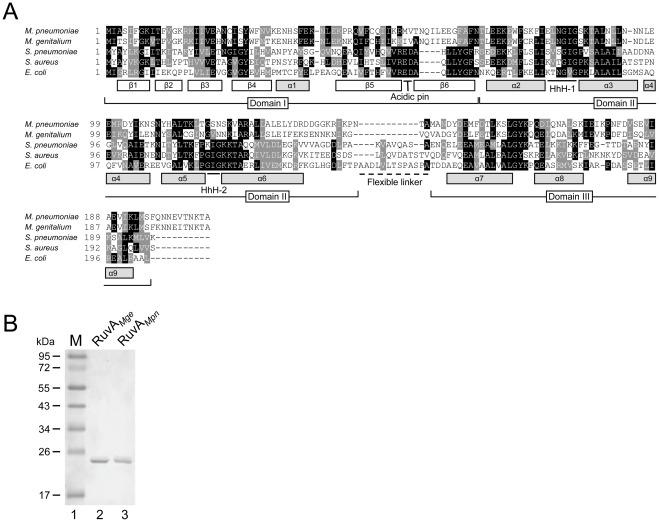
The MPN535 ORF of M. pneumoniae was previously shown to encode a RuvA homologue (RuvAMpn) [17]. A multiple amino acid sequence alignment indeed shows significant similarities between RuvAMpn and other (putative) RuvA proteins from gram-negative and gram-positive bacteria (Fig. 1A). While the similarity between the sequences of RuvAMpn and RuvAEco is relatively low (23.6% identity), a high similarity is observed between the sequences of RuvAMpn and RuvAMge (68.8% identity). In contrast to other members of the putative DNA recombination apparatus of M. pneumoniae, i.e. RecU and RuvB [18], [19], RuvAMpn does not differ in sequence among subtype 1 and subtype 2 strains.
Figure 1. Multiple alignment and purification of RuvAMpn and RuvAMge.
(A) An alignment was generated with amino acid sequences predicted to be encoded by the following ORFs (with GenBank accession numbers in parentheses), M. pneumoniae MPN535 (P75243), M. genitalium G37 MG358 (Q49424), Streptococcus pneumoniae ruvA (Q97SY4), Staphylococcus aureus ruvA (Q5HFC1) and E. coli ruvA (P0A809). Predicted secondary structural features and domains of the RuvA proteins are shown below the alignment and are based on the crystal structure of the RuvA protein from E. coli [27], [28], [30], [33], [54]. The position of the ‘acidic pin’, between β sheets 6 and 7 of RuvAEco, two helix-hairpin-helix (HhH) motifs, and the flexible linker (between domain II and III), are also indicated. The multiple alignment was performed using Clustal W (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The program BOXSHADE 3.21 (http://www.ch.embnet.org/software/BOX_form.html) was used to generate white letters on black boxes (for residues that are identical in at least three out of five sequences) and white letters on grey boxes (for similar residues). (B) Purification of RuvAMge and RuvAMpn. Samples of purified H10-tagged RuvAMge (lane 2) and H10-tagged RuvAMpn (lane 3) were analyzed by SDS-PAGE (12%) and Coomassie brilliant blue (CBB)-staining. The sizes of protein markers (lane 1; PageRulerTM Prestained Protein Ladder [Fermentas]) are shown on the left-hand side of the figure in kDa.
Within the RuvA sequences, a relatively high level of amino acid sequence conservation is found in two so-called helix-hairpin-helix (HhH) motifs (Fig. 1A) [26]. These motifs were previously identified within domain II of RuvAEco and were shown to be crucial for sequence-independent DNA binding by interacting with the DNA phosphate backbone of Holliday junctions (HJs) [27], [28], [29]. The lowest level of sequence conservation was seen in the region defined as the ‘flexible linker’, which separates domain II from domain III in RuvAEco [30].
RuvAMge and RuvAMpn can bind to synthetic oligonucleotide substrates
Both RuvAMge and RuvAMpn were expressed in E. coli as poly histidine (H10)-tagged proteins and were purified to near homogeneity using similar protocols (as described in Materials and Methods). The H10-tagged proteins were found to have activities that were indistinguishable from that of their non-tagged counterparts (data not shown). Because the H10-tagged proteins were obtained at higher concentrations and at a higher purity than their ‘native’ versions (>95% versus ∼90% homogeneity), they were used throughout this study. The estimated molecular masses of the purified proteins matched the theoretical molecular masses of 23.7 kDa for both RuvAMge (Fig. 1B, lane 2) and RuvAMpn (lane 3).
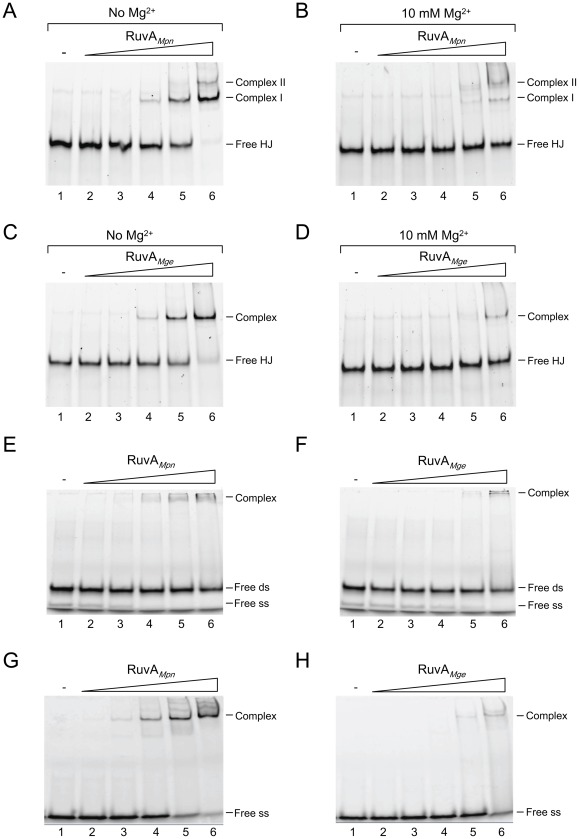
To test and compare the DNA-binding characteristics of RuvAMge and RuvAMpn, both proteins were incubated with HJs, double-stranded (ds) and single-stranded (ss) oligonucleotide substrates, and analyzed by electrophoretic mobility shift assay (EMSA). As described before [17], two distinct complexes (complex I and complex II) were formed between RuvAMpn and HJs in a protein-concentration dependent fashion (Fig. 2A). Similar complexes were reported to be generated between RuvAEco and HJs, and were found to consist of a single protein tetramer (complex I) or a double tetramer (complex II) bound to a HJ [30], [31], [32], [33], [34], [35]. The HJ binding activity of both RuvAMpn and RuvAMge was strongly reduced in the presence of Mg2+ (compare Fig. 2A to Fig. 2B, and Fig. 2C to Fig. 2D). A similar inhibitory effect of Mg2+ on DNA-binding activity has previously also been observed for RuvAEco [31], [36]. In contrast to RuvAEco and RuvAMpn, RuvAMge produced only a single complex with HJs (Fig. 2C, lane 6), even at protein concentrations up to 4 μM (see below). This complex migrated through the gels with a mobility similar to that of RuvAMpn-HJ complex I. These data indicated that: (i) the RuvAMge-HJ complex is composed of a tetramer of RuvAMge bound to a HJ, and (ii) RuvAMge may not stably bind to HJs as an octamer. These notions were supported by gel filtration chromatography data, which indicated that RuvAMge exists as a single, major protein species with a molecular mass of ∼108 kDa (Fig. S1). This molecular mass corresponds to the theoretical molecular mass of a tetramer of RuvAMge (95 kDa). Thus, RuvAMge primarily exists as a homo-tetramer in solution.
Figure 2. Binding of RuvAMpn and RuvAMge to HJs and other oligonucleotide substrates.
(A) Binding of RuvAMpn to HJ substrate HJ 1.1 in the absence of Mg2+. The DNA-binding reactions were performed as indicated in Materials and Methods. Reactions were performed in volumes of 10 μl and contained 12.3 nM DNA substrate and either 0 nM (marked ‘-’, lane 1), 27 nM (lane 2), 81 nM (lane 3), 243 nM (lane 4), 729 nM (lane 5) or 2.2 μM (lane 6) of RuvAMpn. Reaction products were electrophoresed through 8% polyacrylamide gels and analyzed by fluorometry. The positions of unbound HJ 1.1 (Free HJ) and RuvAMpn/HJ complexes (Complex I and II) are indicated at the right-hand side of the gel. (B) Binding of RuvAMpn to HJ substrate HJ 1.1 in the presence of 10 mM Mg2+. Reactions were carried out in a similar fashion as in (A). (C, D). Binding of RuvAMge to HJ substrate HJ 1.1 in the absence (C) or presence of 10 mM Mg2+ (D). (E, F) Binding of RuvAMpn (E) and RuvAMge (F) to double-stranded (ds) oligonucleotide HJ11/HJ11rv. The positions of the ds substrate (Free ds) and residual non-annealed oligonucleotide HJ11 (Free ss) is indicated at the right-hand side of the gels. (G, H) Binding of RuvAMpn (G) and RuvAMge (H) to single-stranded (ss) oligonucleotide HJ11. The reactions shown in panels (C) to (H) were carried out similarly as in (A).
In contrast to RuvAEco, RuvAMpn was previously reported to form stable complexes with linear duplex oligonucleotides [17]. As shown in Fig. 2E and 2F, both RuvAMpn and RuvAMge are able to form DNA-protein complexes in the presence of ds oligonucleotides (substrate HJ11/HJ11rv). Interestingly, at least part of these complexes consisted of RuvA molecules bound to non-annealed, ss oligonucleotide HJ11, which was present as a minor ‘contaminant’ of the ds substrate; this oligonucleotide (designated ‘Free ss’ in Fig. 2E and 2F) was completely complexed by the RuvA proteins at the highest protein concentrations tested (Fig. 2E, lane 4–6 and Fig. 2F, lane 6). In a separate EMSA, we could confirm the binding of RuvAMpn to oligonucleotide HJ11; this binding appeared to occur with an efficiency similar to that observed with the four-stranded HJ substrate (compare Fig. 2G to Fig. 2A). Conversely, while the RuvAMge protein also displayed binding to the ssDNA (Fig. 2H), this binding was considerably less efficient than that observed with the HJ substrate (Fig. 2C).
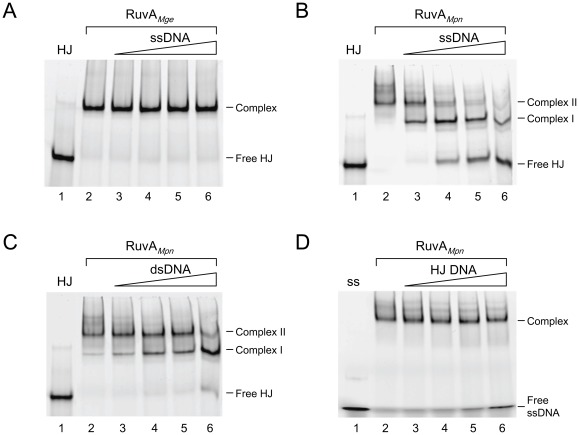
The preferences of RuvAMpn and RuvAMge for binding to either ssDNA or HJ DNA were further investigated in DNA-binding competition experiments, in which a labeled DNA substrate was kept at a constant concentration and another, unlabeled substrate was included at different concentrations. As shown in Fig. 3A, the binding of RuvAMge to the labeled HJ substrate was not significantly influenced by inclusion of up to a 20-fold excess of unlabeled ssDNA in the reaction (lanes 3–6). In contrast, the binding of RuvAMpn to the HJ substrate was already clearly reduced in the presence of a 2.5-fold excess of unlabeled ssDNA in the binding reactions (Fig. 3B, lane 3). Although the dsDNA substrate also competed with the HJ substrate for binding by RuvAMpn, this competition was less efficient than that observed with ssDNA (Fig. 3C). The high affinity of RuvAMpn for ssDNA was further demonstrated in an experiment in which the binding of RuvAMpn to labeled ssDNA was assayed in the presence of different concentrations of unlabeled HJ substrate. As shown in Fig. 3D, the ssDNA-binding of RuvAMpn was only marginally reduced in the presence of a 10-fold (lane 5) or 20-fold (lane 6) molar excess of unlabeled HJ DNA in the reactions. Thus, in contrast to RuvAMge (Fig. 2H and 3A), RuvAMpn is able to bind with a relatively high affinity to ssDNA.
Figure 3. DNA binding preferences of RuvAMge and RuvAMpn.
(A) Binding of RuvAMge (3 µM) to HJ substrate HJ 1.1 (6-FAM-labeled on strand HJ11) in the presence of various concentrations of unlabeled ssDNA (oligonucleotide HJ11). The molar excess of unlabeled DNA over labeled DNA in the reactions was 0× (lane 2), 2.5× (lane 3), 5× (lane 4), 10× (lane 5) and 20× (lane 6). The protein was added as final component in the reactions. Protein was omitted from the reaction shown in lane 1. The positions of the free HJ substrate (Free HJ) and RuvAMge-HJ complexes (Complex) are indicated at the right-hand side of the gel. (B) Binding of RuvAMpn (3 µM) to HJ substrate HJ 1.1 (6-FAM-labeled on strand HJ11) in the presence of various concentrations of unlabeled ssDNA (oligonucleotide HJ11). The experiment was performed similarly as in (A). The two major RuvAMpn-HJ complexes (Complex I and II) are indicated at the right-hand side of the gel. (C) Binding of RuvAMpn (3 µM) to HJ substrate HJ 1.1 (6-FAM-labeled on strand HJ11) in the presence of various concentrations of unlabeled dsDNA (oligonucleotide HJ11/HJ11rv). The experiment was performed similarly as in (A). The two major RuvAMpn-HJ complexes (Complex I and II) are indicated at the right-hand side of the gel. (D) Binding of RuvAMpn (3 µM) to ssDNA (6-FAM-labeled oligonucleotide HJ11) in the presence of various concentrations of HJ DNA (HJ 1.1). The experiment was performed similarly as in (A). Protein was omitted from the reaction shown in lane 1. The positions of the unbound ssDNA (Free ssDNA) and RuvAMpn-ssDNA complex (Complex) are indicated at the right-hand side of the gel.
The interaction between RuvAMge and RecUMge on HJs
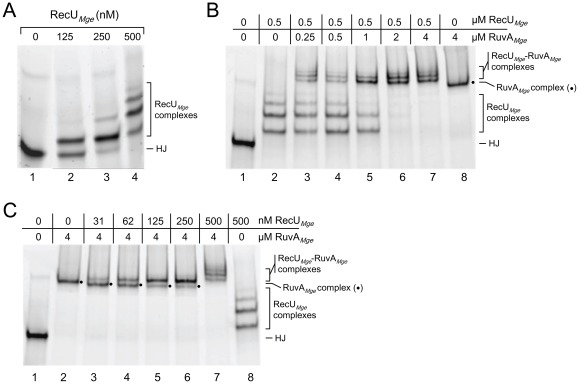
The RecU protein from M. pneumoniae (RecUMpn) was previously found to be inactive in HJ-binding and -cleavage [19]. In contrast, the M. genitalium RecU protein (RecUMge) was reported to be a potent HJ-resolving enzyme [19], [20]. Because it is possible that RecUMge functionally interacts with RuvAMge in the processing of HJs, both proteins were included in HJ binding and resolution assays. The binding of RecUMge to HJ substrate HJ 1.1 was previously demonstrated to result in a single DNA-protein complex [19], [20]. Interestingly, at relatively high RecUMge concentrations and at different binding conditions than those used previously (i.e., binding on ice instead of at room temperature and in the absence of BSA), a range of discrete RecUMge-HJ DNA-protein complexes were generated, with an inverse correlation between protein concentration and mobility of the complexes through EMSA gels (Fig. 4A, lanes 2–4). At 500 nM of RecUMge, three major DNA-protein complexes and one minor complex can be discerned (lane 4). A similar range of complexes was previously also observed after binding of E. coli resolvase RusA to HJ substrates [25]. Due to the distinct nature of the RecUMge-HJ complexes and their relative migration in the gel, we hypothesize that they represent different multimeric forms of RecUMge, bound to a single HJ substrate. Upon addition of RuvAMge to these complexes (after preincubation of RecUMge with the HJ substrate), novel complexes were formed with a considerably slower mobility than the RecUMge-HJ complexes (Fig. 4B, lanes 3–7). At the highest concentration of RuvAMge used (4 μM), all RecUMge-HJ complexes appeared to have shifted to a higher position in the gel (lane 7). Because the novel complexes had a slower mobility than the RuvAMge-HJ complex (Fig. 4B, lane 8), it is likely that they represent HJs bound by both RecUMge and RuvAMge. This notion was corroborated by a reciprocal experiment in which the HJ substrate was preincubated with RuvAMge (at 4 μM), followed by the addition of RecUMge at concentrations ranging from 0 nM to 500 nM (Fig. 4C, lanes 2–7). Already at a RecUMge concentration of 31 nM (lane 3), a ‘supershift’ of the RuvAMge-HJ complex was observed; this supershift was virtually complete at a RecUMge concentration of 250 nM (lane 6). At the latter concentration, a single major supershifted complex was observed. At 500 nM of RecUMge, however, four discrete supershifted complexes were formed, which corresponded in mobility with the complexes generated in the previous experiment (Fig. 4B, lane 7). Again, the supershifted complexes displayed a slower mobility than did the RecUMge-HJ and RuvAMge-HJ complexes (Fig. 4C, lanes 2 and 8), indicating that they indeed represent RecUMge-RuvAMge-HJ complexes. The interactions between RecUMge and RuvAMge on HJ substrates differ significantly from those reported between RuvAEco and the RuvC resolvase from E. coli (RuvCEco). Specifically, RuvAEco appears to have a significantly higher affinity than RuvCEco for HJ substrates, and a fully saturated RuvAEco-HJ complex (complex II) cannot be bound detectably by RuvCEco [32]. As a consequence, RuvACEco-HJ complexes are only observed at relatively low RuvAEco concentrations (1–20 nM); at higher RuvAEco concentrations, RuvACEco-HJ and RuvCEco-HJ complexes are either not formed or rapidly dissociated [32]. In contrast, RecUMge and RuvAMge do not appear to compete with each other in HJ binding, but rather associate readily and stably on a HJ substrate at a wide range of concentrations of both RuvAMge (Fig. 4B) and RecUMge (Fig. 4C). As yet, the multimeric protein composition of the different RecUMge-RuvAMge-HJ complexes is unknown. Nevertheless, while a single stable complex is generated between RuvAMge and HJs, it is likely that each of the RecUMge-RuvAMge-HJ complexes only contains a single tetramer of RuvAMge.
Figure 4. The interaction between RuvAMge and RecUMge on HJs.
(A) HJ-binding by RecUMge. The DNA-binding reactions were performed in a similar fashion as described in Fig. 3. Reactions were performed in volumes of 10 μl and contained 12.3 nM HJ 1.1 and the indicated concentrations of RecUMge. The positions of unbound HJs (HJ) and RecUMge-HJ complexes are depicted at the right-hand side of the gel. (B) The binding of RuvAMge to RecUMge-HJ complexes. RecUMge (0.5 μM) was incubated with HJ 1.1, followed by the addition of RuvAMge (at different concentrations, as indicated above the lanes). The nature of the various protein-DNA complexes is indicated at the right-hand side of the gel; RuvAMge-HJ complexes are indicated with a dot (•). (C) The binding of RecUMge to RuvAMge-HJ complexes. RuvAMge (4 μM) was incubated with HJ 1.1, followed by the addition of RecUMge (at various concentrations, as indicated above the lanes). The labeling of the figure is similar to that shown in (B).
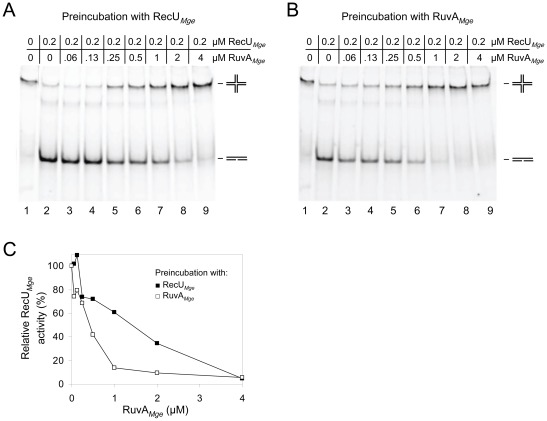
RuvAMge inhibits HJ resolution by RecUMge
Because RuvAMge readily binds to RecUMge-HJ complexes, we investigated the influence of RuvAMge on the activity of RecUMge in HJ resolution assays. In these assays, substrate HJ 1.1 was preincubated on ice with either RecUMge (at 0.2 μM; Fig. 5A) or RuvAMge (at 0 to 4 μM; Fig. 5B), followed by the addition of the other protein. After incubation for 30 min at 37°C, the resolution products were analyzed by polyacrylamide gel electrophoresis. As shown in Fig. 5A and 5B, RuvAMge inhibited the resolution activity of RecUMge in a RuvAMge concentration-dependent fashion. The inhibition of HJ resolution was most effective when RuvAMge was added to the HJ substrate before RecUMge (Fig. 5B and 5C). In that case, HJ resolution by RecUMge was already inhibited by ∼20% at a RuvAMge concentration of 60 nM (Fig. 5B, lane 3 and Fig. 5C). At RuvAMge concentrations of 1 μM or higher, RecUMge activity was reduced by ≥80% (Fig. 5B, lanes 7–9). When the HJ substrate was incubated with RecUMge before the addition of RuvAMge, a significant inhibition of HJ resolution activity (≥20%) was only observed at RuvAMge concentrations of ≥250 nM (Fig. 5A and 5C). Moreover, inhibition levels of >80% were not observed at RuvAMge concentrations lower than 4 μM. When RecUMge and RuvAMge were added simultaneously to the HJ substrates, a similar pattern of HJ resolution was observed as that shown in Fig. 5A (in which RecUMge was added to the reactions before RuvAMge). This finding corroborates the notion that RecUMge-HJ complexes cannot be dissociated by RuvAMge. Despite the significantly different dynamics in the formation of RecUMge-RuvAMge-HJ complexes and RuvACEco-HJ complexes, RuvAMge inhibits the resolution activity of RecUMge in a similar fashion as RuvAEco inhibits RuvCEco activity.
Figure 5. RuvAMge inhibits HJ resolution by RecUMge.
(A, B) HJ resolution assays [19] were performed in volumes of 10 µl and contained 12.3 nM HJ substrate HJ 1.1 (6-FAM-labeled), RecUMge (0.2 µM) and various concentrations of RuvAMge, as indicated above the lanes. Reactions were preincubated for 2 min with either RecUMge (A) or RuvAMge (B), followed by addition of the other protein. After incubation for 30 min at 37°C, the reaction products were separated on 12% polyacrylamide gels, and analyzed by fluorometry. The locations of the HJ substrate and resolution products are indicated schematically at the right-hand side of the gels. (C) Quantification of the influence of RuvAMge on RecUMge activity. The relative RecUMge (resolution) activity was measured from the gels shown in (A) and (B) and expressed as percentage of the protein's activity in the absence of RuvAMge. The data from (A) and (B) are represented by the closed squares (▪) and the open squares (□), respectively.
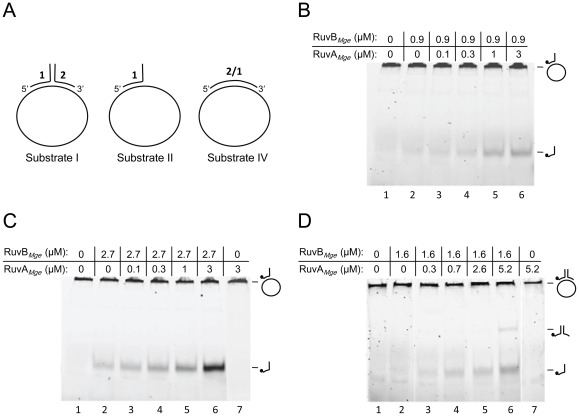
The influence of the RuvA proteins on the activities of RuvBFH and RuvBMge
The RuvB protein that is expressed by M. pneumoniae subtype 2 strains, RuvBFH, was recently reported to act as a DNA helicase on specific, partially double-stranded DNA substrates [18]. Interestingly, while this activity of RuvBFH was not influenced by RuvAMpn, the RuvB protein from M. genitalium, RuvBMge, did show RuvAMge-dependent helicase activity. The latter activity, however, was only detected on a single helicase substrate, i.e. Substrate IV from Fig. 6A [18]. To further delineate the functional interactions between the RuvA and RuvB proteins from M. pneumoniae and M. genitalium, the proteins were combined at various concentrations (including considerably higher RuvA concentrations than used previously) in DNA helicase or branch migration assays, using the DNA helicase substrates shown in Fig. 6A. While the helicase activity of RuvBFH was not influenced by RuvAMpn (data not shown), the helicase activity of RuvBMge on Substrate II (Fig. 6B and 6C) and Substrate I (Fig. 6D) was stimulated in the presence of high concentrations of RuvAMge. As expected, RuvAMge alone did not display any DNA helicase activity (lane 7 in Fig. 6C and 6D). This stimulatory effect of RuvAMge was observed at various concentrations of RuvBMge, from 0.9 μM (Fig. 6B) to 2.7 μM (Fig. 6C). These results indicated that the activation of RuvBMge by RuvAMge is a general phenomenon that is not restricted to a specific DNA substrate. Nevertheless, irrespective of the presence of high concentrations of the RuvA proteins, both RuvBMge and RuvBMpn were unable to unwind small, double-stranded oligonucleotide substrates (data not shown).
Figure 6. The influence of RuvAMge on the DNA helicase activity of RuvBMge.
(A) Schematic illustrations of the DNA substrates used in the DNA helicase assays. The substrates are composed of a combination of oligonucleotides (oligonucleotide 1, oligonucleotide 2 or oligonucleotide 2/1) and single-stranded, circular 5,386-bp φX174 DNA, as described previously [18]. (B, C) RuvAMge stimulates the DNA helicase activity of RuvBMge on Substrate II. Substrate II, 6-FAM-labeled at the 5′ end of oligonucleotide 1, was incubated with either 0 µM, 0.9 µM (B) or 2.7 µM (C) of RuvBMge in the presence of various concentrations of RuvAMge, as indicated above the lanes. (D) RuvAMge stimulates the DNA helicase activity of RuvBMge on Substrate I. Substrate I, 6-FAM-labeled at the 5′ end of oligonucleotide 1, was incubated with either 0 µM (lanes 1 and 7) or 1.6 µM of RuvBMge in the presence of various concentrations of RuvAMge, as indicated above the lanes. After the reaction (5 min at 37°C), the samples were deproteinized, electrophoresed through native 12% polyacrylamide gels, and analyzed by fluorometry. The positions of the substrates, which are too large to enter the gels, as well as the positions of the oligonucleotide reaction products, are indicated at the right-hand side of the gels by schematic illustrations. In these illustrations, the position of the 6-FAM label is indicated by a black dot.
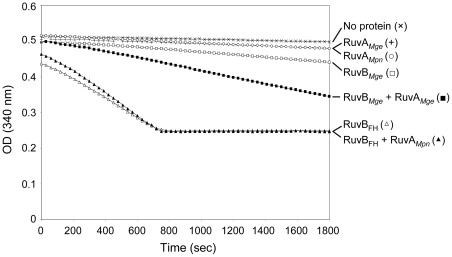
The ATPase activity of RuvBMge is stimulated by RuvAMge
While RuvBFH and RuvBMge were previously found to possess intrinsic ATPase activity, this activity was significantly higher for RuvBFH than for RuvBMge [18]. To investigate whether the ATPase activities of the RuvB proteins can be modulated by their corresponding RuvA proteins, ATPase assays were carried out in which the RuvA and RuvB proteins were tested together. In accordance with previous findings [18], RuvBFH was found to possess a significantly higher ATPase activity than RuvBMge (Fig. 7). However, while the activity of RuvBFH was not significantly influenced by RuvAMpn, the activity of RuvBMge was strongly stimulated by RuvAMge. Thus, the ATPase activities of RuvBFH and RuvBMge directly reflect the DNA helicase activities of these proteins in two important aspects. First, the intrinsic enzymatic activity of RuvBFH is higher than that of RuvBMge. Second, RuvBMge activity can be stimulated by RuvAMge, whereas RuvBFH activity is not influenced by RuvAMpn. As expected, both RuvAMpn and RuvAMge did not show any ATPase activity on their own (Fig. 7).
Figure 7. The influence of RuvAMpn and RuvAMge on the ATPase activities of RuvBFH and RuvBMge, respectively.
ATP hydrolysis by RuvBFH and RuvBMge was measured at a protein concentration of 0.5 µM, either in the absence of presence of the corresponding RuvA protein (at 1 µM). The ATPase activity was determined using an NADH-coupled assay. In this assay, the activity is calculated from the stationary velocities of ATP hydrolysis, as determined by monitoring the absorption of NADH at 340 nm [15], [46]. The ‘no protein’ reaction (×) indicates a control reaction performed in the absence of any protein. (+), RuvAMge alone; (○), RuvAMpn alone; (□), RuvBMge alone; (▪), RuvBMge plus RuvAMge; (▵), RuvBFH alone; (▴), RuvBFH plus RuvAMpn. The graph shows a representative experiment.
Discussion
The DNA recombination and repair machineries of mycoplasmas differ considerably from those of gram-positive and gram-negative bacteria. Most importantly, in contrast to the latter micro-organisms, mycoplasmas do not possess homologues of LexA, RecBCD, AddAB, RecQ, RecJ and RecF [37], [38]. In addition, some components of the putative DNA recombination machineries of M. pneumoniae and M. genitalium were found to have characteristics that diverge from those of their homologues from other bacterial classes. These components include the RecU and RuvB proteins [18], [19], [20]. In Table 1, the characteristics of these as well as the other (putative) components of the DNA recombination machineries of M. pneumoniae and M. genitalium are listed and compared.
Table 1. Compilation of the activities of the RecA, SSB, RuvA, RuvB and RecU proteins from M. pneumoniae, M. genitalium and reference bacteria.
| Protein | Species | ORF | Activities (in vitro) | Divalent cations, nucleotide and protein cofactors | Interacting proteins (physical and/or functional) | Reference | |
| RecA | |||||||
| RecAEco | E. coli | recA | Exchange of homologous DNA strands | Mg2+, ATP, SSBEco | SSBEco | [47] | |
| RecAMpn | M. pneumoniae | MPN490 | Exchange of homologous DNA strands | Mg2+, ATP, SSBMpn | SSBMpn | [15] | |
| RecAMge | M. genitalium | MG339 | Exchange of homologous DNA strands | Mg2+, ATP, SSB3 | SSB3 | [15] | |
| SSB | |||||||
| SSBEco | E. coli | ssb | ssDNA-binding, stimulation of RecAEco, various other roles in DNA replication, repair, and recombination | None | RecAEco, other proteins | [48] | |
| SSBMpn | M. pneumoniae | MPN229 | ssDNA-binding, stimulation of RecAMpn | None | RecAMpn | [16] | |
| SSBMge | M. genitalium | MG091 | Unknown | Unknown | Unknown | n.a. | |
| RuvA | |||||||
| RuvAEco | E. coli | ruvA | HJ-binding, stimulation of RuvBEco, inhibition of RuvCEco | None | RuvBEco, RuvCEco | [31], [32] | |
| RuvAMpn | M. pneumoniae | MPN535 | HJ- and ssDNA-binding | None | Unknown | This study; [17] | |
| RuvAMge | M. genitalium | MG358 | HJ-binding, stimulation of RuvBMge, inhibition of RecUMge | None | RuvBMge, RecUMge | This study | |
| RuvB | |||||||
| RuvBEco | E. coli | ruvB | HJ branch migration, DNA unwinding | Mg2+, ATP, RuvAEco | RuvAEco, RuvCEco | [49], [50], [51] | |
| RuvBFH 1 | M. pneumoniae | MPN536 | DNA unwinding | Mg2+, ATP | Unknown | This study; [18] | |
| RuvBMge | M. genitalium | MG359 | DNA unwinding | Mg2+, ATP, RuvAMge | RuvAMge | This study; [18] | |
| RecU | |||||||
| RecUBsu | Bacillus subtilis | recU | HJ resolution, annealing of homologous DNA substrates, modulation of RecA function | Mg2+ | RuvBBsu, RecABsu | [21], [52], [53] | |
| RecUMpn 2 | M. pneumoniae | MPN528a | None | n.a. | Unknown | [19] | |
| RecUMge | M. genitalium | MG352 | HJ resolution | Mn2+ | RuvAMge | This study; [19], [20] | |
The RuvBFH protein is exclusively expressed by subtype 2 strains of M. pneumoniae. Subtype 1 strains express a RuvB protein (RuvBM129) that differs in a single amino acid residue from RuvBFH. RuvBM129 has significantly lower ATPase and DNA helicase activities than RuvBFH [18].
RecUMpn is only expressed by subtype 2 strains of M. pneumoniae. Subtype 1 strains are unable to express a full-length RecU protein due to the presence of a nonsense mutation in the RecU gene (MPN528a) [19].
The SSB-dependence of the RecAMge protein was determined using SSBMpn and SSBEco [15]. The SSBMge protein has not yet been characterized.
n.a., not applicable.
We here report that the RuvA proteins from both Mycoplasma spp., RuvAMge and RuvAMpn, also possess exceptional properties as opposed to their well-characterized counterpart from E. coli, RuvAEco. While both RuvAMge and RuvAEco [31], [39] preferentially bind to HJs, RuvAMpn displayed a high affinity for both HJ and ssDNA. In addition, while RuvAMpn and RuvAEco are both able to form two distinct complexes with HJ substrates, RuvAMge only formed a single complex with HJs. As this RuvAMge-HJ complex had a similar mobility through polyacrylamide gels as RuvAMpn-HJ complex I and RuvAEco-HJ complex I [17], [30], [31], [32], [33], [34], [35], and because RuvAMge is a tetramer in solution, it is highly likely that this complex is composed of a tetramer of RuvAMge bound to a single HJ. This implies that RuvAMge may only stably bind to HJs as a tetramer. This notion can have important consequences for the interaction of the RuvAMge-HJ complex with other proteins that are potentially targeted to HJs, such as RuvBMge and RecUMge. It was previously reported that the ability of RuvAEco to form stable octamers on HJs was vital for full activity of the protein. This notion was inferred from the activities of four different octamerization-deficient RuvAEco mutants [34], [35], [40]. Three of these mutants carried amino acid substitutions in a protein region known to be involved in tetramer-tetramer interactions [34], [35], [40]. This region was identified within the crystal structure of HJ-bound octamers of the Mycobacterium leprae RuvA protein (RuvAMle) [33]. Within this structure, the two RuvA tetramers make direct protein-protein contacts through specific amino acid side chain interactions at four equivalent points, which are localized to the α6 helix of domain II (Fig. 1A). The interacting α6 helices from two RuvA monomers are in an antiparallel configuration, such that ion pair interactions are formed between three pairs of amino acid residues. On the basis of sequence alignments, we predict that only two of such pairs may be formed between two antiparallel α6 helices of both RuvAMge and RuvAMpn. In RuvAMpn, these pairs would consist of Lys121-Asp133 and Arg124-Glu130, whereas in RuvAMge, they would consist of Lys121-Glu133 and Arg124-Glu130. While this prediction emphasizes the sequence similarity between RuvAMpn and RuvAMge, it does not provide an explanation why RuvAMpn is able to form stable octameric complexes with HJs, and RuvAMge is not. It should be considered, however, that the octamerization signals of RuvAMpn (which are absent from RuvAMge) may differ considerably from those of RuvAMle, and are not (solely) determined by contacts between amino acid residues located in the α6 helix. In this regard, it is relevant to note that one of the reported RuvAEco mutants that is unable to form stable octamers on HJs, RuvAz87, does not carry mutations in helix α6, but in two other regions of the protein, i.e. in the region between helices α2 and α3 and in helix α4 [40].
Despite its inability to octamerize on HJs in a stable fashion, RuvAMge was found to stimulate the DNA helicase and ATPase activities of RuvBMge. The octamerization-competent RuvAMpn protein, however, did not augment RuvBFH activity. It is possible that the relatively high intrinsic DNA helicase activity of RuvBFH obscured the observation of any additional stimulatory effect on this protein by RuvAMpn. An alternative explanation for the inability of RuvAMpn to boost RuvBFH activity is that these proteins are unable to physically interact. In agreement with this notion, we have not yet been able to detect direct or indirect interactions between these proteins in DNA-binding studies.
Another unique feature of RuvAMge is the mode in which this protein forms tripartite complexes with HJ resolvase RecUMge and HJs. This is the first report to demonstrate an interaction between a member of the RecU protein family and a RuvA protein. RuvAMge and RecUMge were found to associate readily and stably on HJ substrates at a broad protein concentration range. In contrast, tripartite complexes of RuvAEco, RuvCEco and HJs were only observed at relatively low concentrations of RuvAEco, because the latter protein has a higher affinity than RuvCEco for HJ DNA [32]. At relatively high RuvAEco concentrations, the HJ DNA will be saturated with protein, such that two RuvAEco tetramers are bound to opposite faces of the junction. Thus, the binding of RuvCEco to the junction is excluded [32], [33], [41]. At low RuvAEco concentrations, however, the main protein-HJ complex that is formed is complex I, which consists of a single tetramer of RuvAEco bound to a single face of the junction. This structure may allow the binding of a RuvCEco dimer to the other face of the DNA substrate, thereby generating a tripartite RuvACEco-HJ complex [32]. In analogy with this model, a tetramer of RuvAMge bound to one side of a HJ may permit the binding of (multimers of) RecUMge at the opposite side of the junction. Because RuvAMge is unable to form stable octameric-HJ complexes, as discussed above, the tetrameric RuvAMge-HJ complex may always be accessible, at one face of the junction, for binding by RecUMge. This may explain why RecUMge and RuvAMge do not compete with each other for binding to HJs, but rather interact readily by forming a stable tripartite complex. This interaction does, however, lead to inhibition of the HJ resolution activity of RecUMge, a phenomenon that parallels the inhibition of RuvCEco-catalyzed HJ resolution by RuvAEco [32]. It remains to be determined whether the RecUMge-RuvAMge-HJ complexes are stabilized exclusively by protein-DNA interactions or also by RecUMge-RuvAMge interactions; experiments aimed at the detection of such protein-protein interactions have hitherto not produced conclusive results. In addition, it is clear that the physiological role will have to be established of the RecUMge-RuvAMge interaction and the RuvAMge-mediated inhibition of the HJ resolution activity of RecUMge. Nevertheless, it is likely that a functional coupling exists between these proteins and that the combined activities of a complex of RuvBMge and RuvAMge may be linked to the resolvase activity of RecUMge. Such a situation could be similar to that in E. coli, in which the RuvAB DNA branch migration complex is coupled to the RuvC resolvase in a RuvABCEco resolvasome complex. In this regard, it is also interesting to note that a close association between RecUMge and RuvAMge (plus RuvBMge) is also reflected in the genome of M. genitalium, in which the ORF encoding RecUMge (MG352) is localized in the vicinity of the ORFs encoding RuvAMge (MG358) and RuvBMge (MG359).
Another issue that remains to be addressed is the nature of the four different RecUMge-HJ complexes that were formed at relatively high concentrations of RecUMge. In previous studies on this protein, only a single RecUMge-HJ complex was observed due to the use of different DNA binding conditions [19], [20]. It was shown by protein crystallography and structure determination that the RecU homologues from Bacillus subtilis [42] and Bacillus stearothermophilus [43] exist as dimers. Based on this information, we speculate that the four RecUMge-HJ complexes that were observed in this study consist of HJs bound by dimers, tetramers, hexamers and octamers, respectively, of RecUMge. How the larger multimers would be accommodated on a single HJ, and how these would also leave room for binding of a RuvAMge tetramer, which was observed for each of the four RecUMge-HJ complexes, are challenging questions. The formation of large assemblies of proteins bound to a junction, however, is not unprecedented, as RuvAEco mutant RuvA3m was reported to generate HJ-protein complexes consisting of six protein tetramers [35].
In conclusion, the studies of the RuvA, RuvB and RecU homologues from mycoplasmas have revealed that these proteins each have distinctive properties as opposed to their counterparts from other bacterial classes. It is possible that these unique features have emerged as a consequence of the evolutionary reduction that the genomes of the mycoplasmas are believed to have undergone. Specifically, the loss of a significant portion of an ancestral set of DNA recombination and repair enzymes may have required an accompanying modification of the function of the RuvA, RuvB and RecU proteins in order to preserve certain functionalities of the recombination and repair system. Nevertheless, the complete set of functions of this system in mycoplasmas is yet to be determined. In this regard, it is particularly interesting to learn how DNA recombination processes are achieved in M. pneumoniae in the absence of a functional RecU resolvase [19]. Although HJ resolvase activities may be exerted by other proteins, such proteins have not yet been identified in M. pneumoniae. Moreover, the lack of a functional RecU was proposed as a possible cause of the relatively low frequency of homologous DNA recombination events in M. pneumoniae [19]. Also, the HJ resolvase deficiency of M. pneumoniae may be associated with the difference between M. pneumoniae and M. genitalium in the specific mechanism by which homologous DNA recombination events occur in these species. In M. genitalium, the repeated DNA elements appear to recombine predominantly in a reciprocal fashion [7], [8], [12], whereas in M. pneumoniae such elements seem to recombine via a gene conversion-like mechanism, in which donor sequences are copied to the acceptor site and the original acceptor sequence is lost [9], [10], [11], [13], [14]. To address these and other issues related to the mechanism of homologous recombination in M. pneumoniae and M. genitalium, it is crucial that the entire set of putative DNA recombination and repair enzymes of these species be delineated. This will therefore be the goal of future studies.
Materials and Methods
Cloning of the M. pneumoniae MPN535 gene and M. genitalium MG358 gene
Bacterial DNA was purified from cultures of M. pneumoniae strain M129 (ATCC® no. 29342TM) and M. genitalium strain G37 (ATCC® no. 33530TM), as described previously [16], [44]. The MPN535 ORF of M. pneumoniae strain M129, which encodes a RuvA homologue, was amplified by PCR. The PCR reaction was performed using the following primers: RuvAmpn_fw (5′-GGTCGTCATATGATTGCTTCAATTTTTGGAA-3′, which overlaps with the translation initiation codon [underlined] of MPN535) and primer RuvAmpn_rev (5′- GCAGCCGGATCC TTAGGCGGTTTTATTTGTAAC-3′, which overlaps with the antisense sequence of the translation termination codon [underlined] of the gene). The resulting 0.6-kilobase pairs (kb) PCR fragment was digested with NdeI and BamHI (the recognition sites for these enzymes are indicated in italics in the sequences of primers RuvAmpn_fw and RuvAmpn_rev, respectively), and cloned into NdeI- and BamHI-digested E. coli protein expression vectors, i.e. pET-11c and pET-16b (Novagen), generating plasmids pET-11c-RuvAMpn and pET-16b-RuvAMpn, respectively. Plasmid pET-11c-RuvAMpn was used for expression of native RuvAMpn, while plasmid pET-16b-RuvAMpn was employed for expression of RuvAMpn as an N-terminally poly histidine (H10)-tagged protein in E. coli.
Before cloning of the MG358 ORF of M. genitalium into E. coli protein expression vectors, a TGA codon within the ORF (encoding the Trp residue at position 27 of RuvAMge) was changed into a TGG codon using a PCR-based mutagenesis procedure [19]. Following mutagenesis, MG358 was amplified by PCR using the primers RuvAmg_pETfw (5′-CGTCACATATGATTACATCTATCTTTGG -3′, which includes an NdeI restriction site [in italics] and the translation initiation codon of MG358 [underlined]) and RuvAmg_pETrv 5′-CGTCAGGATCCGGTATTAGGCGGTTTTATTTG-3′, which includes a BamHI site [in italics] and the antisense sequence of the translation termination codon [underlined] of the gene). The 0.6-kb PCR product was digested with NdeI and BamHI, and ligated into NdeI- and BamHI-digested vectors pET-11c and pET-16b, resulting in plasmids pET-11c-RuvAMge and pET-16b-RuvAMge, respectively. These plasmids were used for expression of native and H10-tagged RuvAMge, respectively, in E. coli. The integrity of all DNA constructs used in this study was checked by dideoxy sequencing, as described before [15].
Protein expression and purification
The various pET-11c- and pET-16b-derived vectors were introduced into E. coli BL21(DE3) and the resulting strains were grown overnight at 37°C in LB medium containing 100 μg/ml ampicillin. The cultures were diluted 1∶100 in 300 ml LB medium with ampicillin and grown at 37°C to an optical density at 600 nm of 0.6. Protein expression was then induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM. After incubation for 2 hr at 30°C, the bacteria were harvested by centrifugation and stored at −20°C.
The H10-tagged RuvAMpn and RuvAMge proteins were both purified using the following protocol. Bacterial pellets were resuspended in 10 ml of buffer A (20 mM Tris-HCl pH 8.0, 1 M NaCl) containing 0.5 mg/ml of lysozyme. The suspension was sonicated on ice and clarified by centrifugation for 20 min at 12,000× g (at 4°C). To the supernatant, imidazole was added to a final concentration of 5 mM. Then, the supernatant was loaded onto a column containing 1 ml of Ni2+-nitroloacetic acid (Ni-NTA)-agarose (Qiagen), which was equilibrated previously in buffer A containing 5 mM imidazole. The column was washed with 5 ml of buffer A plus 5 mM imidazole and with 5 ml of buffer A plus 20 mM imidazole. The specifically bound proteins were eluted from the column with 8 ml of buffer A containing 250 mM imidazole. Fractions of 0.5 ml were collected, analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), pooled, and dialyzed against a solution of 20 mM Tris-HCl (pH 7.4), 0.2 M NaCl, 0.1 mM EDTA, 1 mM DTT and 50% glycerol (buffer B). Aliquots of purified protein, which had an estimated homogeneity of >95%, were stored at −20°C.
The native RuvA proteins were purified by solubilization of the bacterial pellets in a buffer containing 20 mM Tris-HCl pH 7.5, 1 mM EDTA, 1 mM DTT and 0.5 mg/ml of lysozyme. After sonication and centrifugation (using similar procedures as described above), the RuvA proteins were precipitated with ammonium sulphate and resuspended in 20 mM Tris-HCl pH 7.4, 0.1 M NaCl, 0.1 mM EDTA, 1 mM DTT. The proteins were then subjected to affinity chromatography using Heparin Sepharose 6 Fast Flow (GE Healthcare). Proteins were eluted from the column material with a linear gradient from 0 M to 1 M NaCl in 20 mM Tris-HCl pH 7.4, 0.1 mM EDTA and 1 mM DTT. The RuvA-containing fractions were pooled, dialyzed against buffer B, and stored at −20°C.
The purifications of RecUMge, RuvBFH and RuvBMge have been described before [18], [19], [20].
SDS-PAGE
Proteins were separated by SDS-PAGE, as described by Laemmli [45]. Gels were stained with Coomassie brilliant blue (CBB), destained in 40% methanol/10% acetic acid, and recorded using a GelDoc XR system (Bio-Rad). Digital images were processed using Quantity One® 1-D Analysis Software (Bio-Rad).
DNA substrates
The small DNA substrates that were used in the DNA binding experiments consisted of synthetic oligonucleotide substrates that were 5′ 6-FAM-labelled on a single strand. Holliday junction (HJ) substrate HJ 1.1, single-stranded oligonucleotide HJ11 and double-stranded substrate HJ11/HJ11rv have been described by Sluijter et al. [19]. Substrate HJ 1.1 is composed of the following four oligonucleotides: HJ11 (5′-GCGACGTGATCACCAGATGATTGCTAG-GCATGCTTTCCGCAAGAGAAGC-3′), HJ12 (5′-GGCTTCTCTTGCGGAAAGCATGCCTA-GCAATCCTGTCAGCTGCATGGAAC-3′), HJ13 (5′-GGTTCCATGCAGCTGACAGGATT-GCTAGGCTCAAGGCGAACTGCTAACGG-3′) and HJ14 (5′-ACCGTTAGCAGTTCG-CCTTGAGCCTAGCAATCATCTGGTGATCACGTCGC-3′). The sequence of oligonucleotide HJ11rv is 5′-GGCTTCTCTTGCGGAAAGCATGCCTAGCAATCATCTGGTGATCACGTC-GC-3′. The DNA helicase substrates (Fig. 6A) have been described in detail by Estevão and coworkers [18].
DNA-binding assays
Binding of the RuvA proteins to various DNA substrates was carried out in 10-µl volumes and included 20 mM Tris-HCl pH 7.5, 1 mM DTT, 1 mM EDTA, 12.3 nM oligonucleotide substrate and various concentrations of RuvA proteins. After incubation on ice for 10 min, 1 μl was added of a solution containing 40% glycerol and 0.25% bromophenol blue. Then, the reaction mixtures were electrophoresed through 8% polyacrylamide gels in 0.5× TBE buffer (45 mM Tris, 45 mM boric acid, 1 mM EDTA). Following electrophoresis, the polyacrylamide gels were analyzed by fluorometry, using a Typhoon Trio™ 9200 Variable Mode Imager (GE Healthcare) in combination with the Typhoon Scanner Control v4.0 software (Amersham Bioscience). Images were processed using Quantity One® 1-D Analysis Software.
Holliday junction (HJ) resolution assays
HJ resolution assays were carried out as described by Sluijter et al. [19]. Reactions were analyzed by electrophoresis through 12% polyacrylamide/1× TBE mini-gels. The relative RecUMge (resolution) activity (Fig. 5C) was expressed as percentage of the protein's activity in the absence of RuvAMge.
DNA helicase and ATPase assays
DNA helicase assays were performed similarly as described before [18]. After deproteinization, the reactions mixtures were analyzed by electrophoresis through 12% polyacrylamide/1× TBE mini-gel and fluorometry. The ATPase activities of RuvBFH and RuvBMge were determined by using a β-nicotinamide adenine dinucleotide reduced form (NADH)-coupled assay on a VersaMax Tunable Microplate Reader (Molecular Devices) [15], [46].
Supporting Information
RuvA Mge is a tetramer in solution. (A) Gel filtration analysis of RuvAMge. Gel filtration chromatography was performed in a similar fashion as described previously [16], using a Sephadex G-150 column (length, 1.0 m; inner diameter, 1.0 cm). The column was run at 4 ml/h in 50 mM Tris-HCl (pH 7.5)/ 135 mM NaCl, and calibrated with blue dextran (2,000 kDa), bovine serum albumin (BSA, 66.4 kDa), ovalbumin (42.9 kDa), and cytochrome C (12.3 kDa). Fractions of 1.0 ml were collected and monitored by measuring the optical density at 280 nm (OD280, Y-axis at the left-hand side of the graph). The fractions eluted from a subsequent run, containing 15 µg of RuvAMge, were precipitated with trichloroacetic acid, and separated on 12% SDS-PAGE gels. Gels were silver-stained and recorded using the GelDoc XR system. RuvAMge was quantified by densitometry using Quantity One® 1-D Analysis Software (Bio-Rad). The relative concentration of RuvAMge (Y-axis on the right-hand side, in arbitrary units) is shown for column fractions 23 to 39. In all other fractions, RuvAMge was not detected. (B) Calibration curve obtained from the gel filtration experiment shown in (A). The molecular weight of protein size standards (♦) is plotted against the elution volume (Ve) divided by the void volume (V0) of the column (Ve/V0). V0 was determined with blue dextran. The Ve/V0 of RuvAMge is marked on the calibration curve (×).
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: AMCvR is supported by grants of the European Society for Pediatric Infectious Diseases, ZonMW, and the Erasmus MC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Su CJ, Chavoya A, Baseman JB. Regions of Mycoplasma pneumoniae cytadhesin P1 structural gene exist as multiple copies. Infect Immun. 1988;56:3157–3161. doi: 10.1128/iai.56.12.3157-3161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzel R, Herrmann R. Repetitive DNA sequences in Mycoplasma pneumoniae. Nucleic Acids Res. 1988;16:8337–8350. doi: 10.1093/nar/16.17.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruland K, Wenzel R, Herrmann R. Analysis of three different repeated DNA elements present in the P1 operon of Mycoplasma pneumoniae: size, number and distribution on the genome. Nucleic Acids Res. 1990;18:6311–6317. doi: 10.1093/nar/18.21.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson SN, Hu PC, Bott KF, Hutchison CA A survey of the Mycoplasma genitalium genome by using random sequencing. J Bacteriol. 1993;175:7918–7930. doi: 10.1128/jb.175.24.7918-7930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson SN, Bailey CC, Jensen JS, Borre MB, King ES, et al. Characterization of repetitive DNA in the Mycoplasma genitalium genome: possible role in the generation of antigenic variation. Proc Natl Acad Sci USA. 1995;92:11829–11833. doi: 10.1073/pnas.92.25.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 7.Iverson-Cabral SL, Astete SG, Cohen CR, Totten PA. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol Microbiol. 2007;66:55–73. doi: 10.1111/j.1365-2958.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- 8.Iverson-Cabral SL, Astete SG, Cohen CR, Rocha EP, Totten PA. Intrastrain heterogeneity of the mgpB gene in Mycoplasma genitalium is extensive in vitro and in vivo and suggests that variation is generated via recombination with repetitive chromosomal sequences. Infect Immun. 2006;74:3715–3726. doi: 10.1128/IAI.00239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenri T, Taniguchi R, Sasaki Y, Okazaki N, Narita M, et al. Identification of a new variable sequence in the P1 cytadhesin gene of Mycoplasma pneumoniae: evidence for the generation of antigenic variation by DNA recombination between repetitive sequences. Infect Immun. 1999;67:4557–4562. doi: 10.1128/iai.67.9.4557-4562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spuesens EB, Oduber M, Hoogenboezem T, Sluijter M, Hartwig NG, et al. Sequence variations in RepMP2/3 and RepMP4 elements reveal intragenomic homologous DNA recombination events in Mycoplasma pneumoniae. Microbiology. 2009;155:2182–2196. doi: 10.1099/mic.0.028506-0. [DOI] [PubMed] [Google Scholar]
- 11.Spuesens EB, Hartwig NG, van Rossum AM, Vink C. Identification and classification of P1 variants of Mycoplasma pneumoniae. J Clin Microbiol. 2010;48:680. doi: 10.1128/JCM.02078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L, Jensen JS, Myers L, Burnett J, Welch M, et al. Mycoplasma genitalium: an efficient strategy to generate genetic variation from a minimal genome. Mol Microbiol. 2007;66:220–236. doi: 10.1111/j.1365-2958.2007.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spuesens EB, van de Kreeke N, Estevao S, Hoogenboezem T, Sluijter M, et al. Variation in a surface-exposed region of the Mycoplasma pneumoniae P40 protein as a consequence of homologous DNA recombination between RepMP5 elements. Microbiology. 2011;157:473–483. doi: 10.1099/mic.0.045591-0. [DOI] [PubMed] [Google Scholar]
- 14.Vink C, Rudenko G, Seifert HS. Microbial antigenic variation mediated by homologous DNA recombination. 2011. FEMS Microbiol Rev. doi: 10.1111/j.1574-6976.2011.00321.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 15.Sluijter M, Spuesens EB, Hartwig NG, van Rossum AM, Vink C. The Mycoplasma pneumoniae MPN490 and Mycoplasma genitalium MG339 genes encode RecA homologs that promote homologous DNA strand exchange. Infect Immun. 2009;77:4905–4911. doi: 10.1128/IAI.00747-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sluijter M, Hoogenboezem T, Hartwig NG, Vink C. The Mycoplasma pneumoniae MPN229 gene encodes a protein that selectively binds single-stranded DNA and stimulates Recombinase A-mediated DNA strand exchange. BMC Microbiol. 2008;8:167. doi: 10.1186/1471-2180-8-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingleston SM, Dickman MJ, Grasby JA, Hornby DP, Sharples GJ, et al. Holliday junction binding and processing by the RuvA protein of Mycoplasma pneumoniae. Eur J Biochem. 2002;269:1525–1533. doi: 10.1046/j.1432-1033.2002.02805.x. [DOI] [PubMed] [Google Scholar]
- 18.Estevao S, Sluijter M, Hartwig NG, van Rossum AM, Vink C. Functional characterization of the RuvB homologs from Mycoplasma pneumoniae and Mycoplasma genitalium. J Bacteriol. 2011;193:6425–6435. doi: 10.1128/JB.06003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sluijter M, Kaptein E, Spuesens EB, Hoogenboezem T, Hartwig NG, et al. The Mycoplasma genitalium MG352-encoded protein is a Holliday junction resolvase that has a non-functional orthologue in Mycoplasma pneumoniae. Mol Microbiol. 2010;77:1261–1277. doi: 10.1111/j.1365-2958.2010.07288.x. [DOI] [PubMed] [Google Scholar]
- 20.Sluijter M, Aslam M, Hartwig NG, van Rossum AM, Vink C. Identification of amino acid residues critical for catalysis of Holliday junction resolution by Mycoplasma genitalium RecU. J Bacteriol. 2011;193:3941–3948. doi: 10.1128/JB.00247-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayora S, Carrasco B, Doncel-Perez E, Lurz R, Alonso JC. Bacillus subtilis RecU protein cleaves Holliday junctions and anneals single-stranded DNA. Proc Natl Acad Sci USA. 2004;101:452–457. doi: 10.1073/pnas.2533829100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan SN, Harris L, Bolt EL, Whitby MC, Lloyd RG. Sequence specificity and biochemical characterization of the RusA Holliday junction resolvase of Escherichia coli. J Biol Chem. 1997;272:14873–14882. doi: 10.1074/jbc.272.23.14873. [DOI] [PubMed] [Google Scholar]
- 23.Shah R, Bennett RJ, West SC. Activation of RuvC Holliday junction resolvase in vitro. Nucleic Acids Res. 1994;22:2490–2497. doi: 10.1093/nar/22.13.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah R, Bennett RJ, West SC. Genetic recombination in E. coli: RuvC protein cleaves Holliday junctions at resolution hotspots in vitro. Cell. 1994;79:853–864. doi: 10.1016/0092-8674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 25.Sharples GJ, Chan SN, Mahdi AA, Whitby MC, Lloyd RG. Processing of intermediates in recombination and DNA repair: identification of a new endonuclease that specifically cleaves Holliday junctions. EMBO J. 1994;13:6133–6142. doi: 10.1002/j.1460-2075.1994.tb06960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thayer MM, Ahern H, Xing D, Cunningham RP, Tainer JA. Novel DNA binding motifs in the DNA repair enzyme endonuclease III crystal structure. EMBO J. 1995;14:4108–4120. doi: 10.1002/j.1460-2075.1995.tb00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino T, Ariyoshi M, Iwasaki H, Shinagawa H, Morikawa K. Functional analyses of the domain structure in the Holliday junction binding protein RuvA. Structure. 1998;6:11–21. doi: 10.1016/s0969-2126(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 28.Hargreaves D, Rice DW, Sedelnikova SE, Artymiuk PJ, Lloyd RG, et al. Crystal structure of E. coli RuvA with bound DNA Holliday junction at 6 Å resolution. Nat Struct Biol. 1998;5:441–446. doi: 10.1038/nsb0698-441. [DOI] [PubMed] [Google Scholar]
- 29.Rafferty JB, Ingleston SM, Hargreaves D, Artymiuk PJ, Sharples GJ, et al. Structural similarities between Escherichia coli RuvA protein and other DNA-binding proteins and a mutational analysis of its binding to the Holliday junction. J Mol Biol. 1998;278:105–116. doi: 10.1006/jmbi.1998.1697. [DOI] [PubMed] [Google Scholar]
- 30.Rafferty JB, Sedelnikova SE, Hargreaves D, Artymiuk PJ, Baker PJ, et al. Crystal structure of DNA recombination protein RuvA and a model for its binding to the Holliday junction. Science. 1996;274:415–421. doi: 10.1126/science.274.5286.415. [DOI] [PubMed] [Google Scholar]
- 31.Parsons CA, Tsaneva I, Lloyd RG, West SC. Interaction of Escherichia coli RuvA and RuvB proteins with synthetic Holliday junctions. Proc Natl Acad Sci USA. 1992;89:5452–5456. doi: 10.1073/pnas.89.12.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitby MC, Bolt EL, Chan SN, Lloyd RG. Interactions between RuvA and RuvC at Holliday junctions: inhibition of junction cleavage and formation of a RuvA-RuvC-DNA complex. J Mol Biol. 1996;264:878–890. doi: 10.1006/jmbi.1996.0684. [DOI] [PubMed] [Google Scholar]
- 33.Roe SM, Barlow T, Brown T, Oram M, Keeley A, et al. Crystal structure of an octameric RuvA-Holliday junction complex. Mol Cell. 1998;2:361–372. doi: 10.1016/s1097-2765(00)80280-4. [DOI] [PubMed] [Google Scholar]
- 34.Bradley AS, Baharoglu Z, Niewiarowski A, Michel B, Tsaneva IR. Formation of a stable RuvA protein double tetramer is required for efficient branch migration in vitro and for replication fork reversal in vivo. J Biol Chem. 2011;286:22372–22383. doi: 10.1074/jbc.M111.233908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Privezentzev CV, Keeley A, Sigala B, Tsaneva IR. The role of RuvA octamerization for RuvAB function in vitro and in vivo. J Biol Chem. 2005;280:3365–3375. doi: 10.1074/jbc.M409256200. [DOI] [PubMed] [Google Scholar]
- 36.Muller B, Tsaneva IR, West SC. Branch migration of Holliday junctions promoted by the Escherichia coli RuvA and RuvB proteins. II. Interaction of RuvB with DNA. J Biol Chem. 1993;268:17185–17189. [PubMed] [Google Scholar]
- 37.Carvalho FM, Fonseca MM, Batistuzzo De Medeiros S, Scortecci KC, Blaha CA, et al. DNA repair in reduced genome: the Mycoplasma model. Gene. 2005;360:111–119. doi: 10.1016/j.gene.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Rocha EP, Cornet E, Michel B. Comparative and evolutionary analysis of the bacterial homologous recombination systems. PLoS Genet. 2005;1:e15. doi: 10.1371/journal.pgen.0010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd RG, Sharples GJ. Processing of recombination intermediates by the RecG and RuvAB proteins of Escherichia coli. Nucleic Acids Res. 1993;21:1719–1725. doi: 10.1093/nar/21.8.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baharoglu Z, Bradley AS, Le Masson M, Tsaneva I, Michel B. ruvA Mutants that resolve Holliday junctions but do not reverse replication forks. PLoS Genet. 2008;4:e1000012. doi: 10.1371/journal.pgen.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitby MC, Dixon J. Substrate specificity of the SpCCE1 Holliday junction resolvase of Schizosaccharomyces pombe. J Biol Chem. 1998;273:35063–35073. doi: 10.1074/jbc.273.52.35063. [DOI] [PubMed] [Google Scholar]
- 42.McGregor N, Ayora S, Sedelnikova S, Carrasco B, Alonso JC, et al. The structure of Bacillus subtilis RecU Holliday junction resolvase and its role in substrate selection and sequence-specific cleavage. Structure. 2005;13:1341–1351. doi: 10.1016/j.str.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Kelly SJ, Li J, Setlow P, Jedrzejas MJ. Structure, flexibility, and mechanism of the Bacillus stearothermophilus RecU Holliday junction resolvase. Proteins. 2007;68:961–971. doi: 10.1002/prot.21418. [DOI] [PubMed] [Google Scholar]
- 44.Maquelin K, Hoogenboezem T, Jachtenberg JW, Dumke R, Jacobs E, et al. Raman spectroscopic typing reveals the presence of carotenoids in Mycoplasma pneumoniae. Microbiology. 2009;155:2068–2077. doi: 10.1099/mic.0.026724-0. [DOI] [PubMed] [Google Scholar]
- 45.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 46.Morimatsu K, Takahashi M, Norden B. Arrangement of RecA protein in its active filament determined by polarized-light spectroscopy. Proc Natl Acad Sci USA. 2002;99:11688–11693. doi: 10.1073/pnas.142404499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radding CM. Helical RecA nucleoprotein filaments mediate homologous pairing and strand exchange. Biochim Biophys Acta. 1989;1008:131–145. doi: 10.1016/0167-4781(80)90001-9. [DOI] [PubMed] [Google Scholar]
- 48.Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990;54:342–380. doi: 10.1128/mr.54.4.342-380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsaneva IR, Muller B, West SC. ATP-dependent branch migration of Holliday junctions promoted by the RuvA and RuvB proteins of E. coli. Cell. 1992;69:1171–1180. doi: 10.1016/0092-8674(92)90638-s. [DOI] [PubMed] [Google Scholar]
- 50.Tsaneva IR, Muller B, West SC. RuvA and RuvB proteins of Escherichia coli exhibit DNA helicase activity in vitro. Proc Natl Acad Sci USA. 1993;90:1315–1319. doi: 10.1073/pnas.90.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsaneva IR, West SC. Targeted versus non-targeted DNA helicase activity of the RuvA and RuvB proteins of Escherichia coli. J Biol Chem. 1994;269:26552–26558. [PubMed] [Google Scholar]
- 52.Carrasco B, Ayora S, Lurz R, Alonso JC. Bacillus subtilis RecU Holliday-junction resolvase modulates RecA activities. Nucleic Acids Res. 2005;33:3942–3952. doi: 10.1093/nar/gki713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canas C, Carrasco B, Ayora S, Alonso JC. The RecU Holliday junction resolvase acts at early stages of homologous recombination. Nucleic Acids Res. 2008;36:5242–5249. doi: 10.1093/nar/gkn500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ariyoshi M, Nishino T, Iwasaki H, Shinagawa H, Morikawa K. Crystal structure of the holliday junction DNA in complex with a single RuvA tetramer. Proc Natl Acad Sci USA. 2000;97:8257–8262. doi: 10.1073/pnas.140212997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RuvA Mge is a tetramer in solution. (A) Gel filtration analysis of RuvAMge. Gel filtration chromatography was performed in a similar fashion as described previously [16], using a Sephadex G-150 column (length, 1.0 m; inner diameter, 1.0 cm). The column was run at 4 ml/h in 50 mM Tris-HCl (pH 7.5)/ 135 mM NaCl, and calibrated with blue dextran (2,000 kDa), bovine serum albumin (BSA, 66.4 kDa), ovalbumin (42.9 kDa), and cytochrome C (12.3 kDa). Fractions of 1.0 ml were collected and monitored by measuring the optical density at 280 nm (OD280, Y-axis at the left-hand side of the graph). The fractions eluted from a subsequent run, containing 15 µg of RuvAMge, were precipitated with trichloroacetic acid, and separated on 12% SDS-PAGE gels. Gels were silver-stained and recorded using the GelDoc XR system. RuvAMge was quantified by densitometry using Quantity One® 1-D Analysis Software (Bio-Rad). The relative concentration of RuvAMge (Y-axis on the right-hand side, in arbitrary units) is shown for column fractions 23 to 39. In all other fractions, RuvAMge was not detected. (B) Calibration curve obtained from the gel filtration experiment shown in (A). The molecular weight of protein size standards (♦) is plotted against the elution volume (Ve) divided by the void volume (V0) of the column (Ve/V0). V0 was determined with blue dextran. The Ve/V0 of RuvAMge is marked on the calibration curve (×).
(TIF)