Abstract
Background
Rituximab has been widely used off-label as a second line treatment for children with immune thrombocytopenia (ITP). However, its role in the management of pediatric ITP requires clarification. To understand and interpret the available evidence, we conducted a systematic review to assess the efficacy and safety of rituximab for children with ITP.
Methodology/Principal Findings
We searched MEDLINE, EMBASE, Cochrane Library, CBM, CNKI, abstract databases of American Society of Hematology, American Society of Clinical Oncology and Pediatric Academic Society. Clinical studies published in full text or abstract only in any language that met predefined inclusion criteria were eligible. Efficacy analysis was restricted to studies enrolling 5 or more patients. Safety was evaluated from all studies that reported data of toxicity. 14 studies (323 patients) were included for efficacy assessment in children with primary ITP. The pooled complete response (platelet count ≥100×109/L) and response (platelet count ≥30×109/L) rate after rituximab treatment were 39% (95% CI, 30% to 49%) and 68% (95%CI, 58% to 77%), respectively, with median response duration of 12.8 month. 4 studies (29 patients) were included for efficacy assessment in children with secondary ITP. 11 (64.7%) of 17 patients associated with Evans syndrome achieved response. All 6 patients with systemic lupus erythematosus associated ITP and all 6 patients with autoimmune lymphoproliferative syndrome associated ITP achieved response. 91 patients experienced 108 adverse events associated with rituximab, among that, 91 (84.3%) were mild to moderate, and no death was reported.
Conclusions/Significance
Randomized controlled studies on effect of rituximab for children with ITP are urgently needed, although a series of uncontrolled studies found that rituximab resulted in a good platelet count response both in children with primary and children secondary ITP. Most adverse events associated with rituximab were mild to moderate, and no death was reported.
Introduction
Immune thrombocytopenia (ITP) is an immune-mediated disease characterized by transient or persistent decrease of the platelet count and increased risk of bleeding [1]. The incidence of ITP is 1.9∼6.4 per 105 children/year [2] and 23.1%∼47.3% of children with ITP suffer a disease course more than 6 months [3].
The goal of ITP treatment is to achieve a platelet count that is associated with adequate hemostasis, rather than a “normal” platelet count. The recommended first-line drug treatment for children with ITP includes corticosteroids, intravenous immunoglobulin (IVIg) and Anti-D immunoglobulin [4], but for pediatric patients refractory to first line treatment, appropriate second line treatments are needed.
Rituximab is a chimeric, monoclonal anti-CD20 antibody that targets B lymphocytes and causes Fc-mediated cell lysis [5], [6]. It was initially approved for the treatment of lymphoma, as it can substantially decrease the normal and malignant B-cells [7]. It has also been approved for the treatment of rheumatoid arthritis in Europe, as it can destroy B lymphocytes in the joints to help reducing inflammation [8]. In recent years, it has been widely used off-label as the second line treatment in both adults and children with ITP refractory to first line treatment. A systematic review based on studies on adult patients with ITP found that rituximab resulted in overall response (platelet count >50×109/L) and complete response (platelet count >150×109/L) in 62.5% and 43.6% of patients, respectively [9]. Several studies on children suggested that rituximab showed a similar effect as adult ITP, but the reported response rate varied among those studies [10]. In addition, those results may be potentially biased and imprecise, as most studies are case series, and only involved a relatively small number of patients. The role of rituximab in the management of pediatric ITP still requires clarification. To understand and interpret the available evidence, we conducted a systematic review to evaluate the efficacy and safety of rituximab for children with ITP.
Methods
Searching
We searched PUBMED, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) published in Cochran Library (2012, Issue 1) using the search strategy detailed in table S1; we searched Chinese Biomedical Literature Database (CBM) and Chinese National Knowledge Infrastructure (CNKI) for literatures published in Chinese. We also searched the electronic databases of American Society of Hematology, American Society of Clinical Oncology and Abstract Database of Pediatric Academic Society including its Late-Breaker Abstract Presentations,with the search term ritux*, child* and pediatr*. The references of all retrieved articles were scanned for additional relevant citations. We searched all databases from their earliest records to January 2012.
Eligibility Criteria
Clinical studies published in full text or abstract only were both included, and there was no restriction on study design or publication language. We included children aged less than 18 years with primary or secondary ITP, having platelet counts less than 30×109 cell/L. Studies that included both children and adults were excluded if data of children could not be extracted separately. Patients were treated with rituximab irrespective of dosage and schedule. Outcome criteria based on guidance recently provided by international working group (IWG) consensus panel of both adult and pediatric experts [1]. Response (R) was defined as any platelet count ≥30×109/L and at least doubling of the baseline count. Complete response (CR) was defined as any platelet count ≥100×109/L. Time to response was from starting treatment to achievement of response. Duration of response was measured from the achievement to loss of response. All reported adverse events were included for safety assessment. Studies that enrolled fewer than 5 patients but failed to contribute to safety analysis were excluded.
Study Selection and Data Abstraction
Two authors (Yi Liang and Die Hu) independently screened titles and abstracts of all studies identified by the search strategy and assessed the studies for inclusion using predetermined inclusion criteria. The full texts of all potentially relevant articles were retrieved for detailed review. We resolved disagreements by discussion until consensus is achieved.
Two authors (Yi Liang and Die Hu) used a pre-designed data collection form to independently extract data from each included study. The following data were extracted: (1) characteristics of patients, including number of patients that meet the inclusion criteria, country, age, primary/secondary ITP, duration of ITP, splenectomized or not, and platelet count before rituximab treatment; (2) study design and use of controls; (3) dose and schedule of rituximab; (4) number of patients with platelet count response, complete response, and their definitions; (5) time to platelet count responses; (6) follow-up and duration of platelet count responses; (7)toxicities associated with rituximab; (8) source of funding.
Quality Assessment
Quality assessment was based on the checklist developed for assessment of case series by UK national institute for clinical excellence (NICE) [11]. We answered 8 questions included in the checklist with yes, no, or unclear. Quality assessment was only conducted in studies enrolling 5 or more patients, as those studies contributed to efficacy analysis.
Statistical Synthesis
Platelet count responses were analyzed only from those studies enrolling 5 or more patients, as smaller studies may be subject to extreme risk of reporting and selection bias. Adverse effects were considered from all studies, including those enrolling fewer than 5 patients each. We achieved exact binomial 95% confidence interval (CI) of response rate reported in each study. We estimated the between-study variance and determined pooled estimates of response rate using the software STATA 11.1 with a random-effect model [12]. Time to response and response duration were described with medians, minimum and maximum values, and for studies that reported individual level data, we combined and summarized these data with medians and inter quartile ranges (IQR). We assessed publication bias both with Egger and Begg test in STATA 11.1, and P≦0.05 suggested a significant publication bias [13], [14].
We calculated a kappa statistic for measuring agreement between two authors making decisions on study selection. Values of kappa between 0.40 and 0.59 were considered to reflect fair agreement, between 0.60 and 0.74 to reflect good agreement and 0.75 or more to reflect excellent agreement [15]. The design and report of this review has been checked with PRISMA checklist (see table S2).
Results
Study Selection
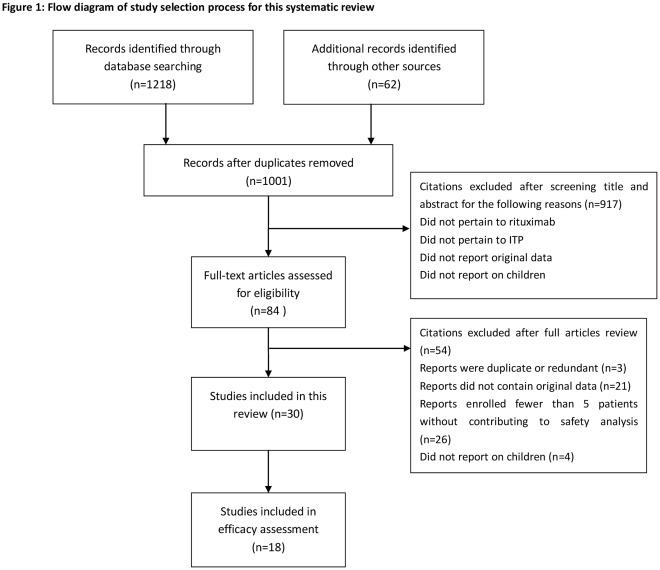
1280 citations were identified from the literature search, and 30 studies [16]–[45] (370 patients) were included in this systematic review. 18 studies [16]–[33] (352 patients) enrolled 5 or more patients each, among which 5 studies [29]–[33] (59 patients) were published only in abstract; 12 studies [34]–[45] (18 patients) enrolled less than 5 patients each, among which 2 studies [34], [38] (6 patients) were published in abstract. Figure 1 shows the literature selection process. Agreement between 2 reviewers for study selection was excellent (Κ = 0.75).
Figure 1. Flow diagram of study selection process for this systematic review.
This is a modified four-phase PRISMA 2009 flow diagram that maps out the number of records identified, included and excluded, and the reasons for exclusions.
Study Design and Source of Funding
17 case series [16]–[27], [29]–[33], 12 case reports [34]–[45] and 1 longitudinal observational cohort study were included in this systematic review. The cohort study included a control group involving patients without rituximab administration, but only reported concerned outcomes in the rituximab group, failing to make a comparison on effects between two groups [28]. 9 studies [17]–[19], [24], [28], [31], [37], [39], [40] were supported by non-profit organizations; 4 study did not receive any funding source [21], [22], [33], [42]; Source of funding was not reported in the other 17 studies.
Quality Assessment
18 studies [16]–[33] enrolling 5 or more patients were involved in the quality assessment (table 1). 10/18 studies were multicenter studies; All studies described their hypothesis/objective and main findings clearly; 14/18 studies clearly defined outcomes; 9/18 studies clearly described patient inclusion criteria; 9/18 studies collected patients data prospectively, 1 cohort study collected data both prospectively and retrospectively; Only 1 study stated consecutive enrollment of patients, none of other studies provided this information, although it is necessary, as consecutive enrollment may reduce risk of selection bias; Platelet count response was stratified in 5 studies by clinical characters of patients to find predictors of response to rituximab.
Table 1. Quality assessment of 18 studies that contributed to efficacy analysis.
| Study | Multi-center study | Objective clearly described | Inclusion/exclusion criteria clearly described | Clear definition of outcomes | Data collected prospectively | Patients recruited consecutively | Main finding clearly described | Outcomes stratified |
| Taube (2005) [16] | NR | Y | N | Y | Y | NR | Y | N |
| Wang (2005) [17] | Y | Y | Y | Y | Y | NR | Y | N |
| Bennett (2006) [18] | Y | Y | Y | Y | Y | NR | Y | Y |
| Bader-Meunier (2007) [19] | Y | Y | Y | Y | N | NR | Y | N |
| Rao (2008) [20] | Y | Y | Y | Y | Y | NR | Y | N |
| Dogan 2009 [21] | N | Y | N | Y | Y | NR | Y | N |
| Kumar (2009) [22] | N | Y | Y | Y | N | Y | Y | N |
| Parodi (2009) [23] | Y | Y | N | Y | Y | NR | Y | Y |
| Rao (2009) [24] | Y | Y | Y | N | N | NR | Y | N |
| Citak (2011) [25] | NR | Y | N | Y | Y | NR | Y | N |
| Xu (2011) [26] | Y | Y | Y | Y | NR | NR | Y | N |
| Yang (2011) [27] | N | Y | Y | Y | Y | NR | Y | N |
| Grace (2012) [28] | Y | Y | Y | Y | Y/N | NR | Y | Y |
| Sharma (2005)* [29] | NR | Y | NR | Y | NR | NR | Y | Y |
| Kim (2009)* [30] | NR | Y | NR | Y | NR | NR | Y | N |
| Pospisilova (2010)* [31] | Y | Y | NR | NR | N | NR | Y | N |
| Sampaio (2010)* [32] | Y | Y | NR | NR | N | NR | Y | N |
| Wang (2011)* [33] | N | Y | NR | NR | Y | NR | Y | Y |
Y: yes; N: no; NR: not reported.
Abstract.
Description of Patients
352 patients were included in 18 studies enrolling 5 or more patients. Characteristics of these patients were described in table 2. 171 (48.6%) patients in 6 studies [17], [18], [20], [22], [24], [28] were from USA/Canada, and 181 (51.4%) patients were from other countries, including Germany [16], Italy [23], France [19] Czech Republic [31],Portugal [32], China [26], [27], [33], Turkey [21], [25], South Korea [30] and India [29]. 304 patients were diagnosed as primary ITP, and 48 cases were diagnosed as secondary ITP associated with other diseases, including Evans syndrome, systemic lupus erythematosus, and autoimmune lymphoproliferative syndrome. The patients were from 0.5 to 19 years old and had a duration of ITP from 0.2 to 175 months, with platelet count from 1 to 75×109/L before rituximab treatment, according to studies that provided data on these items. Treatments before rituximab varied between and within studies. IVIG, steroid, and anti-D were most commonly used, other previous treatment included splenectomy, cyclophosphamide, ciclosporin, azathioprine, vincristine, danazol, hydroxychloroquine and mycophenolate mofetil.
Table 2. Characteristics of studies enrolling 5 or more patients each.
| Study | Country | PrimaryITP, n | secondaryITP, n | Age, y | Splenectomized, n | ITP duration,mo | Platelet Countbefore rituximabtreatment, ×109/L | Previoustreatment | Study design | Dosage ofrituximab,mg/m2/dose/week | Doses, n |
| Taube (2005) [16] | Germany | 22 | None | 5.8 (2.5–15.2)Δ | 2 | 44 (14–103) | 5 (2–27) | IVIG, St, Anti-D, Sp | Case series |

|
1 |
| Wang (2005) [17] | USA | 24 | None | 12.5 (2–19) | 4 | 23 (6–120) | <30 | Sp, IVIG, St, Anti-D, Dan, Az, VCR | Case series |

|
4 |
| Bennett (2006) [18] | USA | 30 | 6 (ES associated) | 11.2 (2.6–18.3) | 7 | 0.6–12.1 | 1–27 | St,IVIG, Sp, Anti-D | Case series |

|
4 |
| Bader-Meunier (2007) [19] | France | None | 11 (ES associated) | 7.7 (0.7–15) | 6 | 1 (0.2–8.5) | NR | IVIG, CSA, Az, Cyc, Sp, VCR, Dan | Case series |

|
3–4 |
| Rao (2008) [20] | USA | 19 | None | 11 (3.8–18.6) | NR | 1–48 | NR | IVIG,St | Case series |
 ; ; 
|
4–6 |
| Dogan (2009) [21] | Turkey | 10 | None | 3.3–13 | 0 | NR | 7 (2–20) | St, IVIG, anti-D | Case series |

|
4–6 |
| Kumar (2009) [22] | Canada | None | 6 (SLE associated) | 14 (8–16) | 0 | 6(2–30) | 31 (2–75) | St, IVIG, Az, HCQ | Case series |
 ; ; 
|
2–4 |
| Parodi (2009) [23] | Italy | 49 | None | 10.7 (1.2–17.7) | 5 | 21(1–175) | 7 (3–23) | IVIG, St,CSA, Sp | Case series |

|
2–6 |
| Rao (2009) [24] | USA | None | 6 (ALPS associated) | 10 (1–15) | 5 | NR | 12 (2–16) | St, IVIG, Sp, MMF; VCR; Cyc; | Case series |

|
4 |
| Citak (2011) [25] | Turkey | 12 | None | 6 (4–14)Δ | NR | 38 (14–98) | 8 (2–28) | St, IVIG | Case series |

|
4 |
| Xu (2011) [26] | China | 9 | None | 6.8 (3–12) | 2 | 16 (8–29) | 14 (3–21) | St, Sp | Case series |

|
4 |
| Yang (2011) [27] | China | 9 | None | 6 | NR | 12–22 | 10–20 | St, IVIG, VCR; CSA | Case series |

|
4 |
| Grace (2012) [28] | USACanada | 61 | 19# | 7.5 (IQR 4.9, 12)Δ | NR | NR | 14–40Δ | St, VIG | Longitudinal, observational, cohort | NR | NR |
| Sharma (2005)* [29] | India | 10 | None | 9 (4–18) | 6 | NR | <10 | St, CSA Cyc, VCR, IVIG Sp | Case series |

|
4 |
| Kim (2009)* [30] | South Korea | 11 | None | 6.5 (0.5–15.4) | 1 | NR | 13.7 (3–46) | IVIG St Sp | Case series |

|
4 |
| Pospisilova (2010)* [31] | Czech Republic | 10 | None | 4–18 | NR | NR | NR | St, IVIG, Az, anti-D | Case series |

|
2–4 |
| Sampaio (2010)* [32] | Portugal | 7 | None | 4–18 | 1 | 27 (4–72) | NR | St, IVIG | Case series | NR | NR |
| Wang (2011)* [33] | China | 21 | None | NR | NR | NR | NR | NR | Case series |
 ; ; 
|
1–4 |
Results are given as median (range), unless otherwise noted.
Abstracts only; NR: not reported; IQR: inter-quartile range.
Dosage of rituximab: ?375 mg/m2/dose/week; ?750 mg/m2/dose/week; ?500 mg/m2/dose every 2 weeks; ?100 mg/dose/weekly.
Age or plate count at diagnosis.
IVIG: intravenous immunoglobulin; anti-D: anti-D immunoglobulins; St: steroids; Cyc: cyclophosphamide; Az: azathioprine; VCR: vincristine; CSA: cyclosporine; Sp: splenectomy; Dan: danazol.
HCQ: Hydroxychloroquine; MMF: mycophenolate mofetil.
ES: Evans syndrome; SLE: Systemic lupus erythematosus; ALPS: Autoimmune Lymphoproliferative Syndrome.
Secondary ITP included 14 Evans syndrome, 1 Lupus-related ITP, and 4 other auto-immune diseases associated ITP.
Rituximab Dose and Schedule
Doses of rituximab were reported in 16 studies (265 patients) (see table 2). 224 (84.5%) patients received intravenously rituximab as a dose of 375 mg/m2/week for 1∼6 doses. Among that, 175 (66.0%) patients received 4 doses; 22 (8.3%) patients received 1 dose; 10 (3.8%) patients received 6 doses; 7 (2.6%) patients received 2 doses; 6 (2.3%) patients received 3 doses; 4 (1.5%) patients received 5 doses. 4 (1.5%) patients received a second course of 3∼4 doses of 375 mg/m2/week rituximab [22], [24], and 9 (3.4%) patients received dose escalation to 750 mg/m2/dose as no response after 3 doses [20]. 18 (6.9%) patients received 100 mg/dose/week for 4 doses [26], [27] considering the high cost of rituximab. 2 patients received 500 mg/m2/dose every 2 weeks for 2 doses [22]. 21 patients received 1 dose of 375 mg/m2 or 4 dose of 100 mg/dose/week, but without further dose information as published in abstract only [33].
Platelet Count Response
Primary ITP
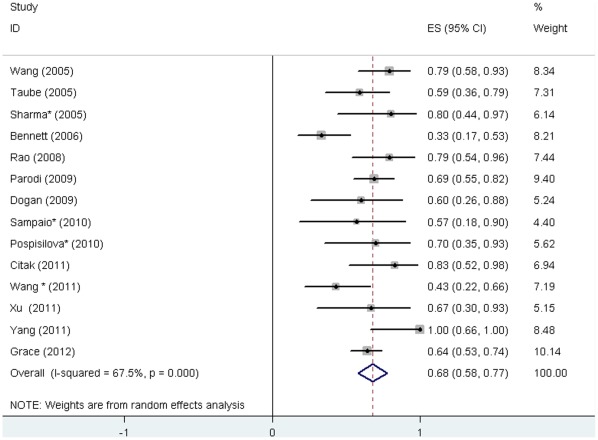
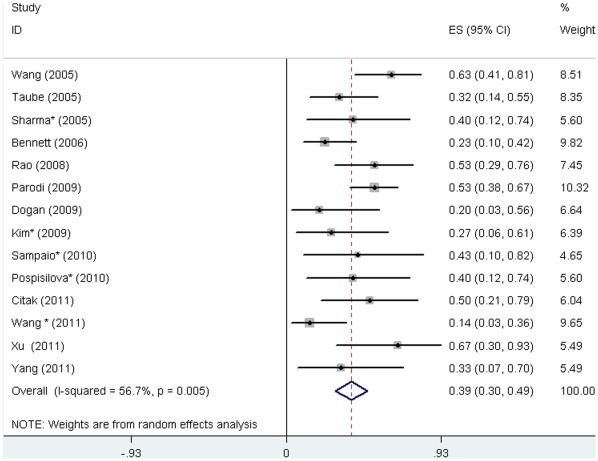
Response and complete response were defined with varied platelet count thresholds in the primary studies. We calculated the pooled response rate according to criteria defined in this systematic review. Response (platelet count ≥30×109/L) after rituximab treatment was reported in 14 studies [16]–[18], [20], [21], [23], [25]–[29], [31]–[33] involving 312 patients, and was achieved in 201 patients (64.4%). 19 (6%) patients, although diagnosed as secondary ITP, were still included in the analysis as their data can not be separated. The response rate reported in the primary studies ranged from 33% [18] to 100% [27], and the pooled rate was 68% (95% CI, 58% to 77%). The heterogeneity between studies was statistically significant (P<0.001) (Figure 2). Complete response was reported in 14 studies [16]–[18], [20], [21], [23], [25]–[27], [29]–[33] involving 243 patients, and was achieved in 99 patients (40.7%). The reported complete response rate ranged from 14% [33] to 67% [26], and the pooled rate was 39% (95%CI, 30% to 49%). The heterogeneity was statistically significant (P = 0.005) (Figure 3).
Figure 2. Response rate to rituximab in children with ITP.
This forest plot is created by the software of STATA 11.1. Solid boxes indicate the response rate in each study. Horizontal lines indicated 95% CIs. The diamond indicates the pooled response rate (68%). Test of heterogeneity: I2 = 67.5%, P<0.001.
Figure 3. Complete response rate to rituximab in children with ITP.
The diamond indicates the pooled complete response rate (39%). Test of heterogeneity: I2 = 56.7%, P = 0.005.
Secondary ITP
4 studies [18], [19], [22], [24] reported data of 29 children with secondary ITP separately. 17 children with Evans syndrome associated ITP were included in 2 studies [18], [19], among that 11 (64.7%) children achieved response and 9 (52.9%) achieved complete response. 6 children with systemic lupus erythematosus and 6 patients with autoimmune lymphoproliferative syndrome associated ITP all (100%) achieved complete response. [22], [24].
Time to Response and Response Duration
Primary ITP
Time to response was reported in 8 studies [17], [18], [21], [23], [25], [26], [27], [30] (97 patients with response), ranging from 0.3 to 17.0 weeks [23]. Individual level data was reported in 2 studies [21], [23] (40 patients with response), the combined median time to response was 3.0 (IQR, 1.0 to 3.6) weeks. Response duration was reported in 8 studies [17], [18], [21], [23], [25], [26], [27], [32] (97 patients with response), ranging from 0.9 to 74.8 months [23], but among that, 63 patients (64.9%) were still having ongoing response when the follow-up ended. Individual level data was reported in 4 studies [17], [21], [23], [32] (62 patients with response), the median response duration was 12.8 (IQR, 4.5 to 25.1) months, among that, 35 patients (56.5%) were having ongoing response when the follow-up ended.
Secondary ITP
7 patients with Evans syndrome associated ITP achieved response with time to response from 0.8 to 8 weeks, and none relapsed after 8 to 20 months follow-up [19]. 6 patients with systemic lupus erythematosus associated ITP achieved response with time to response from 1 to 12 weeks, among that, 2 patients relapsed at 12 and 17 months, but both achieved complete response again after a second course of rituximab, other patients had ongoing response from 6 to 22 months [22]. 2 of 6 patients with autoimmune lymphoproliferative syndrome associated ITP relapsed at 15 and 18 months, the other 4 patients had ongoing response from 5 to 36 months, but data of time to response was not reported [24].
Predictors of Response to Rituximab
4 studies [18], [23], [28], [29] (175 patients) analyzed clinical characteristics associated with platelet count response to rituximab, however, their results varied. Bennett 2005 [18] (36 patients) found that attainment of response was weakly associated with diagnosis of Evans syndrome (P = 0.06), female sex (P = 0.14) and black race (P = 0.09), but the reported P value did not show significance association (P>0.05). Parodi 2009 [23] (49 patients) founded that median duration of ITP was significantly shorter in responders than in non-responders (P = 0.01). Grace 2012 [28], which was the largest (80 patients), showed that secondary ITP and response to steroid were strong predictors of response to rituximab, and was both suggested by result of univariate and multivariable analysis. Sharma 2005 [29] reported that patients showing a higher degree of response continued to be in remission for a longer period compared to ones with lesser degree of response.
Toxicities
23 studies reported 108 adverse events in 91 patients. In 11 studies (190 patients) enrolling more than 5 patients, 78 (41.1%) patients experienced adverse events. Adverse events were classified from Grade 1 to 5 according to Common Terminology Criteria for Adverse Events (CTCAE) [46], and were described in table 3. In all reported 108 adverse events, 91 (84.3%) were mild to moderate, and the most frequently described adverse events are mild allergic reactions, including pruritus, urticaria, chills and fever. 7 patients developed serum sickness after 1 or 2 doses of rituximab, presented with fever, rash, arthralgia and fatigue, and in which, 3 cases were assigned to Grade 3–4, as 1 patient has an acute reaction followed by persistent arthralgia and 2 patients discontinued rituximab for serum sickness [17], [18]. 2 patients experienced immediate hypersensitivity reaction during rituximab infusion, which caused termination of the treatment [20], [45]. 4 patients developed infections that may be associated with rituximab, including 2 patients with varicella [18], [34], 1 patient with pneumonia [19], and 1 patient with life-threatening enteroviral meningoencephalitis presented with a progressive alteration in cognitive functions associated with aphasia and sensorimotor deafness [36]. 1 patient developed common variable immunodeficiency which caused prolonged hypogammaglobulinemia and increased susceptibility to infections [41]. 1 patient developed headache with white matter changes on brain MRI, as only described in abstract, no further information was reported [38]. No death associated with rituximab was reported.
Table 3. Adverse events observed after rituximab infusion in children with ITP.
| Study | ITP Patients, n | Patients experienced adverse events, n | Adverse events (n) | ||
| Grade 1–2 | Grade 3–4 | Grade 5 | |||
| Lorenzana, (2002)* [34] | 3 | 1 | NR | Viral infection (varicella) (1); Centralvenous line infection (1) | None |
| Bengtson, (2003) [35] | 1 | 1 | Rash(1); Elevated serum IgE level (1) | None | None |
| Quartier (2003) [36] | 1 | 1 | NR | Enteroviral meningoencephalitis (1) | None |
| Pusiol (2004) [37] | 2 | 1 | Fever and chill (1) | None | None |
| Sharma (2005)* [29] | 10 | 3 | Urticaria (1); Fever(2) | None | None |
| Wang, (2005) [17] | 24 | 9 | Pruritus (1); Throat tightness (1);Urticaria(3); Headache (3); Chest pain (1);Serum sickness (2); Low ANC (3) | Serum sickness (1) | None |
| Bennett, (2006) [18] | 36 | 23 | Chills, fever, and respiratorysymptoms (17) | Serum sickness (2); Viral infection(varicella) (1); Hypotension (1) | None |
| Yadav, (2006)* [38] | 3 | 1 | Drowsiness (1) | Hypotension(1); White matterchanges on MRI (1) | None |
| Bader-Meunier(2007) [19] | 11 | 7 | Vomiting(1); Facial edema(1);Urticarial rash(1) | Transient neutropenia (3); Pneumonia(1) | None |
| Bisogno (2007) [39] | 1 | 1 | Persistent B-cell depletion andhypogammaglobulinemia (1) | NR | None |
| Kim (2007) [40] | 1 | 1 | Fatigue and malaise (1) | NR | None |
| Gentner,(2008) [41] | 1 | 1 | NR | Common variable immunodeficiency (1) | None |
| Parodi, (2009) [23] | 49 | 9 | Urticaria (4); Mild headache (3);Chills (3); Fever (2) | None | None |
| Rao,(2008) [20] | 19 | 15 | Cough (4); Rash (3); Muscle cramps (3);Headache (2); Shortness of breath (1);Hypertension (1); Chills (1); Fever (1);Angioedema (1) | Immediate hypersensitivity reaction(1) | None |
| Adeli,(2009) [42] | 2 | 2 | Persistent hypogammaglobulinemia (2) | NR | None |
| Cooper (2009) [43] | 1 | 1 | Persistent B-celldepletion andhypogammaglobulinemia (1) | NR | None |
| Dogan, (2009) [21] | 10 | 3 | Itching and scraps(3) | None | None |
| Goto (2009) [44] | 1 | 1 | Serum sickness (1) | NR | None |
| Rao (2009) [24] | 6 | 3 | Persistent hypogammaglobulinemia (3) | NR | None |
| Sampaio (2010)* [32] | 7 | 3 | Pruritus (1); Urticaria (1); Vomiting (1) | None | None |
| Romanos-Sirakis (2011) [45] | 1 | 1 | Serum sickness (1) | Immediate hypersensitivity reaction(1) | None |
| Xu (2011) [26] | 9 | 2 | Fever and chill (2); Urticaria (2) | None | None |
| Yang (2011) [27] | 9 | 1 | Pruritus (1) | None | None |
NR: not reported * Abstract.
Grade 1-Grade 2: Mild to moderate; Grade 3: Severe but not immediately life-threatening; Grade 4: Life-threatening; Grade 5: Death.
ANC: absolute neutrophil count; MRI: magnetic resonance imaging.
Publication Bias
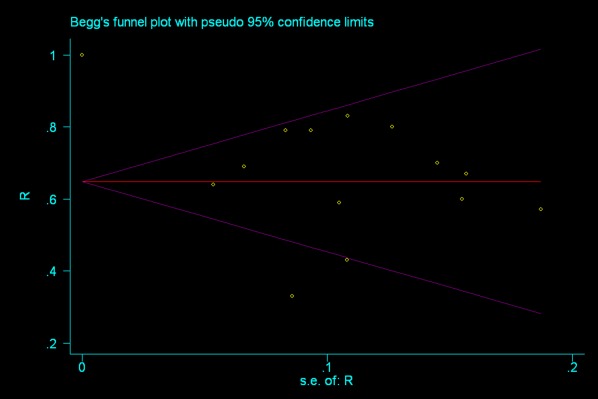
The Egger and Begg test were conducted to investigate publication bias in 14 studies reporting response in patients with primary ITP. The Egger test indicated no evidence of publication bias (P = 0.987). Begg’s funnel plot with pseudo 95% (figure 4) was graphed in the logic that studies with smaller sample size have larger random error, thus are more dispersed, and the plot of effect estimates against standard errors would be skewed and asymmetrical in the presence of publication bias. In this review, the funnel plot suggests no significant asymmetry, indicating no evidence of substantial publication bias (P = 0.583).
Figure 4. Begg funnel plot with pseudo 95% for studies reporting response.
This plot is created by STATA 11.1. For each study, the response rate is plotted against its standard error. The funnel plot suggests no significant asymmetry, indicating no evidence of substantial publication bias.
Discussion
This is the first systematic review that summarizes the efficacy and safety of rituximab in children with ITP, and this review is very important at this time as rituximab has been widely used off-label for children with ITP. The treatment of rituximab achieved response in 68% and complete response in 39% of patients with primary ITP. According to studies reporting individual data, the median time to response was 3.0 weeks and the median response duration was 12.8 months. Studies with a small number of patients with secondary ITP reported that response rate to rituximab was from 64.7%∼100%. Most adverse events were mild to moderate and no death was reported.
The response and complete response rate in children with primary ITP obtained in this review was 68% and 39%, respectively. It was similar to that reported in the previous systematic review on adults, which reported that overall response (platelet count >50×109/L) and complete response (platelet count >150×109/L) rate was 63% and 46% respectively [9]. Although response criteria differ in these two reviews, it may suggest that rituximab results in a similar response in adults and children with primary ITP.
The frequently administrated dose of rituximab is 375 mg/m2 per week for 4 weeks for both children and adult with ITP [9], although this dose was developed and approved for the treatment of lymphoma [47]. 1 study investigated the efficacy of a single dose of rituximab (375 mg/m2) in children, and reported a similar response and complete response rate as 4 doses [16]. 2 studies investigated a low dose rituximab of 100 mg/dose/week for 4 weeks in 18 children with primary ITP, which suggested that the lower dose of rituximab still reached a good response [26], [27]. Treatment of rituximab is still costly in the present time, and thus sometimes 4 doses of 375 mg/m2 are not affordable, especially for patients with poor accessibility to health resources. In addition, high dose and multiple dosing may increase risk of adverse events. Low dose of rituximab may be promising for patients with ITP, and more studies are needed to investigate that.
Most of adverse events associated with rituximab in children with ITP were mild to moderate infusional reactions. More severe adverse effects included serum sickness, common variable immunodeficiency, severe virus infection including enteroviral meningoencephalitis, and white matter changes. 2 children developed viral infection after rituximab treatment, but they were both previously treated with other immunosuppressive agents like steroids, vincristine, and cyclosporine A, it is difficult to establish a direct association with rituximab [18], [34]. In addition, a systematic review in cancer patients did not find any increase of infection caused by monoclonal antibodies [48]. The case of common variable immunodeficiency in patients with ITP after rituximab treatment was reported in an 8-year-old child [41], however, it was not clear whether it was caused by rituximab, as ITP might be the first clinical manifestation before development of common variable immunodeficiency. 1 patient developed headache and MRI showing white matter changes [38], but without detailed information as published only in abstract, it is not clear whether the children developed progressive multifocal leukoencephalopathy, although it is reported that there was a potential of progressive multifocal leukoencephalopathy among rituximab-treated patients [49]. No death was found in this review, but 9 cases of death (2.9%) were reported in studies on adults [9]. The mortality reported in adults treated with rituximab might be overestimated and these cases might be explained by long courses of complex treatment regimens or the selection of patients with advanced disease.
All the studies eligible for efficacy analysis in this review failed to compare effect between rituximab with non-rituximab group. The response rate obtained may be influenced by many potential factors, such as treatments before and combined with rituximab, cause and development of ITP. Studies on this topic with better methodological design like randomized controlled studies are urgently needed. Consecutive enrolling of patients can help to reduce selection bias in prospective case series studies, unfortunately, few studies provided information on that, and this needs improvement in future studies.
Supporting Information
Search strategy for PUBMED, EMBASE and CENTRAL.
(DOC)
PRISMA checklist.
(DOC)
Acknowledgments
We thank Dr Jin Wen from Department of Hospital Management and Health Policy in West China Hospital of Sichuan University for providing statistic support for this review.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, et al. Standardization of terminology, definitions and outcome criteria in an international working group immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 2.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, et al. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85:174–180. doi: 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 3.Kühne T, Buchanan GR, Zimmerman S, Michaels LA, Kohan R, et al. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143:605–608. doi: 10.1067/s0022-3476(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 4.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 5.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 6.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–3908. [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 8.Eupropean Medicine Agency website. Summary of European public assessment report for Mabthera. 20 Available: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000165/human_med_000897.jsp&mid=WC0b01ac058001d124. Accessed 2012 Jan. [Google Scholar]
- 9.Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cooper N, Bussel JB. The long-term impact of rituximab for childhood immune thrombocytopenia. Curr Rheumatol Rep. 2010;12:94–100. doi: 10.1007/s11926-010-0090-5. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Clinical Excellence (NICE) website. Quality assessment for case series. 11 Available: http://www.nice.org.uk/nicemedia/pdf/Appendix_04_qualityofcase_series_form_preop.pdf. Accessed 2011 Apr. [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1011. [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1997;33:159–174. [PubMed] [Google Scholar]
- 16.Taube T, Schmid H, Reinhard H, von Stackelberg A, Overberg US. Effect of a single dose of rituximab in chronic immune thrombocytopenic purpura in childhood. Haematologica. 2005;90:281–283. [PubMed] [Google Scholar]
- 17.Wang J, Wiley JM, Luddy R, Greenberg J, Feuerstein MA, et al. Chronic immune thrombocytopenic purpura in children: assessment of rituximab treatment. J Pediatr. 2005;146:217–221. doi: 10.1016/j.jpeds.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Bennet CM, Rogers ZR, Kinnamon DD Bussel JB, Mahoney DH, et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107:2639–2642. doi: 10.1182/blood-2005-08-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bader-Meunier B, Aladjidi N, Bellmann F, Monpoux F, Nelken B, et al. Rituximab therapy for childhood Evans syndrome. Haematologica. 2007;92:1691–1694. doi: 10.3324/haematol.11540. [DOI] [PubMed] [Google Scholar]
- 20.Rao A, Kelly M, Musselman M, Ramadas J, Wilson D, et al. Safety, efficacy, and immune reconstitution after rituximab therapy in pediatric patients with chronic or refractory hematologic autoimmune cytopenias. Pediatr Blood Cancer. 2008;50:822–825. doi: 10.1002/pbc.21264. [DOI] [PubMed] [Google Scholar]
- 21.Dogan M, Oner AF, Acikgoz M, Uner A. Treatment of chronic immune thrombocytopenic purpura with rituximab in children. Indian J Pediatr. 2009;76:1141–1144. doi: 10.1007/s12098-009-0230-y. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Benseler SM, Kirby-Allen M, Silverman ED. B-cell depletion for autoimmune thrombocytopenia and autoimmune hemolytic anemia in pediatric systemic lupus erythematosus. Pediatrics. 2009;123:e159–63. doi: 10.1542/peds.2008-2361. [DOI] [PubMed] [Google Scholar]
- 23.Parodi E, Rivetti E, Amendola G, Bisogno G, Calabrese R, et al. Long-term follow-up analysis after rituximab therapy in children with refractory symptomatic ITP: identification of factors predictive of a sustained response. Br J Haematol. 2008;144:552–558. doi: 10.1111/j.1365-2141.2008.07487.x. [DOI] [PubMed] [Google Scholar]
- 24.Rao VK, Price S, Perkins K, Aldridge P, Tretler J, et al. Use of rituximab for refractory cytopenias associated with autoimmune lymphoproliferativesyndrome (ALPS). Pediatr Blood Cancer. 2009;52:847–852. doi: 10.1002/pbc.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Citak EC, Citak FE. Treatment results of children with chronic immune thrombocytopenic purpura (ITP) treated with rituximab. J Trop Pediatr. 2011;57:71–72. doi: 10.1093/tropej/fmq033. [DOI] [PubMed] [Google Scholar]
- 26.Xu S, Bai H, Wang C, Zhang Q, Xi R, et al. The effect of rituximab in chronic refractory immune thrombocytopenic purpura in children. J China Pediatr Blood Cancer. 2011;16:129–131. [Google Scholar]
- 27.Yang J, Wang X, Wang H, Li XQ. Low dose of rituximab for children with chronic immune thrombocytopenia and a review of the literatures. Chinese Journal of Obstetrics & Gynecology and Pediatrics. 2011;7:474–475. [Google Scholar]
- 28.Grace RF, Bennett CM, Ritchey AK, Jeng M, Thornburg CD, et al. Pediatr Blood Cancer Jun 14 [Epub ahead of print]; 2011. Response to steroids predicts response to rituximab in pediatric chronic immune thrombocytopenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma A, Nair V, Mishra DK Kotwal J Chopra GS, et al. Chimeric anti CD-20 monoclonal antibody-rituximab in refractory childhood idiopathic thrombocytopenic purpura: an Indian experience.[Abstract] Blood. 2005;106:4010. [Google Scholar]
- 30.Kim JY, Lee, KS, Kang HJ, Kook H, Koo HH, et al. Rituximab treatment in childhood chronic immune thrombocytopenic purpura (ITP). [Abstract] Blood. 2009;114:4461. [Google Scholar]
- 31.Pospisilova D, Smisek P, Su kova M, Votava T, Hak J, et al. Experience with rituximab treatment in children with severe refractory immune thrombocytopenic purpura in the Czech Republic. Ann Hematol 89 (Suppl 1): S105–. 2010;S109 [Google Scholar]
- 32.Sampaio I, Palaré MJ, Ferrão A, Morais A. Saianda A. Rrituximab in the treatment of immune thrombocytopenia: follow-up in seven cases. Haematologica: 2010;95:750. [Google Scholar]
- 33.Wang H-M, Sui T, Yang Y, Zhou Z, Liu X, et al. Low-dose rituximab treatment in Chinese patients with immune thrombocytopenia. J Pediatr. 2011;146:330. [Google Scholar]
- 34.Lorenzana AN, Mularoni PJ, Doyle, M Greenwood R, Sawaf H. Therapeutic effect of rituximab (Rituxan) in childhood immune thrombocytopenia and Evan’s syndrome. [Absract] 2002;1043 [Google Scholar]
- 35.Bengtson KL, Skinner MA, Ware RE. Successful use of anti-CD20 (rituximab) in severe, life-threatening childhood immune thrombocytopenic purpura. J Pediatr. 2003;143:670–673. doi: 10.1067/S0022-3476(03)00446-3. [DOI] [PubMed] [Google Scholar]
- 36.Quartier P, Tournilhac O, Archimbaud C, Lazaro L, Chaleteix C, et al. Enteroviral meningoencephalitis after anti-CD20 (rituximab) treatment. Clin Infect Dis: 2003;36:e47–49. doi: 10.1086/345746. [DOI] [PubMed] [Google Scholar]
- 37.Pusiol A, Cesaro S, Nocerino A, Picco G, Zanesco L. Successful treatment with the monoclonal antibody rituximab in two children with refractory autoimmune thrombocytopenia. Eur J Pediatr. 2004;163:305–307. doi: 10.1007/s00431-004-1417-x. [DOI] [PubMed] [Google Scholar]
- 38.Yadav SP, Misra R, Gaurav K, Sachdeva A. Rituximab use in children – a single center experience. [Absract] Blood. 2006;108:3892. [Google Scholar]
- 39.Bisogno G. Persistent B-cell depletion after rituximab for thrombocytopenic purpura. Eur J Pediatr. 2007;166:85–86. doi: 10.1007/s00431-006-0222-0. [DOI] [PubMed] [Google Scholar]
- 40.Kim JJ, Thrasher AJ, Jones AM, Davies EG, Cale CM. Rituximab for the treatment of autoimmune cytopenias in children with immune deficiency. Br J Haematol. 2007;138:94–96. doi: 10.1111/j.1365-2141.2007.06616.x. [DOI] [PubMed] [Google Scholar]
- 41.Gentner J, Morra M, Knutsen AP. Development of common variable immunodeficiency in an 8-year-old boy treated with rituximab for idiopathic thrombocytopenia. Pediatr Asthma Allergy Immunol. 2008;21:99–104. [Google Scholar]
- 42.Adeli MM, Eichner BH, Thornburg C, Williams L. Persistent antibody depletion after rituximab in three children with autoimmune cytopenias. Pediatr Hematol Oncol. 2009;26:566–572. doi: 10.3109/08880010903271697. [DOI] [PubMed] [Google Scholar]
- 43.Cooper N, Davies EG, Thrasher A. Repeated courses of rituximab for autoimmune cytopenias may precipitate profound hypogammaglobulinemia requiring replacement intravenous immunoglobulin. British Journal of Haematology. 2009;146:120–122. doi: 10.1111/j.1365-2141.2009.07715.x. [DOI] [PubMed] [Google Scholar]
- 44.Goto S, Goto H, Tanoshima R, Kato H, Takahashi H, et al. Serum sickness with an elevated level of human anti-chimeric antibody following treatment with rituximab in a child with chronic immune thrombocytopenic purpura. Int J Hematol. 2009;89:305–309. doi: 10.1007/s12185-009-0269-6. [DOI] [PubMed] [Google Scholar]
- 45.Romanos-Sirakis E, Feigenbaum BA. Immediate hypersensitivity reaction and successful rapid desensitization to rituximab followed by serum sickness in a 4 year old. Journal of Allergy and Clinical Immunology. 2011;127:AB198. [Google Scholar]
- 46.National Institute of Health website. National Cancer Institute common terminology criteria for adverse events, Version 4.0. 25 Available: http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed 2011 Apr. [Google Scholar]
- 47.Pescovitz MD. Rituximab, an Anti-CD20 Monoclonal antibody: history and mechanism of action. Am. J. Transplant. 2006;6:859–866. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 48.Rafailidis PI, Kakisi OK, Vardakas K, Falagas ME. Infectious complications of monoclonal antibodies used in cancer therapy: a systematic review of the evidence from randomized controlled trials. Cancer. 2007;109:2182–2189. doi: 10.1002/cncr.22666. [DOI] [PubMed] [Google Scholar]
- 49.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy for PUBMED, EMBASE and CENTRAL.
(DOC)
PRISMA checklist.
(DOC)