Abstract
Walleye dermal sarcoma (WDS) is a benign tumor of walleye fish that develops and completely regresses seasonally. The retrovirus associated with this disease, walleye dermal sarcoma virus, encodes three accessory genes, two of which, rv-cyclin (orfA) and orfb, are thought to play a role in tumor development. In this study, we attempted to recapitulate WDS development by expressing rv-cyclin in chimeric and stable transgenic zebrafish. Six stable transgenic lines expressing rv-cyclin from the constitutive CMVtk promoter were generated. Immunohistochemistry and quantitative reverse transcriptase polymerase chain reaction demonstrate that rv-cyclin is widely expressed in different tissues in these fish. These lines were viable and histologically normal for up to 2 years. No increase in tumors or tissue proliferation was observed following N-ethyl N-nitrosourea exposure or following tail wounding and subsequent tissue regeneration compared to controls. These data indicate that rvcyclin is not independently sufficient for tumor induction in zebrafish.
Keywords: Walleye dermal sarcoma virus, orfA, rv-cyclin, Fish retrovirus, Walleye dermal sarcoma, Zebrafish
Introduction
Walleye dermal sarcoma virus (WDSV), an Epsilonretrovirus, was first identified in association with benign cutaneous proliferative lesions in walleyes (Sander vitreus) (Martineau et al. 1992), known as walleye dermal sarcoma (WDS), and was found to be highly enriched within neoplastic cells (Poulet et al. 1995). Subsequent molecular characterization of WDSV identified three novel open reading frames: rv-cyclin (orfA), orfB, and orfC. The accessory genes rv-cyclin and orfb are located downstream of env in the 3′ proximal region of the genome, and orfC lies between the 5′ long terminal repeat and gag (Holzschu et al. 1995).
Several observations suggest that rv-cyclin and OrfB may play a central role in inducing cell proliferation, leading to WDS. In developing WDS fall tumors, low levels of spliced viral transcripts are present that are capable of encoding rv-cyclin and OrfB exclusively (Quackenbush et al. 1997). The rv-cyclin protein contains a region homologous to the cyclin box fold (LaPierre et al. 1998). Functionally, rv-cyclin complements cyclin function in G1 cyclin-deficient yeast (Saccharomyces cerevisiae) to promote cell cycle progression (LaPierre et al. 1998). Expression of rv-cyclin in transgenic mice from the bovine keratin-5 promoter causes wound-associated severe squamous epithelial hyperplasia and dysplasia (Lairmore et al. 2000). An additional function for rv-cyclin in transcriptional regulation may be mediated through an interaction with cyclin-dependent kinase 8 (CDK8), a component of the mediator complex (Rovnak and Quackenbush 2002). The rv-cyclin protein also has a functional transcription activation domain in the carboxy end, outside of the cyclin box fold, which directly contacts TATA-binding protein-associated factor 9 (TAF9) (Rovnak and Quackenbush 2002, 2006). A functional activation domain is necessary for both the inhibition and the activation of transcription by the rv-cyclin protein in vitro (Rovnak and Quackenbush 2006; Quackenbush et al. 2009).
Zebrafish (Danio rerio) have been utilized in cancer research in both chemical carcinogen and in genetic studies (Amatruda and Patton 2008; Feitsma and Cuppen 2008; Stern and Zon 2003). In these systems, zebrafish develop a wide spectrum of proliferative diseases including epidermal hyperplasias and neoplasias (Spitsbergen et al. 2000a, b; Beckwith et al. 2000). To investigate the ability of WDSV rv-cyclin to induce cell proliferation, we have expressed this viral accessory gene in transgenic zebrafish and monitored for proliferative changes. In this study, we report the creation of stable transgenic zebrafish lines that express WDSV rv-cyclin from the constitutive CMVtk promoter. In this system, rv-cyclin expression is insufficient to induce tissue proliferation or increase the incidence of cancer, even after exposure to chemical mutagens.
Materials and Methods
Plasmid Constructs
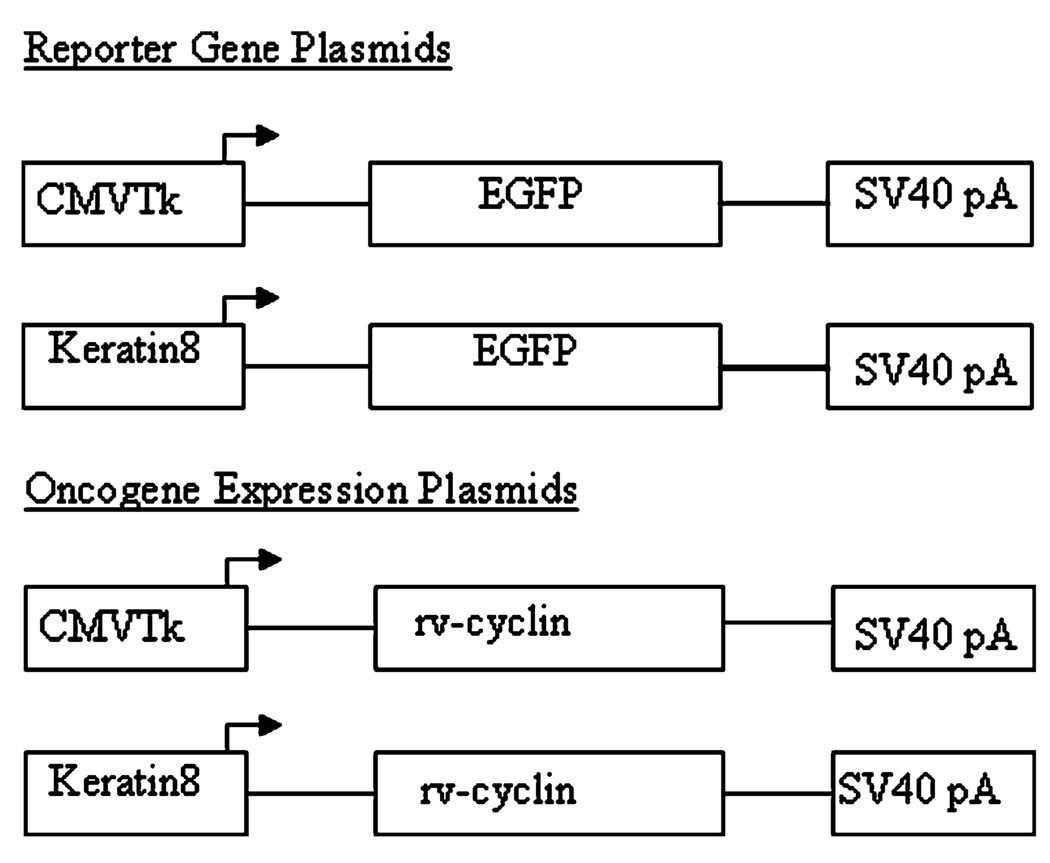
Plasmids pKH3orfA, which expresses WDSV rv-cyclin (OrfA) with an N-terminal HA tag from a CMV promoter, and pcDNA3.1orfA have been previously characterized (Rovnak et al. 2001; Rovnak and Quackenbush 2002). pCMVtkmrkYY (gift of C. Winkler, U. Würzburg) contains the Xiphophorus Xmrk mini-gene driven by a CMVtk promoter (Winkler et al. 1994). The CMVtk promoter was isolated by polymerase chain reaction (PCR) cloning using forward primer CMVtkF 5′GCTCTAGATCTGCTTATA TAGACCTCCCACCGTA3′ with an XbaI site added and reverse primer CMVtkR 5′AGATCTGCGGCACGCTGTTGA3′ with a BglII site added. The DNA sequence of the promoter was verified, and it was cloned into shuttle vector pSF1 using BglII and XbaI sites to generate pSF1CMVtk. pCMVtk rv-cyclin was constructed by removing orfA and the polyadenylation (pA) signal from retroviral vector pLNACL with a HindIII and partial XhoI digestion and ligating into HindIII and SalI digested pSF1CMVtk. To generate pCMVtkGFP, the EGFP gene and pA signal were PCR cloned from pEGFPN-1 using forward primer (EGFP-F) 5′CTCAAGCTTCGAATTCTGACG3′ and reverse primer (EGFP-R) 5′CCGCTCGAGACGCCTTAAGATACATTGATGAGTT3′. HindIII and XhoI sites were added to the forward and reverse primers, respectively. The fragment was cloned into the HindIII and SalI sites of pSF1CMVtk (Fig. 1).
Fig. 1.
Plasmid DNA constructs used for oncogene microinjections. Reporter gene constructs express enhanced green fluorescent protein (EGFP) under the control of a ubiquitous promoter CMVtk (Herpes Simplex Virus-1 thymidine kinase core promoter elements fused to enhancer sequences derived from the cytomegalovirus immediate–early promoter) or the zebrafish krt8 gene promoter that expresses in the superficial layer of epithelium (Ju et al. 1999)
The plasmid pKrt8GFP (originally named pCK-EGFP), which expresses green fluorescent protein (GFP) from the zebrafish krt8 gene promoter, has been previously described (Ju et al. 1999). To construct pKrt8 rv-cyclin, the GFP gene was removed from pKrt8GFP using BamHI and NotI enzymes. The rv-cyclin gene was removed from pcDNA3.1orfA with BamHI and NotI enzymes and ligated into the corresponding sites from pKrt8GFP (Fig. 1). Control plasmid Δptr rv-cyclin was constructed by excising the krt8 promoter from pKrt8orfA by self-ligation following digestion with BamHI and BglII.
Zebrafish Egg Production and Microinjection
We used wild-type zebrafish of the Cornell line (K.E. Whitlock; Cornell University) which were AB fish (University of Oregon) crossed to descendants of wild-type commercial fish that underwent a selection against deleterious lethals. Fish were reared in a recirculating system (Aquatic Habitats) in a 14-h light, 10-h dark cycle at 28°C. The fish were fed twice daily with either crushed TetraMin Tropical Flake (Blacksburg, VA) or live artemia from INVE (Grantsville, UT). Single-pair crosses were set up in divided breeder traps, and embryos were collected typically 15 min after the divider was removed.
Before injection, plasmids were linearized upstream of the promoter region and purified using GeneClean (Qbiogene; Carlsbad, CA).
Micropipets with a 1.2-mm outside diameter and 0.94-mm inside diameter were pulled on a Sutter Brown-Flaming micropipet puller and beveled on a World Precision Instruments microbeveler. For microinjection, linearized DNA was resuspended at a concentration of 500 µg/mL in DNA injection solution (100 mM KCl, 0.02% phenol red, pH 7.0). GFP plasmids (Fig. 1), pCMVtkGFP and pKrt8-GFP, were mixed 1:1 with oncogene-expressing constructs, such that each plasmid was at a final concentration of 250 µg/mL when injected. Approximately 1 nL of DNA was microinjected using a Narishige foot-pedal-operated gas injector into the cytoplasm of single-celled, fertilized embryos. Injected embryos were maintained in Holtfreter's solution (60 mM NaCl, 2.4 mM sodium bicarbonate, 0.8 mM calcium chloride, 0.67 mM potassium chloride) supplemented with 1 µg/mL of methylene blue at 28°C.
Generation of Stable Transgenic Zebrafish
The walleye accessory gene expression plasmid pCMVtk rv-cyclin was co-injected with pKrt8-GFP. At 5 days post-injection, embryos with the highest levels of GFP fluorescence were isolated and maintained according to The Zebrafish Book (Westerfield 1994). At sexual maturity, fish were mated and the progeny examined for GFP fluorescence. One hundred embryos were also collected from each cross and genomic DNA prepared by a Qiagen DNA blood kit (Qiagen Inc). DNA (100 ng) was analyzed by PCR for the presence of the pCMVtk rv-cyclin transgene using forward primer CMVtkF (see above) and reverse primer orfA anti-135 (5′CCATGTGTTCGGGGTAGCTT3′). PCR reaction conditions were: 30 s denaturation at 94°C, annealing at 55°C for 30 s, and extension for 1 min at 72°C. After 35 cycles, the samples were incubated 10 min. at 72°C and analyzed on a 1.5% agarose gel.
Western Blot
Zebrafish co-injected with pCMVtk rv-cyclin and pKrt8GFP were screened for GFP fluorescence at 24 h post-injection. GFP-positive embryos were grown to adults. At 3 days, 1 month, and 3 months, tissues were collected from injected fish for western blot analysis of protein expression. Zebrafish tissues were suspended in lysis buffer containing 1% Triton-X 100, 150 mM NaCl, 10 mM Tris (pH 7.5), 1 mM EDTA, pH 8.0, 1 mM EGTA, pH 8.0, 0.2 mM sodium orthovanadate, 0.2 mM phenylmethylsulfonyl fluoride, and 0.5% NP-40. Tissues were homogenized on ice with a dounce homogenizer and frozen in liquid nitrogen. After centrifugation, protein concentrations were determined using a Bradford assay (Bio-Rad). For a positive control, zebrafish ZF4 cells (Driever and Rangini 1993) were transfected with pCMVtk rv-cyclin using Lipofectamine reagent. Approximately 10 µg of denatured protein for each sample were loaded onto a 10% Bis-Tris gel (Invitrogen) and run in NuPAGE MOPS SDS running buffer (Invitrogen). Protein was transferred to a nitrocellulose membrane and blocked in TBST (150 mM NaCl, 10 mM Tri, pH 8.0, 0.1% Tween-20) containing 5% milk. The blot was then incubated with a polyclonal rv-cyclin antibody (Rovnak et al. 2001) for 1 h at 37°C at a 1:100 dilution and treated with a goat anti-rabbit IgG secondary antibody conjugated to horseradish peroxidase (KPL) and detected with 3, 3′, 5, 5′-tetramethylbenzidine (KPL).
Quantitative Reverse Transcriptase-PCR
For quantitative reverse transcriptase-PCR (qRT-PCR) analysis of transgene expression, cross-sections of tissue were collected from one zebrafish from each WDSV rv-cyclin transgenic line. From founder 26, we removed tissues from the skin, tail fin, muscle, brain, and intestine. Tissue samples were homogenized in glass tissue grinders on ice in 1 mL of RNAzol (Tel-test, Inc.); RNA was extracted and pelleted from the homogenate according to the manufacturer's protocol. The total RNA was treated with RNase-free DNase I for 15 min at room temperature, then heat-inactivated for 15 min at 65°C.
qRT-PCR was performed with the Taqman one-step RT-PCR kit and ABI 7700 quantitative PCR instrument (both Applied Biosystems). Primers and probes were designed by Primer Express Software (Applied Biosystems) to amplify a 100-bp region of the rv-cyclin gene. The qRT-PCR reaction was performed with 50 ng of total RNA in 1× Master Mix without UNG, 1× multiscribe, and RNase inhibitor mix. Forward primer 5′CCAGCACAATTACCTCTTGTGG3′, reverse primer 5′CCAGCCTTGAATGTGCCTCT3′, and probe 5′ [6-FAM]-CGCATGCCTCCAGATAGCTGCTAAACAC-[TAMRA]3′ were at final concentrations of 300, 300, and 100 nM, respectively. Thermal cycling began with a 30-min RT step at 48°C, followed by a 10-min hold at 95°C for AmpliTaq activation, and 40 cycles at 15 s 95° C denaturing and 1 min 60°C annealing and extension.
For an rv-cyclin RNA standard, pCMVtk rv-cyclin was linearized by digestion with BamHI. The rv-cyclin gene was then transcribed from the vector's T7 promoter using the MAXIscript T7 kit (Ambion). Resulting RNA was precipitated and redissolved according to the manufacturer's protocol. Final RNA concentration was determined by an OD260 measurement with a spectrophotometer. Tenfold serial dilutions from a starting concentration of 1 × 106 copies were prepared for a standard curve. Copy numbers in unknown samples were determined from a standard regression fit using the supplier's software (Applied Biosystems).
N-Ethyl N-Nitrosourea Mutagenesis and Tail-Fin Regeneration
N-Ethyl N-nitrosourea (ENU) treatment was based on conditions used in the study by Moore et al. (2006), previously shown to induce both somatic and germ-line mutations at a high frequencies. ENU (Sigma) was dissolved in 10 mM sodium phosphate, pH 6.5, by injection into bottles yielding a solution of final concentration of approximately 100 mM. The dissolved ENU solution was used within 1 h of being dissolved. One-month-old rv-cyclin transgenic, GFP transgenic, or control zebrafish was treated with 3.0 mM ENU at 25°C for 1 h every 3 days, for a total of three treatments. Following each treatment, fish were placed in a series of recovery baths and returned to the recirculating system.
Tail-fin amputations were conducted on fish anesthetized in tricaine (MS222; Sigma). A scalpel cut was made half way between where the scales end and the tip of the caudal fin. The fish were returned to fresh water and monitored for 48 h before being returned to the recirculating system.
Histology and Immunohistochemistry
Zebrafish were initially examined stereomicroscopically for presence of skin proliferative lesions or lesions consistent with walleye dermal sarcoma. Tissue histology was done according to published protocols (Luna 1968; Bowser and Wooster 1991). The fish were preserved in 10% neutral buffered formalin and then decalcified in sodium EDTA prior to sectioning, which consisted of five sagittal sections of whole fish at the following locations starting from the left side: (1) one-fourth way to midline, (2) one-half way to midline, and (3) at midline sagittal plane for untreated transgenic and control zebrafish. ENU-treated zebrafish caudal fin was initially removed, and the remainder of the fish was sectioned in half sagittally. The two halves and tail were then sectioned three times. All sections were treated with hematoxylin and eosin stains.
Immunohistochemistry was conducted on 5-µm transverse sections made midway through the caudal fin. For antibody staining, sections were deparaffinized in graded ethanol solutions and incubated with 1:500 dilutions of polyclonal rabbit antiserum reactive to rv-cyclin or normal rabbit serum for 1 h at 37°C (Rovnak et al. 2001; Daniels et al. 2008) followed by a 1:40 dilution of affinity purified goat anti-rabbit rhodamine isothiocyanine conjugate (RITC; Kirkegaard & Perry Laboratories). Nuclei were counterstained for 1 h in 300 µM 4′,6′-diamidino-2-phenylindole (DAPI). Individual images were captured in color on a Nikon Eclipse 80i and overlaid and composed with Photoshop 7.0 software (Adobe).
Results
Evaluation of the CMVtk Promoter in Zebrafish
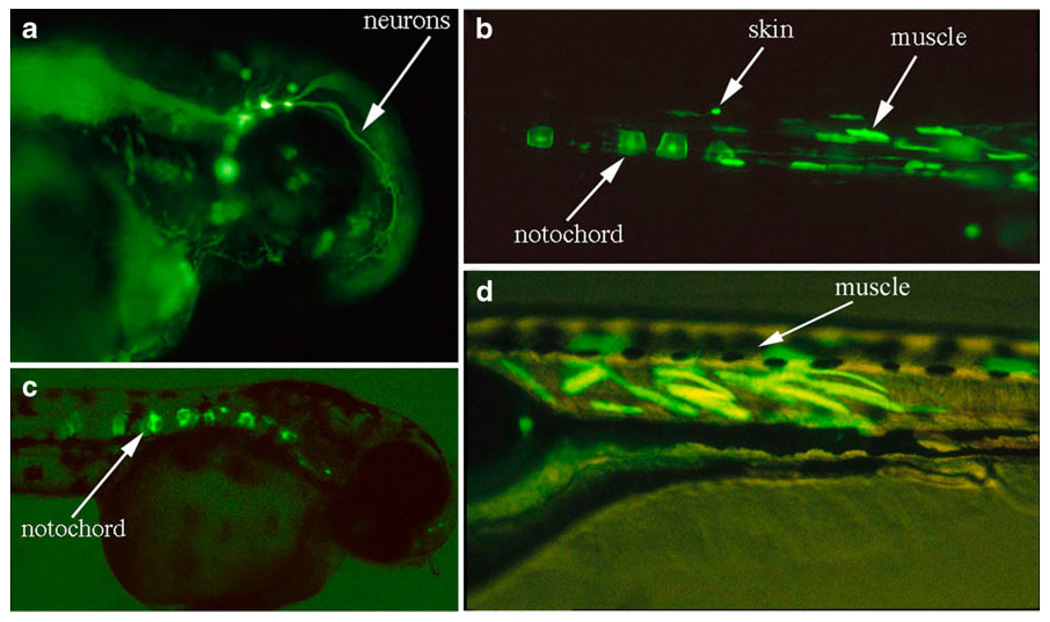
The CMVtk promoter has previously been demonstrated to drive strong, constitutive expression of reporter genes in medaka (Oryzias latipes) from late blastula onwards to posthatching stages (Winkler et al. 1992). To evaluate the activity of this promoter in zebrafish, we fused promoter sequences to the enhanced green fluorescent protein (EGFP) gene (Fig. 1). When the DNA is microinjected into zebrafish embryos, expression of GFP is first visible during the late gastrula period (8–10 h post-injection). Expression appears to be mosaic, i.e., certain patches of cells express the reporter gene, and almost all injected embryos expressed GFP to some level (Fig. 2a–d). After screening several embryos, we found that the activity of this promoter appears ubiquitous in vivo. However, certain cell types such as muscle and notochord appear to express GFP more frequently (Fig. 2a–d).
Fig. 2.
GFP expression from the CMVtk promoter in zebrafish. Three days post-injection, GFP expression in 3-day-old embryos appears highly mosaic with highest levels (indicated by arrows) in the neurons (a), skin (b), muscle (b, d), and notochord (b, c)
Chimeric Expression of Walleye Cyclin Homologs
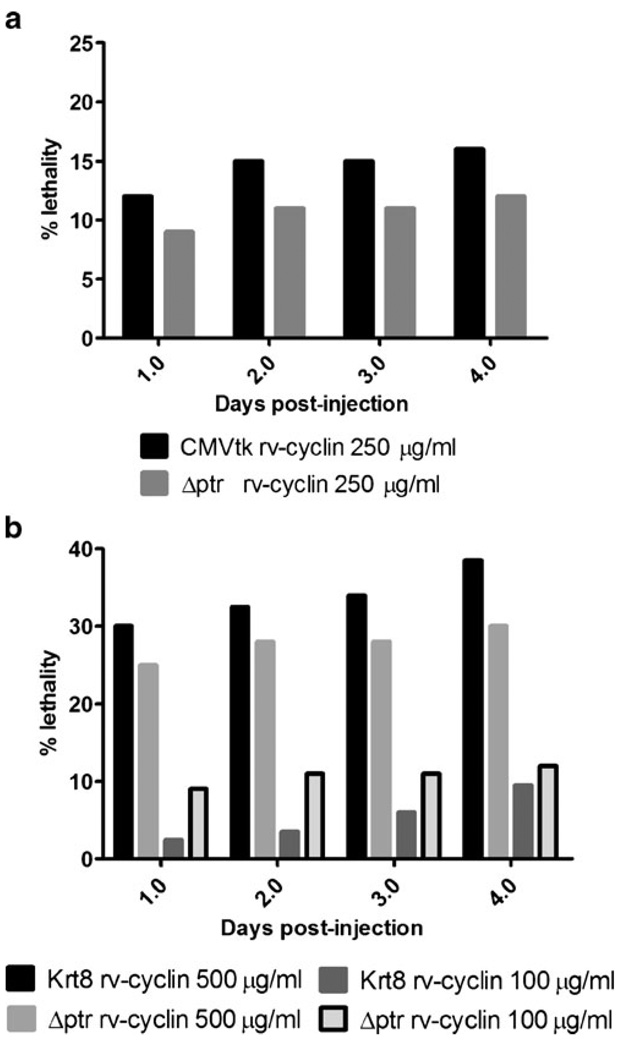
We evaluated the effects of rv-cyclin expression from the CMVtk promoter or from a skin-specific zebrafish promoter, krt8, in developing zebrafish. Linearized expression constructs (250 µg/mL) were microinjected into two-cell stage zebrafish embryos with a GFP reporter plasmid, pCMVtkGFP or Krt8GFP. Viable embryos selected for GFP expression post-injection were found to reliably express rv-cyclin. Developmental defects and lethality were scored in GFP-expressing fish for 4 days. Over a 4-day period, there appeared to be a slight increase in lethality in embryos expressing rv-cyclin (12% day 1; 16% day 4) from the CMVtk promoter; however, lethality was not statistically higher in embryos injected with CMVtk rv-cyclin compared to embryos injected with control constructs at any time point (Fisher's exact test) (Fig. 3a). Embryos expressing rv-cyclin (3% day 1; 10% day 4) from the krt8 promoter did not have increased lethality compared to control constructs (Fig. 3b). Numbers of developmental defects observed in rv-cyclin-expressing fish over the 4-day period were not significantly different from levels seen in control injected embryos (data not shown). Observed developmental defects included pericardial edema, bent axis, subcutaneous edema of the yolk sac, and disorganized tissue, typically seen in areas of high GFP expression. Microscopically, we did not observe signs of proliferation in the tissues of any of these fish.
Fig. 3.
Lethality resulting from rv-cyclin expression in injected zebrafish embryos during the first 4 days of development. a Percentage of lethality in embryos injected with CMVtk rv-cyclin at 250 µg/ml. Krt8 rv-cyclin (b) DNA was injected at 500 and 100 µg/ml and percentages of nonsurviving embryos recorded. All embryos were co-injected with a GFP reporter construct to monitor for successful injection and scored over the first 4 days of development. Control embryos were co-injected with a promoter-deletion plasmid (Δptr) and a GFP reporter plasmid. Data are presented as percentage of lethality of 200 injected embryos
To determine if a higher concentration of rv-cyclin affects the levels of lethality, we titrated injected Krt8 rv-cyclin DNA at three concentrations (500, 100, and 10 µg/mL) while maintaining the same injection volume. Higher concentrations of injected DNA nonspecifically increased lethality in embryos within 24 h of development in Krt8 rv-cyclin and control construct injected embryos (Fig. 3b). Levels of lethality in embryos injected with 500 µg/mL of Krt8 rv-cyclin (30%) at 1 day were not substantially higher with control DNA (25% Δptr rv-cyclin). Similarly, lower concentrations of Krt8 rv-cyclin injected DNA (100 and 10 µg/mL) did not increase lethality in developing embryos compared to control DNA. Over 4 days of development, slight increases in the level of lethality occurred for all injected constructs including control DNA (Fig. 3b). No increase in development defects was observed in rv-cyclin-expressing embryos compared to control injected embryos.
Production of Transgenic Zebrafish Expressing rv-cyclin
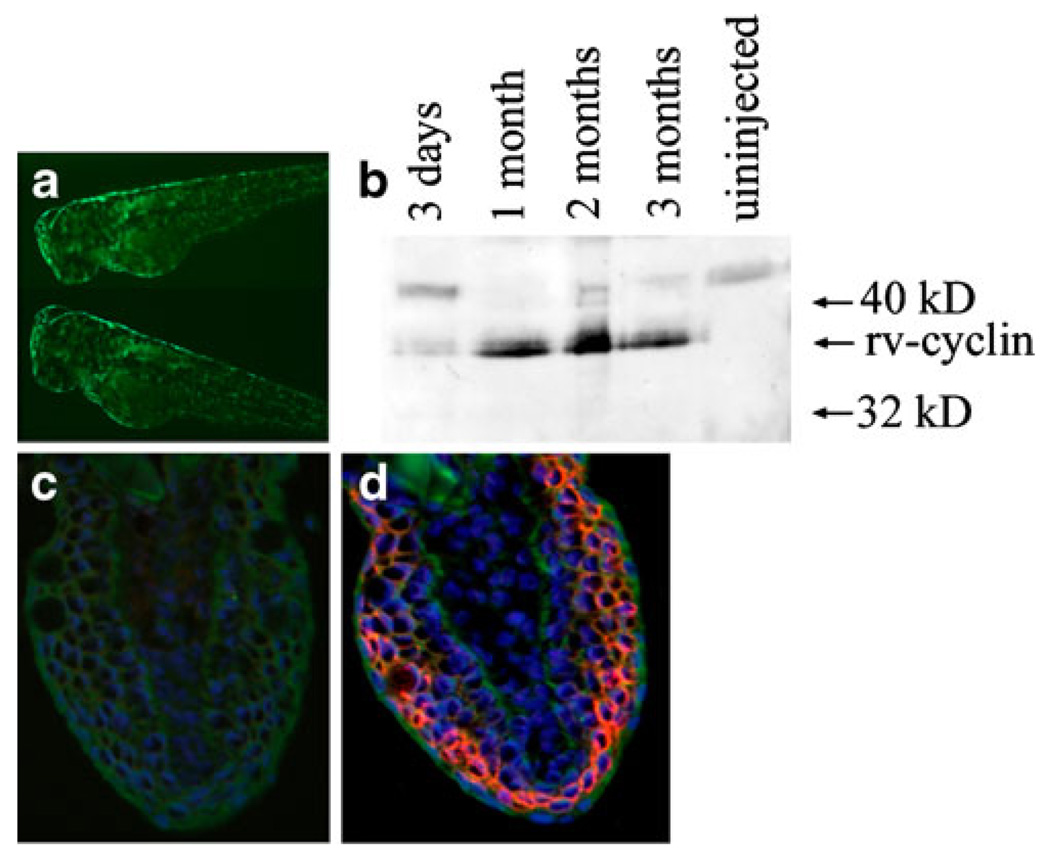
To generate lines of transgenic zebrafish that stably express rv-cyclin and also co-express GFP as a sortable genetic marker, we injected single-cell zebrafish embryos with two separate plasmid DNAs expressing the rv-cyclin protein from the CMVtk promoter or GFP from the krt8 promoter. Embryos co-injected with DNAs expressing the rv-cyclin protein from the CMVtk promoter and GFP from the krt8 promoter were selected for GFP expression after 24 h (Fig. 4a). We found that co-injected zebrafish embryos selected for GFP expression in most cases also express rv-cyclin for up to 3 months post-injection (Fig. 4b).
Fig. 4.
Expression of rv-cyclin in embryos co-injected with Krt8GFP. a Expression of GFP in the skin of co-injected embryos. b Expression of GFP in injected chimeric embryos correlates with rv-cyclin protein expression detected by western blot in zebrafish at 3 days, 1 month, 2 months, and 3 months of age. Immunohistochemistry of caudal tail transverse sections (40×-dorsal side up) from adult stable transgenic zebrafish (F1 offspring from founder 26) for Krt8-GFP and CMVtk rv-cyclin (c, d), treated with control antiserum (c) or rv-cyclin antibody (d). GFP expression in green, DAPI staining in blue, and rv-cyclin expression in red
GFP-expressing zebrafish that express rv-cyclin from the CMVtk promoter were selected 5 days post-injection for the highest distribution of GFP expression and grown to maturity. In many cases, GFP expression covered one half of the fish extending laterally and was predictably limited to the skin. Of the 42 GFP-expressing chimeric adults we isolated and bred, nine transmitted the GFP transgene to their progeny. Six out of nine GFP-expressing lines were also PCR positive for the CMVtk rv-cyclin transgene. Twelve additional fish produced embryos that were PCR positive for CMVtk rv-cyclin but did not express GFP. The frequency of transgenic offspring from rv-cyclin founder fish co-expressing GFP ranged from 1% to 29%, indicating that founders are mosaic in their germ line.
Transgenic lines of rv-cyclin-expressing zebrafish have been carried to the F2 generation. In all cases, the CMVtkrv-cyclin and Krt8GFP genes co-segregate at Mendelian frequencies. This provides evidence that these genes have stably integrated into the same locus in the zebrafish genome.
Characterization of Stable Transgenic Lines
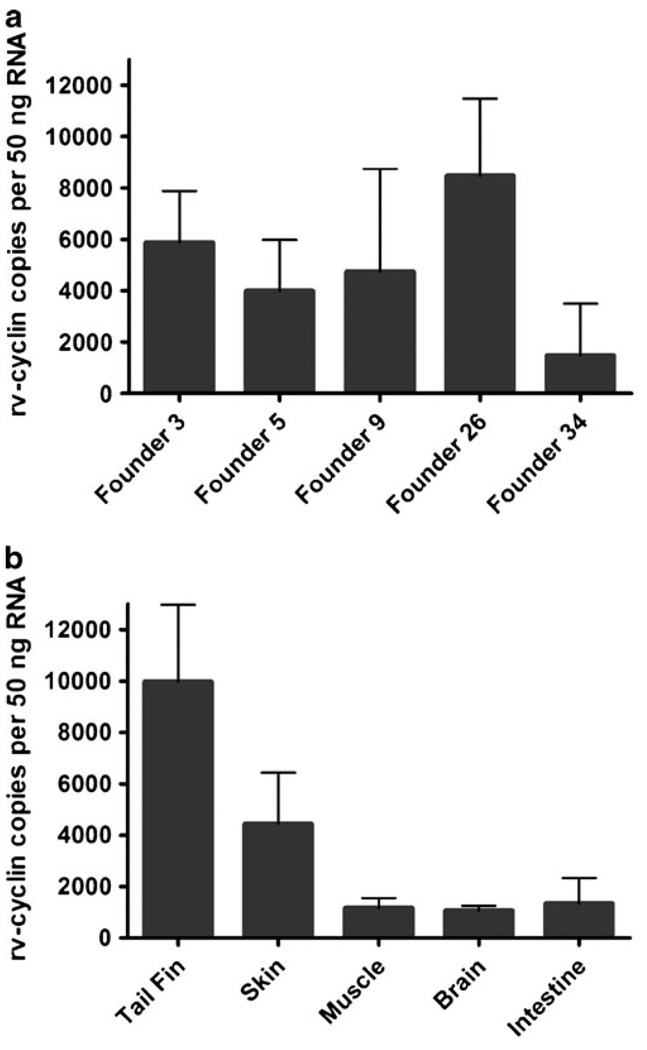
Transgenic lines of rv-cyclin-expressing zebrafish were analyzed by RT-PCR for the expression of the rv-cyclin transgene. For analysis, levels of rv-cyclin RNA isolated from transgenic founders 3, 5, 9, 26, and 34 were directly compared against a standard of in vitro transcribed rv-cyclin. The rv-cyclin transcript was present in all five transgenic lines and ranged from 1,490 copies per 50 ng total RNA in founder 34 to 8,470 copies per 50 ng total RNA in founder 26 (Fig. 5a). Overall, there appeared to be no significant difference between rv-cyclin-expression levels in different founders.
Fig. 5.
Comparison of rv-cyclin RNA levels between different CMVtk rv-cyclin transgenic founders F1 offspring (a) and different tissue samples isolated from the F1 offspring of transgenic founder 26 (b). RNA copy numbers were determined by plotting the threshold cycle (CT) value of each sample against that of a standard curve constructed from rv-cyclin standards. Numbers indicate rv-cyclin copies per 50 ng total RNA. Bars represent ± 1 standard deviation
To characterize the tissue distribution of rv-cyclin expression from the constitutive CMVtk promoter, we isolated RNA from the tail fin, skin, muscle, brain, and internal organs of transgenic fish from one F1 transgenic fish from founder 26. qRT-PCR analysis indicated that rv-cyclin expression appeared in all tissues at similar rates ranging from an average of 9,970 copies per 50 ng RNA in tail fin to 1,060 copies per 50 ng RNA in brain (Fig. 5b). Rv-cyclin protein was detected in CMVtk rv-cyclin transgenic zebrafish by immunohistochemistry of transverse sections of caudal tail region. In rv-cyclin transgenic zebrafish, protein levels were highest in muscle and surface epithelium of the skin (Fig. 4c–d).
Histologic Evaluation of Tumor Incidence in Transgenic Zebrafish
Transgenic zebrafish stocks of 100 to 150 fish expressing rv-cyclin from the CMVtk promoter have been maintained for a 3-year period with no visible tumor formation. These fish were killed at 3 years of age and assessed grossly and microscopically for lesions in the skin and other tissues by histological evaluation for tumor development. No external abnormalities were observed in CMVtk rv-cyclin or control fish. Additionally, histology conducted on sagittal sections of CMVtk rv-cyclin or control transgenic fish identified no internal proliferative lesions. These data suggest that rv-cyclin expression is not sufficient for the induction of proliferative changes in zebrafish (Table 1).
Table 1.
Tumor incidence in control and transgenic zebrafish
| Treatment group | Fraction of fish with histologic tumors (%) |
Tumor histologic types | Sex ratio M/ total (%M) |
|---|---|---|---|
| Control (3 year) | 0/11 (0%) | None | 6/11 (54%) |
| Krt8-GFP and CMVtk rv-cyclin (3 years) | 0/14 (0%) | None | 7/14 (50%) |
| Krt8-GFP and CMVtk rv-cyclin ENU-treated (2 years) | 3/20 (15%) | 2/20 spermatocytic seminomas of testis; 1/20 malignant peripheral nerve-sheath tumor of gas bladder and ovary | 14/20 (70%) |
| Krt8-GFP ENU-treated (2 years) | 2/13 (15%) | 2/13 spermatocytic seminomas of testis | 11/13 (85%) |
| Control zebrafish ENU-treated (2 years) | 1/22 (5%) | 1/22 hepatocellular adenoma | 11/22 (50%) |
| Krt8-GFP tail regeneration (1 year) | 0/16 (0%) | None | 8/16 (50%) |
| CMVtk rv-cyclin tail regeneration (1 year) | 0/16 (0%) | None | 8/16 (50%) |
To assess the necessity for secondary mutations to collaborate with WDSV rv-cyclin in inducing tumor formation, we treated rv-cyclin-expressing zebrafish with ENU at 1 month of age. These fish were maintained for a period of 2 years with no incidence of grossly or stereomicroscopically visible tumor formation. Histological evaluation of these ENU-treated fish revealed spermatocytic seminomas of the testis in two CMVtk rv-cyclin, two Krt8-GFP, and one control zebrafish. Rv-cyclin fish had a similar incidence of tumor formation (15%) compared to GFP-expressing fish (15%). One rv-cyclin fish exhibited a malignant peripheral nerve-sheath tumor, a tumor not observed in control fish in this study. Given the low incidence of this tumor and the fact that malignant peripheral nerve-sheath tumors are relatively common in many laboratory colonies of untreated zebrafish, this tumor is most likely an incidental finding (Kent et al. 2007; Spitsbergen and Kent 2003) (Table 1).
Skin-specific expression of WDSV rv-cyclin in a transgenic mouse model has been demonstrated to induce hyperplasia in response to wound healing (Lairmore et al. 2000). To address the effects of tissue regeneration in CMVtk rv-cyclin in the transgenic zebrafish model, we amputated the tail fin of adult zebrafish and followed tissue regeneration. Full regeneration occurred within 1 month. We observed these fish for 6 months, and they appeared grossly and histologically similar to control fish (Table 1).
Discussion
We have utilized an experimental system with zebrafish to study the capacity of the WDSV accessory gene, rv-cyclin, to induce proliferative changes in developing embryos and in transgenic adult fish. We demonstrate that rv-cyclin expression in developing embryos as well as in transgenic adults does not cause developmental defects or proliferative changes. In support of these results, we have maintained rv-cyclin expressing chimeric and transgenic zebrafish for 3 years and successive generations without apparent phenotypic change. Consistent with these observations, Zhan et al. (2010, submitted with this manuscript) found that rv-cyclin expression protects the fish liver from carcinogen damage and delays onset of malignancy.
Translucent zebrafish embryos are especially well suited for these studies since early developmental phenotypes resulting from oncogene expression can be visualized. Expression of injected DNA in embryos is initially highly mosaic; however, integration into cells of the germ line allows the production of stable transgenic lines of oncogene-expressing zebrafish. Although expression of genes in chimeric fish is limited to certain areas, it may be a more representative model of viral or chemical tumorigenesis in situ, in which isolated groups of cells containing activated oncogenes interact with nontransformed neighbor cells. The chimeric expression system has previously been utilized to demonstrate that the Xmrk receptor tyrosine kinase from Xiphophorus induces neoplastic growth in injected medaka fish at 1 week post-injection (Winkler et al. 1994).
One explanation for the inability of the rv-cyclin to induce neoplastic transformation in zebrafish is that these genes may require additional genetic changes or tumor promotion in order to induce proliferative changes. A multistep model for tumorigenesis is well established in mammalian systems in both clinical and experimental studies (Hanahan and Weinberg 2000). The requirement for secondary genetic events in transgenic mice is evident by the latency periods between oncogene expression and tumor formation. The introduction of secondary oncogenes or application of tumor promoters has been shown to reduce latency periods and cause more severe phenotypes (Macleod and Jacks 1999). Interestingly, transgenic mice expressing WDSV rv-cyclin from the bovine keratin-5 promoter require wounding to induce neoplastic growth, supporting the need for a secondary promotion event (Lairmore et al. 2000). However, in this model, induced hyperplastic skin lesions do not fully recapitulate WDS tumors.
Dermal sarcoma development and regression are seasonal in infected fish populations and are associated with qualitative and quantitative changes in viral gene expression (Quackenbush et al. 1997). To assess if additional components of WDSV are necessary for tumor formation, we have recently created transgenic zebrafish lines expressing OrfB individually or in combination with rv-cyclin. Both transgenic lines have been maintained with no increase in tumor incidence over a 2-year period. Although we cannot rule out that additional components of WDSV are necessary for WDS, it is likely that additional host factors are involved in disease progression. In our system, a long latency period (3 years) or chemical mutagenesis was not sufficient to increase tumor formation in rv-cyclin transgenic zebrafish. Additionally, tail-fin wounding and subsequent tissue regeneration were unable to promote abnormal cell proliferation or cell differentiation. The possibility exists that the function of walleye accessory genes may not be conserved in zebrafish or that the zebrafish transgenic systems may not faithfully recapitulate the WDSV target-cell population in nature. It is of interest that transgenic expression of mammalian oncogenes including mouse c-myc (Langenau et al. 2003), human NOTCH (Chen et al. 2007), and the human translocation product TEL-AML1 (Sabaawy et al. 2006) have conserved oncogenic function in zebrafish. An alternative explanation is that zebrafish, as a species, may be more resistant to neoplasia than inbred strains of mice. Lifetime rates of spontaneous tumor development in certain inbred lines of mice can be as high as 100% (Näf et al. 2002). Comparatively, studies by Spitsbergen et al. (2000a, b), found that the incidence of spontaneous neoplasia in wild-type zebrafish grown under optimal dietary and husbandry conditions may be as low as 1% at 1 to 1.5 years of age and 2% at 2 years of age. In tumor-prone zebrafish mutants, cancer incidence is close to 30% (Haramis et al. 2006; Berghmans et al. 2005; Faucherre et al. 2008). Lastly, it is possible that genome duplication before the teleost radiation (Taylor et al. 2003) may provide a degree of redundancy in tumor suppressor loci that make zebrafish more resistant to cancer.
Although the causal role of WDSV in dermal sarcoma development has not been conclusively established, there is substantial evidence that rv-cyclin interacts with host proteins involved in cell cycle progression and transcription, the dysregulation of which may be involved in tumorigenesis. The establishment of a cell-culture-based system in which infectious WDSV particles can be propagated (Rovnak et al. 2007) may provide a more compelling experimental model for clarifying whether WDSV is exclusively involved in WDS development.
Acknowledgements
TAP was supported by NIH training grant 5T32CA09682. This work was supported in part by NIH grant CA095056 to SLQ. We appreciate the administrative support from the Department of Microbiology, host of the Center for Fish Disease Research and John Fryer Salmon Disease Laboratory at Oregon State University. Facilities supporting the carcinogenesis and spontaneous tumor studies of JS were funded by US Public Health Service Grants ES03850 and ES00210 from the National Institutes of Environmental Health Sciences and RR12546 from the National Center for Research Resources. Z.G. was funded by the Biomedical Research Council of Singapore.
Contributor Information
Thomas A. Paul, Department of Microbiology and Immunology, Cornell University, Ithaca, NY, USA
Joel Rovnak, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, CO, USA.
Sandra L. Quackenbush, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, CO, USA
Kathleen Whitlock, Centro de Genómica de la Célula, Centro de Neurociencia, Universidad de Valparaíso, Valparaíso, Chile.
Huiqing Zhan, Department of Biological Sciences, National University of Singapore, Singapore, Singapore.
Zhiyuan Gong, Department of Biological Sciences, National University of Singapore, Singapore, Singapore.
Jan Spitsbergen, Department of Microbiology and Marine and Freshwater Biomedical Sciences Center, Oregon State University, Corvallis, OR, USA.
Paul R. Bowser, Department of Microbiology and Immunology, Cornell University, Ithaca, NY, USA
James W. Casey, Email: jwc3@cornell.edu, Department of Microbiology and Immunology, Cornell University, Ithaca, NY, USA.
References
- Amatruda JF, Patton EE. Genetic models of cancer in zebrafish. Int Rev Cell Mol Biol. 2008;271:1–34. doi: 10.1016/S1937-6448(08)01201-X. [DOI] [PubMed] [Google Scholar]
- Beckwith LG, Moore JL, Tsao-Wu GS, Harshbarger JC, Cheng KC. Ethylnitrosurea induces neoplasia in zebrafish (Danio rerio) Lab Invest. 2000;80:379–385. doi: 10.1038/labinvest.3780042. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD, Morris JP, Liu TX, Schulte-Merker S, Kanki JP, Plasterk R, Zon LI, Look AT. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser PR, Wooster GA. Regression of dermal sarcoma in adult walleyes (Stizostedion vitreum) J Aqut Anim Health. 1991;3:147–150. [Google Scholar]
- Chen J, Jette C, Kanki JP, Aster JC, Look AT, Griffin JD. NOTCH1-induced T-cell leukemia in transgenic zebrafish. Leukemia. 2007;21:462–471. doi: 10.1038/sj.leu.2404546. [DOI] [PubMed] [Google Scholar]
- Daniels CC, Rovnak J, Quackenbush SL. Walleye dermal sarcoma virus Orf B functions through receptor for activated C kinase (RACK1) and protein kinase C. Virology. 2008;375:550–560. doi: 10.1016/j.virol.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Rangini Z. Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In Vitro Cell Dev Biol Anim. 1993;29A:749–754. doi: 10.1007/BF02631432. [DOI] [PubMed] [Google Scholar]
- Faucherre A, Taylor GS, Overvoorde J, Dixon JE, Hertog J. Zebrafish pten genes have overlapping and non-redundant functions in tumorigenesis and embryonic development. Oncogene. 2008;27:1079–1086. doi: 10.1038/sj.onc.1210730. [DOI] [PubMed] [Google Scholar]
- Feitsma H, Cuppen E. Zebrafish as a cancer model. Mol Cancer Res. 2008;6:685–694. doi: 10.1158/1541-7786.MCR-07-2167. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Haramis AP, Hurlstone A, van der Velden Y, Begthel H, van den Born M, Offerhaus GJ, Clevers HC. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006;7:444–449. doi: 10.1038/sj.embor.7400638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschu DL, Martineau D, Fodor SK, Vogt VM, Bowser PR, Casey JW. Nucleotide sequence and protein analysis of a complex piscine retrovirus, walleye dermal sarcoma virus. J Virol. 1995;69:5320–5331. doi: 10.1128/jvi.69.9.5320-5331.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B, Xu Y, He J, Liao J, Yan T, Hew CL, Lam TJ, Gong Z. Faithful expression of green fluorescent protein (GFP) in transgenic zebrafish embryos under control of zebrafish gene promoters. Dev Genet. 1999;25:158–167. doi: 10.1002/(SICI)1520-6408(1999)25:2<158::AID-DVG10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kent ML, Spitsbergen JM, Matthews, Fournie JW, Westerfield M. Diseases of zebrafish in research facilities. ZIRC Health Services Zebrafish Disease Manual, Zebrafish International Resources Center. 2007 [Google Scholar]
- Lairmore MD, Stanley JR, Weber SA, Holzschu DL. Squamous epithelial proliferation induced by walleye dermal sarcoma retrovirus cyclin in transgenic mice. Proc Natl Acad Sci. 2000;97:6114–6119. doi: 10.1073/pnas.110024497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, Lin S, Prochownik E, Trede NS, Zon LI, Look AT. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- LaPierre LA, Casey JW, Holzschu DL. Walleye retroviruses associated with skin tumors and hyperplasias encode cyclin D homologs. J Virol. 1998;72:8765–8771. doi: 10.1128/jvi.72.11.8765-8771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna LG. Manual of histological staining methods of the Armed Forces Institute of Pathology. 3rd edn. New York: McGraw Hill Publications; 1968. [Google Scholar]
- Macleod KF, Jacks T. Insights into cancer from transgenic mouse models. J Pathol. 1999;187:43–60. doi: 10.1002/(SICI)1096-9896(199901)187:1<43::AID-PATH246>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Martineau D, Bowser PR, Renshaw RR, Casey JW. Molecular characterization of a unique retrovirus associated with a fish tumor. J Virol. 1992;66:596–599. doi: 10.1128/jvi.66.1.596-599.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JL, Rush LM, Breneman C, Mohideen MA, Cheng KC. Zebrafish genomic instability mutants and cancer susceptibility. Genetics. 2006;174:585–600. doi: 10.1534/genetics.106.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näf D, Krupke DM, Sundberg JP, Eppig JT, Bult CJ. The mouse tumor biology database: a public resource for cancer genetics and pathology of the mouse. Cancer Res. 2002;62:1235–1240. [PubMed] [Google Scholar]
- Poulet FM, Vogt VM, Bowser PR, Casey JW. In situ hybridization and immunohistochemical study of walleye dermal sarcoma virus (WDSV) nucleic acids and proteins in spontaneous sarcomas of adult walleyes (Stizostedion vitreum) Vet Pathol. 1995;32:162–172. doi: 10.1177/030098589503200210. [DOI] [PubMed] [Google Scholar]
- Quackenbush SL, Holzschu DL, Bowser PR, Casey JW. Transcriptional analysis of walleye dermal sarcoma virus (WDSV) Virology. 1997;237:107–112. doi: 10.1006/viro.1997.8755. [DOI] [PubMed] [Google Scholar]
- Quackenbush SL, Linton A, Brewster CD, Rovnak J. Walleye dermal sarcoma virus rv-cyclin inhibits NF-kappaB-dependent transcription. Virology. 2009;386:55–60. doi: 10.1016/j.virol.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovnak J, Casey JW, Quackenbush SL. Intracellular targeting of walleye dermal sarcoma virus Orf A (rv-cyclin) Virology. 2001;280:31–40. doi: 10.1006/viro.2000.0731. [DOI] [PubMed] [Google Scholar]
- Rovnak J, Quackenbush SL. Walleye dermal sarcoma virus cyclin interacts with components of the mediator complex and the RNA polymerase II holoenzyme. J Virol. 2002;76:8031–8039. doi: 10.1128/JVI.76.16.8031-8039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovnak J, Quackenbush SL. Walleye dermal sarcoma virus retroviral cyclin directly contacts TAF9. J Virol. 2006;80:12041–12048. doi: 10.1128/JVI.01425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovnak J, Casey RN, Brewster CD, Casey JW, Quackenbush SL. Establishment of productively infected walleye dermal sarcoma explant cells. J Gen Virol. 2007;88:2583–2599. doi: 10.1099/vir.0.82967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaawy HE, Azuma M, Embree LJ, Tsai HJ, Starost MF, Hickstein DD. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci. 2006;103:15166–15171. doi: 10.1073/pnas.0603349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research—advantages and current limitations. Toxicol Pathol. 2003;31S:62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Tsai H-W, Reddy A, Miller T, Arbogast D, Hendricks JD, Bailey GS. Neoplsia in zebrafish (Danio rerio) treated with 7, 12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol Pathol. 2000a;28:705–715. doi: 10.1177/019262330002800511. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Tsai H-W, Reddy A, Miller T, Arbogast D, Hendricks JD, Bailey GS. Neoplasia in zebrafish (Danio rerio) treated with N-methyl-N′-nitro-N-nitrosoguanidine by three exposure routes at different developmental stages. Toxicol Pathol. 2000b;28:716–725. doi: 10.1177/019262330002800512. [DOI] [PubMed] [Google Scholar]
- Stern HM, Zon LI. Cancer genetics and drug discovery in the zebrafish. Nat Rev Cancer. 2003;3:1–7. doi: 10.1038/nrc1126. [DOI] [PubMed] [Google Scholar]
- Taylor J, Braasch I, Frickey T, Meyer A, Van De Peer Y. Genome duplication, a trait shared by 22, 000 species of rayfinned fish. Genome Res. 2003;13:382–390. doi: 10.1101/gr.640303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish (Brachydanio rerio) Eugene: University of Oregon Press; 1994. The zebrafish book. [Google Scholar]
- Winkler C, Hong Y, Wittbrodt J, Schartl M. Analysis of heterologous and homologous promoters and enhancers in vitro and in vivo by gene transfer into Japanese medaka (Oryzias latipes) and Xiphophorus. Mol Mar Biol Biotechnol. 1992;1:326–337. [PubMed] [Google Scholar]
- Winkler C, Wittbrodt J, Lammers R, Ullrich A, Schartl M. Ligand-independent tumor induction in medakfish embryos by a Xmrk receptor tyrosine kinase transgene. Oncogene. 1994;9:1517–1525. [PubMed] [Google Scholar]
- Zhan H, Spitsbergen JM, Qing W, Wu YL, Paul TA, Casey JW, Her GM, Gong Z. Transgenic expression of walleye dermal sarcoma virus rv-cyclin gene in zebrafish and its suppressive effect on liver tumor development after carcinogen treatment. Marine Biotechnology. 2010 doi: 10.1007/s10126-009-9251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]