Abstract
Total hip arthroplasty (THA) in developmental dysplasia of the hip (DDH) presents many challenges to the reconstructive surgeon. The complex femoral and acetabular anatomy makes standard reconstruction technically challenging. Acetabular coverage can be improved by medialization of the component or augmentation of the deficient areas with bone graft. Femoral shortening osteotomies are considered in cases of severe dysplasia and frankly dislocated hips. Each patient’s unique anatomy dictates what options of reconstruction are available. The functional outcomes of THA in DDH are generally excellent, though higher rates of mechanical failure have been reported in this group. This article reviews the anatomy, classification, technical considerations, and outcomes of THA in patients with DDH.
Keywords: Developmental dysplasia of the hip, Total hip arthroplasty, Hip, Arthritis, Hip replacement
INTRODUCTION
Developmental dysplasia of the hip (DDH) is a leading cause of hip arthritis in young adults. Although several non-arthroplasty options exist prior to the development of end stage osteoarthritis in these patients including proximal femoral and periacetabular osteotomies[1], total hip arthroplasty (THA) remains the standard of care when end stage osteoarthritis leads to significant pain and loss of function[2]. Abnormal contact stresses in the dysplastic hip predisposes patients with DDH to develop arthritic changes earlier than seen for patients without dysplasia[3]. There are many challenges in considering THA in patients with DDH including patient factors such as young age, distorted anatomy[4], and documented higher failure and revision rates[5].
ANATOMY
Although every patient with DDH has unique anatomy, there are well described trends seen for the acetabulum and the proximal femur. The acetabulum usually is characterized by deficiencies anterolaterally and superiorly. The proximal femur has been characterized by increased anteversion, decreased intramedullary canal size, straight contour, and either coxa vara or valga. Recent computed tomography (CT) studies have demonstrated that dysplastic femurs had consistently increased anteversion, shorter necks, and smaller canals than non-dysplastic femurs, and that the anterior bow of the femur displaced further distally with increasing degree of dysplasia[6]. The decreased canal width and thinner cortical diameters in dysplastic hips also may make them more prone to fracture[7]. Hence, particular attention to detail to each patient’s anatomic factors need to be made prior to proceeding with THA.
Soft tissue considerations in patients with DDH are also important. Patients with severe DDH often have inefficient abductor musculature leading to limp or frank trendelenburg gait. Musculature around the hip including the adductors, hip flexors, and hip extensors are shortened due to chronic dislocation. The sciatic nerve also is prone to injury if excessive limb lengthening occurs greater than 3 cm. Sciatic nerve palsy has been reported to range from 5.2% to 13% for patients with hip dysplasia treated with arthroplasty[8].
DIAGNOSIS AND CLASSIFICATION
Patients with DDH commonly present as young patients who develop an insidious onset of activity related groin pain or lateral hip pain. Many patients have a leg length discrepancy, and the development of a limp is the most commonly reported functional loss in this population[9]. Patients with high dislocation have a decreased lever arm for the hip abductors which reduces gait efficiency and can lead to a limp. The development of advanced osteoarthritis secondary to abnormal biomechanics including acetabular rim overload eventually leads to significant pain and functional limitations necessitating THA. Radiographic evaluation confirms diagnosis and the characteristic anatomic abnormalities of the acetabulum and proximal femur.
Radiographic evaluation of the patient with DDH is essential for surgical planning. Standard radiographic series include an AP view of the pelvis and a false profile view of the hip, which conveys information regarding the amount of lateral and anterior acetabular coverage of the femoral head respectively. The center edge angle, normally > 25°, is measured as the angle between a vertical line through the center of the femoral head and a line going through the center of the head and the lateral edge of the acetabulum on an AP view of the hip. The vertical-center-anterior angle, normally > 25°, is measured similarly as the angle between a vertical line through the center of the femoral head and a line going through the center of the head and the anterior edge of the acetabulum on a false profile view of the hip[10]. An AP view of the hip also provides a general assessment of neck shaft angle of the proximal femur. CT scans are also helpful for assessment of acetabular bone stock and anteversion.
Several classification systems exist that are helpful for considering surgical treatment. The most commonly used classification scheme is that of Crowe et al[11] which characterizes severity based on the amount of femoral head displacement from the acetabulum as follows: Type I: < 50% femoral head subluxation, Type II: 50%-75% subluxation, Type III: 75%-100% subluxation, Type IV: > 100% subluxation. This classification scheme can be used as a general guideline for the acetabular component reconstruction in THA. The Hartofilakidis classification describes three characteristic types: dysplasia in which the femoral head is contained in the true acetabulum, low dislocation in which the femoral head articulates with a false acetabulum that partially covers the true acetabulum, and high dislocation in which the femoral head does not articulate with a true or false acetabulum[12]. Many surgeons find this classification to be more practical in guiding surgical treatment.
SURGICAL TECHNIQUES
Approach
The surgical approach is often dictated by the surgeon’s preference. In mildly dysplastic hips in which extensive exposure of the acetabulum is not necessary, standard approaches including anterior, anterolateral, and posterior approaches of the hip can be utilized. In cases of severe dysplasia with significant subluxation of the femoral head, a posterior approach is favored in order to gain enough exposure to the femoral head and acetabulum. Furthermore, in cases of severe dysplasia, a subtrochanteric shortening osteotomy is often needed for which a direct approach to the proximal femur can be extended from the posterior approach. Another option for severe dysplasia is a trochanteric slide osteotomy that provides excellent exposure to the acetabulum, and allows for trochanteric advancement to improve the biomechanics of the abductor mechanism.
ACETABULAR RECONSTRUCTION
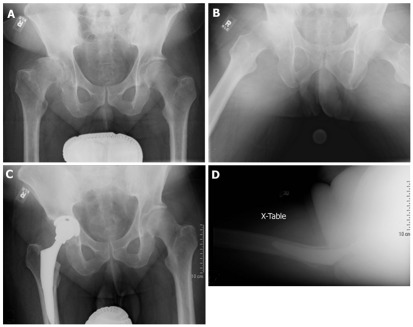
In Crowe I hips, the acetabular component can usually be placed in the true acetabulum without augmentation (Figure 1). If necessary, medialization of the cup can generally provide adequate coverage. Garvin et al[13] suggested that approximately 20% of the superolateral aspect of the acetabular cup could be left uncovered without significant risk for failure. However, no clear guideline exists regarding the amount of adequate acetabular cup coverage. Although cemented and press fit acetabular components can be considered in Crowe I hips, cemented fixation is associated with higher rates of mechanical failure as discussed later.
Figure 1.
Treatment of Crowe I hip using an anatomic hip center. A and B: Pre-op X-rays; C and D: Post-op X-rays.
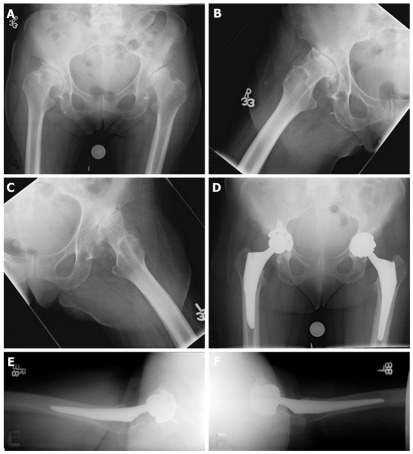
In Crowe II and III dysplastic hips, the superolateral acetabular deficiency prevents placement of a standard cup due to inadequate coverage. These hips are the most challenging for reconstruction. Special components including extra small cups and metal augments may be necessary to address inadequate osseous coverage of the acetabulum. The acetabular deficiency can be addressed in one of several methods: (1) Acetabular reconstruction at the anatomic hip center with augmentation using bone graft or augments; (2) Medialization of the anatomic hip joint to obtain sufficient lateral coverage (Figure 2); or (3) Acetabular reconstruction at a high hip center in a false acetabulum. Acetabular augmentation with bone graft allows for more anatomic position of the cup as well as increased bone stock for future revisions. However, the use of bone grafts for acetabular augmentation is associated with significant complications including bone graft resorption, nonunion, mechanical failure of the graft, and cup loosening[14-17]. The use of bulk femoral head autograft from the resected femoral head is an effectively utilized technique in which the deficient acetabulum bone is reamed to prepare a vascular bed of bone, and the cancellous portion of the femoral head is shaped to match the convexity of the prepared area of deficient acetabulum then impacted and secured by two or more screws into the ilium[18]. With this technique, no grafts were observed to fail at 10 years. To prevent mechanical graft failure, Mulroy and Harris[14] recommended > 70% coverage of the cup by host bone, while Rodriguez et al[19] suggested that < 60% of structural support of the cup should be from the graft.
Figure 2.
Treatment of Crowe II (right) and III (left) hips using an anatomic hip center with medialization. A, B and C: Pre-op X-rays; D, E and F: Post-op X-rays.
Medialization of the anatomic hip joint involves controlled reaming of the acetabulum through the medial acetabular wall to create enough coverage for the cup. The cortical edge of the cotyloid notch is palpated. Any intervening soft tissue in the acetabulum is removed. The anterior wall is protected during the reaming as it is hypoplastic and prone to fracture. Undersized reamers are used first to create a hemispheric acetabulum, with the appropriate degree of anteversion. The protrusio technique involves careful reaming through the medial wall until the medial periostium is seen[20]. Successful medialization allows for the use of a porous coated press fit component and avoids the use of bone graft augmentation.
It may not be feasible to recreate the anatomic hip center during reconstruction due to excessive acetabular deficiencies. In these situations, placement of the acetabular component at a high hip center in a false acetabulum can be performed using a small cementless cup affixed with screws. This technique is biomechanically unfavorable compared to anatomic hip center reconstruction, as it leads to increased joint contact forces, less mechanical advantage of the abductors, and increased rates of acetabular component loosening[21,22]. Superolateral displacement of hip center decreases the abductor moment arm by 28%[23]. If a high hip center is chosen for reconstruction, it is recommended to avoid lateral positioning of the hip center, with which Kaneuji et al[24] showed no acetabular loosening in their series at 10 years follow up.
In Crowe IV hips, the acetabulum is also hypoplastic, however the superior rim is less eroded than Crowe II-III hips. Therefore, placement of the hip in the anatomic hip center is possible using a small uncemented acetabular cup in the anatomic hip center. Augmentation with bone graft is usually not needed.
FEMORAL RECONSTRUCTION
Crowe I and II hips do not require femoral shortening for safe positioning into the anatomic hip center. Due to the narrow medullary canal and commonly observed anteversion of the femur in hip dysplasia, both cemented narrow DDH specific femoral stems and uncemented stems can be utilized. Narrow stemmed cemented components allow for improved surgeon control of anteversion. If an uncemented press fit component is used, the surgeon must be careful to avoid excessive anteversion of the femoral component as the femur already carries some degree anteversion. Often the proximal femoral anatomy is significantly distorted, or biomechanically inadequate for fixation. In these situations, diaphyseal fixed components as well as modular components can be used. Modular components allow for easier control of anteversion. Techniques regarding use of modular systems are described elsewhere in this specific topic highlight symposium.
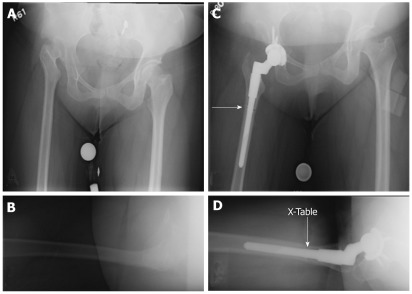
Femoral reconstruction of Crowe III hips follows the same general principles as in Crowe I and II hips, however some may require femoral shortening if the anatomic hip center is used for reconstruction. Femoral shortening is more often the norm in Crowe IV hips, for which one of two techniques are often utilized: (1) subtrochanteric femoral shortening osteotomy with the use of an uncemented component (Figure 3); or (2) greater trochanteric osteotomy with proximal femoral shortening with the use of a cemented DDH specific stem. Recently, the second technique has fallen out of favor as subtrochanteric femoral shortening osteotomy preserves the proximal femoral metaphysis, which allows for the use of an uncemented component due to inherent rotational stability when affixed proximally. Furthermore, subtrochanteric osteotomy allows for correction of rotation, and obviates the need for trochanteric osteotomy which can be subject to nonunion. Kyrch et al[25] described a technique in which a subtrochanteric shortening osteotomy is considered when templating leads to limb lengthening of more than 3-4 cm. Based on templating, the osteotomy site is planned at a level distal to the metaphyseal flare of the implant though proximal enough for distal stem engagement. The femur is reamed or broached prior to the osteotomy. A lateral approach is made to the subtrochanteric femur, and a transverse osteotomy is created. A trial component is inserted to the proximal fragment, and the hip is reduced. The amount of femoral shortening can be verified intraoperatively by overlapping the two fragments, and a second cut is made on the distal fragment. The proximal edge of the distal fragment is then prepared to create well opposed edges. The end of the trial component is then inserted and reduced into the distal fragment while adjusting for anteversion.
Figure 3.
Treatment of Crowe IV hip using an anatomic hip center with subtrochanteric shortening osteotomy (arrow) and a modular femoral component. A and B: Pre-op X-rays; C and D: Post-op X-rays.
POST-OPERATIVE CARE
Postoperative care generally follows routine care for hip arthroplasty. Prophylactic anticoagulation should be initiated postoperatively to reduce the risk of deep venous thrombosis. A plan for an adequate pain control regimen to allow for postoperative mobilization should be made. Physical therapists are essential to help safely mobilize the patient after surgery. If femoral or trochanteric osteotomy was not required, then patients can bear weight as tolerated after surgery with hip restrictions based on the surgical approach. If a trochanteric osteotomy was used in Crowe IV hips for proximal femoral shortening or during the approach, trochanteric precautions should include limitations in active hip abduction for at least six weeks or until trochanteric union has been achieved. If a subtrochanteric osteotomy was used in Crowe IV hips for proximal femoral shortening, it is recommended to keep the patient toe touch weight bearing for 6-8 wk to allow for healing of the osteotomy.
OUTCOMES
Total hip arthroplasty improves both Harris hip scores and pain levels in patients with hip dysplasia. The outcomes for mildly dysplastic Crowe I and II hips are generally good, and are similar to results seen for THA in patients without dysplasia. Revision rates for THA in severely dysplastic hips, however, are significantly higher than revision rates for THA in non-dysplastic hips[2,26,27]. Patients with severe DDH may continue to walk with a limp after surgery due to the inherent abductor weakness, although overall function including walking distance, hip pain, range of motion generally improves after hip replacement. There are only a few large studies that directly compare THA outcomes in dysplastic and non-dysplastic patients. Recent studies show that short-term THA outcomes (6 mo follow-up) are similar for dysplastic and non-dysplastic hips with regards to Oxford hip score and revision rate[28]. At 15 year follow-up, however, THA revisions are 1.5-2.0 times more likely in dysplastic hips than in non-dysplastic hips[5].
Cemented acetabular reconstruction has fallen out of favor because of reported revision rates up to 37%[29-31]. Uncemented acetabular reconstruction without acetabular augmentation is now the standard of care in mildly dysplastic hips with lower rates of aseptic loosening and revision at mid to long term follow-up. When acetabular augmentation is necessary, uncemented acetabular components with augmentation have revision rates of 0-5% and aseptic loosening of up to 26% at short term follow up[15,32], while cemented components have revision rates of 10%-35% at long term follow-up[14,19,33]. The longest term follow-up of uncemented acetabular fixation in combination with bulk femoral allograft showed 94% survival at 10 years[18]. Overall, there is strong support in the literature for uncemented acetabular fixation, even when acetabular augmentation is required.
There are few reports on the outcomes of using a high hip center, which is limited by small sample size. Nevertheless, these reports show a wide range of acetabular component mechanical failure or loosening rates from 16%-83.3%[22,34,35]. Higher rates of loosening were correlated with lateral displacement of the hip center[22]. A recent report of 30 hips treated with slight elevation of the hip center without lateralization using an uncemented cup showed no evidence of loosening at average 15.2 year follow up, which implies the potential importance of preventing lateralization when using this technique[24].
Cemented femoral reconstruction has shown more favorable results compared to acetabular reconstruction, though the results are inconsistent. Two studies with favorable results with long term follow up of at least 9.9 years showed femoral revision due to mechanical failure or loosening to range from 3%-10%[29,31]. One other study reported a 15%-40% incidence of radiographic loosening of cemented femoral components at 16 year follow-up when used in dysplastic hips[36].
Uncemented femoral components have had excellent survivorship in patients without DDH[37,38]. Nevertheless, definitive long term results in hip dysplasia are lacking. Mortazavi et al[39] evaluated the outcome of cementless femoral reconstruction in patients with proximal femoral deformity and found that the overall mechanical failure rate was 9% at an average four years follow up in 58 hips, though only 48.5% of hips evaluated had deformity due to dysplasia. The use of proximally fit uncemented components in hip dysplasia is challenging due to significant deformity, and often osteotomies and modular components are necessary to achieve an optimal fit.
Femoral shortening via proximal femoral osteotomy and distal greater trochanteric advancement can be associated with significant complications. Anwar et al[40] reported up to 29% nonunion of the greater trochanter, as well as increased frequency of Trendelenburg gait. Hence, recent attention has been directed towards subtrochanteric osteotomy which allows for maintenance of abductor mechanism as well as more flexibility in correcting for rotational deformities.
Short to medium term results of patients with Crowe IV hips treated with femoral shortening subtrochanteric osteotomy using uncemented components and anatomic hip center reconstruction generally show excellent healing rates of the osteotomy, ranging from 0-7%[41,42]. The overall outcome of reconstruction in these patients showed a 75% survivorship rate at 14 years follow up, with failure mostly attributable to polyethylene wear and osteolysis likely secondary to the use of older generation polyethylene components as well as thin liners[42]. A ten year follow up study also revealed improved lasting Harris hip scores in patients with dysplasia treated with modular femoral components and subtrochanteric osteotomy compared to preoperatively[43]. Long term results using newer generation highly cross linked polyethylene components need to be evaluated for this technique.
CONCLUSION
THA in patients with DDH is a complex procedure that requires an understanding of the complex acetabular and proximal femoral anatomy of each patient. The complex anatomy dictates what surgical techniques are necessary to create a mechanically stable and functional outcome. Patients can expect significant improvement in function and quality of life after THA, although complication rates are understandably higher in this patient group due to their increased complexity.
ACKNOWLEDGMENTS
The series and guest editors would like to thank Dr. Michael R Schuck, Premier Orthopedics, Colorado Springs, Colorado for his critical review and editing of this manuscript.
Footnotes
Peer reviewers: Zoran Vukasinovic, PhD, Pediatric Orthopaedics Department, Institute for Orthopaedic Surgery “Banjica”28, Mihajla Avramovica, 11040 Belgrade, Serbia; Dror Lakstein, MD, Consultant Orthopaedic Surgeon, Hip and Knee Arthroplasty Service, Orthopaedic Department, E. Wolfson Medical Center, Holon, Israel
S- Editor Yang XC L- Editor A E- Editor Yang XC
References
- 1.Sanchez-Sotelo J, Trousdale RT, Berry DJ, Cabanela ME. Surgical treatment of developmental dysplasia of the hip in adults: I. Nonarthroplasty options. J Am Acad Orthop Surg. 2002;10:321–333. doi: 10.5435/00124635-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Sotelo J, Berry DJ, Trousdale RT, Cabanela ME. Surgical treatment of developmental dysplasia of the hip in adults: II. Arthroplasty options. J Am Acad Orthop Surg. 2002;10:334–344. doi: 10.5435/00124635-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Brand RA. Hip Osteotomies: A Biomechanical Consideration. J Am Acad Orthop Surg. 1997;5:282–291. doi: 10.5435/00124635-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Argenson JN, Flecher X, Parratte S, Aubaniac JM. Anatomy of the dysplastic hip and consequences for total hip arthroplasty. Clin Orthop Relat Res. 2007;465:40–45. doi: 10.1097/BLO.0b013e3181576052. [DOI] [PubMed] [Google Scholar]
- 5.Engesaeter LB, Furnes O, Havelin LI. Developmental dysplasia of the hip--good results of later total hip arthroplasty: 7135 primary total hip arthroplasties after developmental dysplasia of the hip compared with 59774 total hip arthroplasties in idiopathic coxarthrosis followed for 0 to 15 years in the Norwegian Arthroplasty Register. J Arthroplasty. 2008;23:235–240. doi: 10.1016/j.arth.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Noble PC, Kamaric E, Sugano N, Matsubara M, Harada Y, Ohzono K, Paravic V. Three-dimensional shape of the dysplastic femur: implications for THR. Clin Orthop Relat Res. 2003;417:27–40. [PubMed] [Google Scholar]
- 7.Perka C, Fischer U, Taylor WR, Matziolis G. Developmental hip dysplasia treated with total hip arthroplasty with a straight stem and a threaded cup. J Bone Joint Surg Am. 2004;86-A:312–319. doi: 10.2106/00004623-200402000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Lewallen DG. Neurovascular injury associated with hip arthroplasty. Instr Course Lect. 1998;47:275–283. [PubMed] [Google Scholar]
- 9.Nunley RM, Prather H, Hunt D, Schoenecker PL, Clohisy JC. Clinical presentation of symptomatic acetabular dysplasia in skeletally mature patients. J Bone Joint Surg Am. 2011;93 Suppl 2:17–21. doi: 10.2106/JBJS.J.01735. [DOI] [PubMed] [Google Scholar]
- 10.Delaunay S, Dussault RG, Kaplan PA, Alford BA. Radiographic measurements of dysplastic adult hips. Skeletal Radiol. 1997;26:75–81. doi: 10.1007/s002560050197. [DOI] [PubMed] [Google Scholar]
- 11.Crowe JF, Mani VJ, Ranawat CS. Total hip replacement in congenital dislocation and dysplasia of the hip. J Bone Joint Surg Am. 1979;61:15–23. [PubMed] [Google Scholar]
- 12.Hartofilakidis G, Stamos K, Karachalios T, Ioannidis TT, Zacharakis N. Congenital hip disease in adults. Classification of acetabular deficiencies and operative treatment with acetabuloplasty combined with total hip arthroplasty. J Bone Joint Surg Am. 1996;78:683–692. doi: 10.2106/00004623-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Garvin KL, Bowen MK, Salvati EA, Ranawat CS. Long-term results of total hip arthroplasty in congenital dislocation and dysplasia of the hip. A follow-up note. J Bone Joint Surg Am. 1991;73:1348–1354. [PubMed] [Google Scholar]
- 14.Mulroy RD, Harris WH. Failure of acetabular autogenous grafts in total hip arthroplasty. Increasing incidence: a follow-up note. J Bone Joint Surg Am. 1990;72:1536–1540. [PubMed] [Google Scholar]
- 15.Morsi E, Garbuz D, Gross AE. Total hip arthroplasty with shelf grafts using uncemented cups. A long-term follow-up study. J Arthroplasty. 1996;11:81–85. doi: 10.1016/s0883-5403(96)80164-1. [DOI] [PubMed] [Google Scholar]
- 16.Gross AE, Catre MG. The use of femoral head autograft shelf reconstruction and cemented acetabular components in the dysplastic hip. Clin Orthop Relat Res. 1994;298:60–66. [PubMed] [Google Scholar]
- 17.Gerber SD, Harris WH. Femoral head autografting to augment acetabular deficiency in patients requiring total hip replacement. A minimum five-year and an average seven-year follow-up study. J Bone Joint Surg Am. 1986;68:1241–1248. [PubMed] [Google Scholar]
- 18.Kim M, Kadowaki T. High long-term survival of bulk femoral head autograft for acetabular reconstruction in cementless THA for developmental hip dysplasia. Clin Orthop Relat Res. 2010;468:1611–1620. doi: 10.1007/s11999-010-1288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez JA, Huk OL, Pellicci PM, Wilson PD. Autogenous bone grafts from the femoral head for the treatment of acetabular deficiency in primary total hip arthroplasty with cement. Long-term results. J Bone Joint Surg Am. 1995;77:1227–1233. doi: 10.2106/00004623-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Dorr LD, Tawakkol S, Moorthy M, Long W, Wan Z. Medial protrusio technique for placement of a porous-coated, hemispherical acetabular component without cement in a total hip arthroplasty in patients who have acetabular dysplasia. J Bone Joint Surg Am. 1999;81:83–92. doi: 10.2106/00004623-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Linde F, Jensen J. Socket loosening in arthroplasty for congenital dislocation of the hip. Acta Orthop Scand. 1988;59:254–257. doi: 10.3109/17453678809149356. [DOI] [PubMed] [Google Scholar]
- 22.Stans AA, Pagnano MW, Shaughnessy WJ, Hanssen AD. Results of total hip arthroplasty for Crowe Type III developmental hip dysplasia. Clin Orthop Relat Res. 1998;348:149–157. [PubMed] [Google Scholar]
- 23.Delp SL, Wixson RL, Komattu AV, Kocmond JH. How superior placement of the joint center in hip arthroplasty affects the abductor muscles. Clin Orthop Relat Res. 1996;328:137–146. doi: 10.1097/00003086-199607000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Kaneuji A, Sugimori T, Ichiseki T, Yamada K, Fukui K, Matsumoto T. Minimum ten-year results of a porous acetabular component for Crowe I to III hip dysplasia using an elevated hip center. J Arthroplasty. 2009;24:187–194. doi: 10.1016/j.arth.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Krych AJ, Howard JL, Trousdale RT, Cabanela ME, Berry DJ. Total hip arthroplasty with shortening subtrochanteric osteotomy in Crowe type-IV developmental dysplasia: surgical technique. J Bone Joint Surg Am. 2010;92 Suppl 1 Pt 2:176–187. doi: 10.2106/JBJS.J.00061. [DOI] [PubMed] [Google Scholar]
- 26.Thillemann TM, Pedersen AB, Johnsen SP, Søballe K. Implant survival after primary total hip arthroplasty due to childhood hip disorders: results from the Danish Hip Arthroplasty Registry. Acta Orthop. 2008;79:769–776. doi: 10.1080/17453670810016830. [DOI] [PubMed] [Google Scholar]
- 27.Charnley J, Feagin JA. Low-friction arthroplasty in congenital subluxation of the hip. Clin Orthop Relat Res. 1973;91:98–113. doi: 10.1097/00003086-197303000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Boyle MJ, Frampton CM, Crawford HA. Early results of total hip arthroplasty in patients with developmental dysplasia of the hip compared with patients with osteoarthritis. J Arthroplasty. 2012;27:386–390. doi: 10.1016/j.arth.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 29.MacKenzie JR, Kelley SS, Johnston RC. Total hip replacement for coxarthrosis secondary to congenital dysplasia and dislocation of the hip. Long-term results. J Bone Joint Surg Am. 1996;78:55–61. doi: 10.2106/00004623-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Sochart DH, Porter ML. The long-term results of Charnley low-friction arthroplasty in young patients who have congenital dislocation, degenerative osteoarthrosis, or rheumatoid arthritis. J Bone Joint Surg Am. 1997;79:1599–1617. doi: 10.2106/00004623-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Numair J, Joshi AB, Murphy JC, Porter ML, Hardinge K. Total hip arthroplasty for congenital dysplasia or dislocation of the hip. Survivorship analysis and long-term results. J Bone Joint Surg Am. 1997;79:1352–1360. doi: 10.2106/00004623-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Silber DA, Engh CA. Cementless total hip arthroplasty with femoral head bone grafting for hip dysplasia. J Arthroplasty. 1990;5:231–240. doi: 10.1016/s0883-5403(08)80077-0. [DOI] [PubMed] [Google Scholar]
- 33.Lee BP, Cabanela ME, Wallrichs SL, Ilstrup DM. Bone-graft augmentation for acetabular deficiencies in total hip arthroplasty. Results of long-term follow-up evaluation. J Arthroplasty. 1997;12:503–510. doi: 10.1016/s0883-5403(97)90172-8. [DOI] [PubMed] [Google Scholar]
- 34.Schutzer SF, Harris WH. High placement of porous-coated acetabular components in complex total hip arthroplasty. J Arthroplasty. 1994;9:359–367. doi: 10.1016/0883-5403(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 35.Russotti GM, Harris WH. Proximal placement of the acetabular component in total hip arthroplasty. A long-term follow-up study. J Bone Joint Surg Am. 1991;73:587–592. [PubMed] [Google Scholar]
- 36.Klapach AS, Callaghan JJ, Miller KA, Goetz DD, Sullivan PM, Pedersen DR, Johnston RC. Total hip arthroplasty with cement and without acetabular bone graft for severe hip dysplasia. A concise follow-up, at a minimum of twenty years, of a previous report. J Bone Joint Surg Am. 2005;87:280–285. doi: 10.2106/JBJS.D.02130. [DOI] [PubMed] [Google Scholar]
- 37.Kim YH. The results of a proximally-coated cementless femoral component in total hip replacement: a five- to 12-year follow-up. J Bone Joint Surg Br. 2008;90:299–305. doi: 10.1302/0301-620X.90B3.20096. [DOI] [PubMed] [Google Scholar]
- 38.Hallan G, Lie SA, Furnes O, Engesaeter LB, Vollset SE, Havelin LI. Medium- and long-term performance of 11,516 uncemented primary femoral stems from the Norwegian arthroplasty register. J Bone Joint Surg Br. 2007;89:1574–1580. doi: 10.1302/0301-620X.89B12.18969. [DOI] [PubMed] [Google Scholar]
- 39.Mortazavi SM, Restrepo C, Kim PJ, Parvizi J, Hozack WJ. Cementless femoral reconstruction in patients with proximal femoral deformity. J Arthroplasty. 2011;26:354–359. doi: 10.1016/j.arth.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Anwar MM, Sugano N, Masuhara K, Kadowaki T, Takaoka K, Ono K. Total hip arthroplasty in the neglected congenital dislocation of the hip. A five- to 14-year follow-up study. Clin Orthop Relat Res. 1993;295:127–134. [PubMed] [Google Scholar]
- 41.Krych AJ, Howard JL, Trousdale RT, Cabanela ME, Berry DJ. Total hip arthroplasty with shortening subtrochanteric osteotomy in Crowe type-IV developmental dysplasia. J Bone Joint Surg Am. 2009;91:2213–2221. doi: 10.2106/JBJS.H.01024. [DOI] [PubMed] [Google Scholar]
- 42.Bernasek TL, Haidukewych GJ, Gustke KA, Hill O, Levering M. Total hip arthroplasty requiring subtrochanteric osteotomy for developmental hip dysplasia: 5- to 14-year results. J Arthroplasty. 2007;22:145–150. doi: 10.1016/j.arth.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Biant LC, Bruce WJ, Assini JB, Walker PM, Walsh WR. Primary total hip arthroplasty in severe developmental dysplasia of the hip. Ten-year results using a cementless modular stem. J Arthroplasty. 2009;24:27–32. doi: 10.1016/j.arth.2007.12.016. [DOI] [PubMed] [Google Scholar]