Abstract
Osteonecrosis is a phenomenon involving disruption to the vascular supply to the femoral head, resulting in articular surface collapse and eventual osteoarthritis. Although alcoholism, steroid use, and hip trauma remain the most common causes, several other etiologies for osteonecrosis have been identified. Basic science research utilizing animal models and stem cell applications continue to further elucidate the pathophysiology of osteonecrosis and promise novel treatment options in the future. Clinical studies evaluating modern joint-sparing procedures have demonstrated significant improvements in outcomes, but hip arthroplasty is still the most common procedure performed in these affected younger adults. Further advances in joint-preserving procedures are required and will be widely studied in the coming decade.
Keywords: Osteonecrosis, Avascular necrosis, Femoral head, Total hip arthroplasty, Core decompression, Hip
INTRODUCTION
Osteonecrosis of the femoral head, also referred to as avascular necrosis, is a pathological state with multiple possible etiologies that causes decreased vascular supply to the subchondral bone of the femoral head, resulting in osteocyte death and collapse of the articular surface. The ischemic injury upregulates tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts to resorb dead trabeculae of subchondral bone[1]. These trabeculae eventually fail under the repetitive loads of weight bearing during walking and other activities[2-4].
Total hip arthroplasty (THA) is commonly utilized as a definitive treatment for high-grade osteonecrosis with articular collapse. However, as this disorder is commonly seen in young adults, joint-sparing therapeutic techniques have been studied extensively in the past decade and will be a major focus of orthopaedic research in the coming years. Osteonecrosis is undoubtedly a challenging condition to treat, but ongoing basic science and clinical investigations are progressing toward effective future treatment options.
EPIDEMIOLOGY
Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis[4-6]. Patients are generally younger adults age 35 years to 45 years, and risk factors for 75%-90% of cases include chronic steroid use, alcoholism, smoking, hip trauma including femoral neck fractures and hip dislocations, and prior hip surgery. Other potential etiologies for osteonecrosis include childhood history of slipped capital femoral epiphysis (SCFE), deep sea diving or other hyperbaric conditions, systemic lupus erythematosus (SLE) and other connective tissue disorders, autoimmune diseases causing vasculitis, sickle cell anemia, coagulopathy such as thrombophilia or disseminated intravascular coagulation, human immunodeficiency virus (HIV) infection, hyperlipidemia, fat embolus syndrome, treatment of developmental hip dysplasia, chemotherapy and/or radiation, organ transplantation, chronic liver disease, Gaucher disease, gout, and metabolic bone disease[3,4,6-10]. Males are affected up to three times more than females, and bilateral femoral head osteonecrosis is found in up to 75% of cases[3,5]. Incidence in the late 1990’s was reported to be 10 000 to 20 000 new patients per year, but this incidence has almost certainly increased over the past decade[4].
PATHOPHYSIOLOGY
The blood supply to the femoral head originates primarily from the basicervical extracapsular articular ring and ascending branch of the medial femoral circumflex artery, as well as smaller secondary contributions from inferior and superior gluteal arteries and artery of the ligamentum teres[11]. The interruption of this blood supply can be multifactorial, either extravascular or intravascular. Extravascular disruption is commonly attributed to traumatic causes. Proximal femur fractures resulting in displacement of the neck affect the basicervical arterial ring, whereas hip dislocations can disrupt the ligamentum teres and cause intracapsular hematoma, making the integrity of the extracapsular ring an important factor in the survival of the femoral head. Intravascular embolic matter such as clots, lipids, immune complexes, or sickle cells can also occlude the terminal arterioles in the subchondral bone of the femoral head[2-4,12].
Regardless of the underlying etiology of osteonecrosis, several studies suggest a common pathogenic pathway involving apoptosis of osteoblasts and osteocytes[13-15]. Following infarction, oxygen- and nutrient-deprived osteocytes and marrow cells die unless they can receive blood supply from collateral circulation. As the collateral circulation supplying the epiphyses is limited, capillary arterialization may not restore sufficient blood flow to the tissues[16]. In addition to vascular compromise and programmed cell death, defective bone repair is also a key component of osteonecrosis[17]. Adipogenesis has been shown to be a causal factor in steroid- and alcohol-related osteonecrosis, as it leads to compression of venous sinusoids and congestion. The venous congestion increases intraosseous pressure, preventing adequate arterial blood flow, eventually leading to bone infarction[18,19]. In certain cases, genetic factors, such as mutations in the COL2A1 gene, have been associated with the pathogenesis of osteonecrosis[20].
Weight bearing during walking generates loads 2 to 3 times body weight on the anterosuperior femoral head articular cartilage and superior acetabular dome and 5 to 6 times body weight during running or jumping[21]. Ischemic disruption of the weight-bearing surface in an osteonecrotic hip significantly affects a person’s ability to complete pain-free activities of daily living. Infarcted subchondral bone has trabeculae that become thinned by osteoclastic activity, and the hypoxic environment does not allow for osteoblastic repair or remodeling. The area of bone necrosis becomes surrounded by a reactive, sclerotic rim, and the weakened cancellous bone eventually fails under the repetitive loads of weight bearing, leading to subchondral fracture (the “crescent sign” on radiographs). Subchondral collapse eventually leads to articular degeneration[2,22].
DIAGNOSIS AND CLASSIFICATION
Medial thigh or groin pain with limitation of hip motion in patients less than 50 years of age should raise the suspicion of osteonecrosis. Patients usually present with slow-onset, insidious groin pain that may be unilateral or bilateral. Symptoms are generally amplified with weight bearing and relieved with rest. The pain may also be in the buttocks, knees, or anterior and lateral thigh. Range of motion becomes limited, particularly hip abduction and internal rotation, and logrolling (passive internal and external rotation) elicits pain. Early stages of the disease can often be asymptomatic, and some patients present after articular surface collapse has already occurred. Hip prognosis can be significantly improved with early diagnosis, before articular collapse[4,22].
Laboratory values such as activated partial thromboplastin time (aPTT) and prothrombin time (PT) are generally normal in hip osteonecrosis, although more extensive workup of the etiology may reveal coagulopathy or inflammatory joint disease such as SLE[23]. Plain radiographs should include anteroposterior and frog-leg lateral views of both hips, as the pathology commonly also presents in the contralateral hip. Radiographs may demonstrate normal findings (Ficat stage I) or subchondral cyst formation and sclerosis (Ficat stage II), but more advanced disease involves femoral head flattening and subchondral collapse, as seen with the “crescent sign” (Ficat stage III). Osteoarthritic joint space narrowing with osteophyte formation are findings of untreated osteonecrosis (Ficat stage IV)[24] (Table 1). Radiographs are highly specific for more advanced osteonecrosis (Ficat II or III) but not very sensitive for early changes (Ficat I)[3].
Table 1.
Osteoarthritic joint space narrowing with osteophyte formation findings of untreated osteonecrosis
| System | |||
| Ficat/Arlet | Steinberg/U Penn | ARCO | |
| Stage I | Normal radiographs | Normal radiographs | Normal radiographs |
| Stage II | Subchondral cyst formation and sclerosis | Femoral head lucency/sclerosis | Demarcating sclerosis in femoral head, no collapse |
| Stage III | Femoral head flattening, subchondral collapse, "crescent sign" | Subchondral collapse without femoral head flattening, "crescent sign" | Femoral head collapse, "crescent sign", no joint space narrowing |
| IIIA | Collapse < 3 mm | ||
| IIIB | Collapse > 3 mm | ||
| Stage IV | Osteoarthritic joint space narrowing, degenerative changes | Subchondral collapse, femoral head flattening, normal joint space | Osteoarthritic degenerative changes |
| Stage V | Flattening with joint space narrowing, acetabular changes, or both | ||
| Stage VI | Advanced degenerative changes, secondary osteoarthritis | ||
ARCO: Association Research Circulation Osseous.
The advent of magnetic resonance imaging (MRI) and its widespread use gave rise to the Steinberg or University of Pennsylvania osteonecrosis classification system[22,25], which differentiates subchondral collapse from femoral head articular cartilage collapse (flattening) (Table 1). Stages I through IV are classified by percent of femoral head involvement: A < 15%, B 15%-30%, C > 30%. These size modifiers are considered predictors of femoral head collapse. Small lesion size and more medial location are considered prognostically favorable[25].
Another commonly used classification system that utilizes MRI and other radiographic modalities is the Association Research Circulation Osseous (ARCO) staging system, which was introduced in 1992 and is summarized in Table 1[26].
MRI has become the imaging modality of choice, as it is highly sensitive and specific for osteonecrosis[3]. T1 images on MRI typically demonstrate a serpiginous “band-like” lesion with low signal intensity in the anterosuperior femoral head, and a “double-line sign” can be seen on T2 sequences, which depicts a high signal intensity reparative interface of vascular reactive bone adjacent to necrotic subchondral bone[3]. Radionuclide bone scans are less sensitive and specific than MRI but can be used to detect inflammatory activity in the femoral head when MRI is contraindicated. CT is also less sensitive than MRI in detecting osteonecrosis and has a significant radiation burden. Angiography and biopsy are invasive methods to confirm osteonecrosis and therefore are only used as research modalities[25,27].
CURRENT TREATMENT OPTIONS
Non-operative treatments for osteonecrosis include measures to offload force on the affected hip by limiting weight bearing with a cane or walker, activity modification, and physical therapy. However, these methods have no role in treatment of late stage osteonecrosis and show limited success in preventing disease progression, even in early stage (Steinberg stage I and II) disease[9,28]. Patients can be encouraged to abstain from or decrease alcohol consumption and smoking[29]. Other conservative options include lipid-reducing agents, bisphosphonates, and hyperbaric oxygen, but these therapies have minimal utility after subchondral collapse has occurred in the femoral head, as seen in a meta-analysis when compared with core decompression[30].
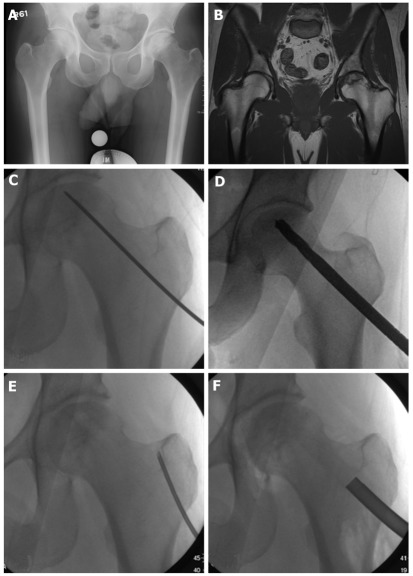
Core decompression is a commonly used prophylactic surgery used in pre-collapse osteonecrosis (prior to Ficat and ARCO stage II, Steinberg stage III), in which necrotic cancellous bone in the femoral head is drilled and removed from a lateral femoral cortical entry point (Figure 1). This is often stabilized with structural allograft or with autograft by harvesting cancellous bone from the greater trochanter and proximal femur. This cancellous graft contains osteoprogenitor cells that aid in healing. The results for core decompression alone generally deteriorate with more advanced lesions[27]. However, augmentation of the core decompression can be achieved with the addition of bone morphogenic proteins, electromagnetic stimulation, or demineralized bone matrix[27,31]. Although core decompression for Steinberg stage I disease was successful as a definitive procedure in > 80% of patients, Steinberg stage II and III osteonecrosis treated with decompression required further surgical reconstructive intervention in 37% and 71% of patients, respectively[28,30]. Multiple drilling of the femoral head osteonecrotic lesion can be an alternative, and comparable results have been reported[32]. Another biologic option that has met with some success is the harvesting and in vitro culture of autologous mesenchymal stem cells (MSCs) and reimplantation in the core decompression site[33,34]. Studies of the long-term success of using bone morphogenic proteins and autologous MSCs are still underway[35].
Figure 1.
Forty-one year old male with pre-collapse osteonecrosis of left femoral head as evidenced by (A) plain radiograph and (B) magnetic resonance imaging of pelvis. Patient underwent core decompression: (C) Kirschner wire to localize to affected subchondral bone; D: Drilling of lesion; E: Aspiration of bone marrow from cancellous bone in greater trochanter; F: Insertion of bone graft mixed with bone marrow aspirate.
Vascularized fibular graft supplementation during core decompression and other salvage procedures has also been studied extensively and implemented for higher stages of osteonecrosis. These grafts deter progression of pre-collapse (Steinberg stage I and II) lesions and can also delay the development of end stage osteonecrosis after mild collapse (Steinberg stage III through V) has occurred. The cortical graft not only offers structural stability, but also biologic incorporation, as the vascularized bone promotes callus formation and remodeling in the femoral head[36-39]. Although certain methods such as the patient-specific Ioannina aiming device increase optimal graft placement in the anterosuperior aspect of the femoral head, vascularized grafting still remains technically challenging[40]. Non-vascularized fibular grafts have also been studied as an alternative, but vascularized grafts appear to have better clinical results for prevention of femoral head collapse[41].
Several osteotomies have been studied for the treatment of pre-collapse and early post-collapse (Steinberg stage II to IV) osteonecrosis, with the goal of transferring weight-bearing forces away from necrotic subchondral bone toward other areas of the articular surface. These include flexion, extension, varus, valgus, rotation, or combined osteotomies, and subtrochanteric and intertrochanteric osteotomies have also been described[42-45]. These procedures have met with favorable success rates but can have a moderate risk of nonunion, and they can make conversion to total hip arthroplasty more difficult.
The emergence of hip arthroscopy has improved surgeons’ ability to visualize and quantify or stage the degree of chondrosis in osteonecrosis[46]. Small-diameter core decompression has been described as an arthroscopic procedure for early stages[47], but arthroscopy is not considered an effective option to treat advanced osteonecrosis.
Advanced osteonecrosis with significant arthrosis is commonly treated with prosthetic replacement, including femoral resurfacing arthroplasty, hemiarthroplasty, and THA. THA has excellent clinical results for pain relief and functional improvement, but these reconstructive arthroplasties in young patients can be problematic, as they are often associated with early failure from loosening and other complications[48-51].
Femoral resurfacing can be used in younger patients with osteonecrosis involving less than one third of the femoral head, and this arthroplasty option has been studied and used more outside of the United States. Technical issues with these implants have been associated in the past with femoral neck fracture, high failure rate, and conversion to THA[48,52]. Newer designs and uncemented implants have yielded more favorable results, but other complications such as ionic metal wear in metal-on-metal implants can still occur[53]. Hemiarthroplasty with unipolar or bipolar implants have been utilized but by design do not address pathology at the acetabular surface in late-stage osteonecrosis and are also associated with wear, loosening, groin pain, and conversion to THA[48]; therefore hemiarthroplasty is not recommended.
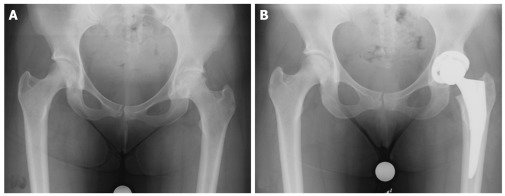
Ultimately, advanced osteonecrosis and failure of the other aggressive interventions mentioned above may necessitate total hip arthroplasty. THA is the most commonly performed procedure for Ficat and ARCO stage III and IV (Steinberg stage IV to VI) osteonecrosis and is highly successful for symptomatic improvement[49,50] (Figure 2). The durability of THA, however, is inferior to the same procedure performed for osteoarthritis, as patients with osteonecrosis are generally younger and have higher functional demands[54]. Associated comorbidities such as alcohol abuse and inflammatory disorders associated with steroid use such as SLE may also contribute to the inferior outcomes. Cemented THA has been documented to have higher complication rates relative to cementless prosthesis with improved modern press-fit designs[55]. THA complications in osteonecrosis include infection (particularly in SLE, sickle cell, and immunocompromised patients), high risk of dislocation (notably in alcohol abusers), compromise in soft tissue healing, and implant loosening. Despite these risk factors and potential complications, however, modern advancements in hip arthroplasty over the past decade have improved outcomes of THA in osteonecrosis, as seen in a recent meta-analysis by Myers et al[51].
Figure 2.
Forty-eight year old female with osteonecrotic left femoral head, with evidence of left hip osteoarthritis (A). Patient underwent left uncemented total hip arthroplasty (B).
In summary, there are several pre-collapse treatment options available for symptomatic osteonecrosis; however, the majority of patients progress to advanced stages with articular collapse, requiring total hip arthroplasty. The future of osteonecrosis treatment depends on finding alternative joint-sparing procedures and treatments to delay the need for hip arthroplasty. Basic science and clinical research in this field over the past decade has focused on developing animal models to understand pathophysiologic mechanisms, as well as testing novel growth factor- and cell-based therapeutic options that may prevent or postpone the progression of osteonecrosis.
UPDATE ON BENCH RESEARCH AND CLINICAL APPLICATIONS
As the research and development of new osteonecrosis treatments are continuously being explored, one of the limiting factors that prevents the systemic evaluation of the effectiveness of the treatments is the lack of an animal model that replicates the natural history and progression of osteonecrosis in humans. Nevertheless, several animal models have been developed to evaluate various treatment strategies. Vascular damage is a crucial event in trauma-induced osteonecrosis, and many animal models have attempted to mimic this injury by surgically inducing vascular deprivation. A rat osteonecrosis model in which the femoral head is temporarily dislocated after the ligamentum teres is cut is the most common surgical osteonecrosis model[56-58]. An adult rabbit model of traumatic osteonecrosis has been established by complete surgical removal of the hip joint capsule followed by circumferential cauterization of the periosteum and blood vessels covering the femoral neck to interrupt the blood supply to the femoral head[59]. Cryogenic and thermal insults have been used to induce osteonecrosis in quadrupeds such as canines[60] and bipeds such as emus[61]. Intramuscular injection of methylprednisolone has been used to develop steroid-induced osteonecrosis in mouse[62], rat[63], rabbit[64,65], pig[66], and chicken models[18], where the percent incidence of induced osteonecrosis is dependent on the amount of methylprednisolone injected. These animal models have been used to study the molecular mechanisms of osteonecrosis and assess the usefulness of several therapies over the last few decades.
Some of the more recent efforts in treatment development have focused on the use of cellular therapies for osteonecrosis. In one study, CD34+ cells, known to be both vasculogenic and osteogenic, were intravenously transplanted after G-CSF mobilization in a rat model, resulting in improved outcomes[67]. Since it has been reported that MSC proliferation is affected during osteonecrosis[68], several studies have attempted to treat osteonecrosis by transplanting MSCs either systemically or locally in various animal models. Li et al[69] investigated the efficacy of giving allogeneic MSCs derived from the bone marrow to rabbits with heat-induced femoral head necrosis and showed the directional migration of GFP-labeled MSCs to the defect site. Yan et al[70] showed that transplanted MSCs differentiated into osteoblasts and aided in the repair process of traumatic osteonecrosis in a skeletally mature canine model. Another recent study evaluated the effectiveness of biphasic calcium phosphate (BCP) ceramic scaffolds seeded with MSCs on inducing osteointegration and new bone formation in a canine model[71]. These studies indicate the efficacy of exogenous stem cells in osteonecrosis treatment. Early trials outside the United States have also utilized MSCs in humans and preliminarily show good results. For instance, the percutaneous injection of autologous adipose-derived MSCs with hyaluronic acid, platelet-rich plasma, and calcium chloride demonstrate MRI evidence of improvement in osteonecrosis and cartilage regeneration[72]. However, this is an uncontrolled clinical trial, and further study is needed.
Another branch of osteonecrosis research has focused on the effectiveness of bisphosphonates, growth factors, lipid-lowering agents, and combined drug therapies. Lipid-lowering drugs such as statins decrease the incidence of steroid-induced osteonecrosis[73,74]. Other studies have also shown that the simultaneous use of anticoagulants along with lipid-lowering agents can decrease the prevalence of steroid-induced osteonecrosis in rabbits[75,76]. Bisphosphonates, used regularly for the treatment of osteoporosis and other pathologic conditions of bone, have been found to be promising for clinically treating osteonecrosis to postpone surgical interventions[77]. Lai et al[78] developed a randomized study that showed that alendronate delays or prevents progression of femoral head collapse in Steinberg stage II and III disease, and may ultimately reduce the need for joint arthroplasty, although longer term follow-up was needed. Agarwala et al[79] showed that a daily dose of alendronate resulted in improved hip function and decreased dependency on nonsteroidal antiinflammatory drugs over a period of 2.5 years. In addition, there was decreased femoral head edema on MRI, suggesting slower progression of osteonecrosis. Along with preservation, it is crucial that the bone undergoes remodeling during osteonecrosis. While bisphosphonates have been known to do the former, Vandermeer et al[80] show that combining these drugs with bone morphogenetic protein-2 improved the epiphyseal quotient and trabecular bone remodeling in immature pigs that had surgically-induced ischemic ON. The impact of such combination drug therapies is yet to be fully evaluated in human subjects.
On the surgical forefront, clinical studies have scrutinized older techniques and evaluated novel techniques for treatment of osteonecrosis. Transtrochanteric rotational osteotomy has historically demonstrated variable success for avoidance of femoral head collapse[43,45,81]. Exploration of the risk factors revealed that higher age, higher BMI, and higher stages of osteonecrosis were determinants of likelihood of conversion of osteotomies to THA[82]. These factors can be useful during patient selection for joint-sparing procedures. Advancements in hip resurfacing have made this procedure a viable option in younger patients under the age of 25 years and can help reduce the need for THA, but there are still risks of ionic wear, fracture, and loosening[83]. Total hip arthroplasty itself has undergone technical improvements over the last two decades, and implant survival is significantly higher. Uncemented ceramic-on-ceramic THA has demonstrated some promise for improved outcomes and implant durability in younger patients[84].
Improvements in microsurgical techniques have enhanced outcomes for free vascularized fibula grafting to the osteonecrotic hip. This procedure has been shown to be successful for younger patients in pre-collapse stages and generally delays the need for THA, even in post-collapse osteonecrosis[85]. Other grafting techniques, such as bone graft pedicled with quadratus femoris in a titanium mesh, have also been developed, but long-term effectiveness has not yet been studied[86]. Augmentation of core decompression with porous tantalum rods has also been explored as a treatment method for early stages of osteonecrosis, with some favorable results; however, the release of high-density metal particles as well as progression to femoral head collapse are frequent complications[87]. Other clinical trials involving the use of trabecular metal rods[88] and mesh cages[86] have also been published.
CONCLUSION
Osteonecrosis is a pathology commonly seen in younger adults, in which collapse of the femoral head and early onset of osteoarthritis may eventually necessitate hip arthroplasty when non-operative measures and joint-sparing procedures fail. Basic science research to understand the pathophysiology and to develop therapies that can be translated to clinical application has progressed rapidly, and these advances offer great promise for the future treatment of osteonecrosis. Similarly, technological improvements in surgical treatment methods have also improved outcomes over the past two decades and will continue to help patients recover from this functionally debilitating joint disease.
ACKNOWLEDGMENTS
The series and guest editors would like to thank Dr. Lynne C Jones, Johns Hopkins University, Baltimore, Maryland for her critical review and editing of this manuscript.
Footnotes
Peer reviewers: Seung-Hoon Baek, MD, PhD, Assistant Professor, Department of Orthopedic Surgery, Daegu Catholic University Medical Center, 3056-6 Dae-myung-4-dong, Nam-gu, Daegu 705-718, South Korea; George C Babis, Associate Professor, University of Athens Medical School, Rimini 1, 12462 Chaidari, Greece
S- Editor Yang XC L- Editor A E- Editor Yang XC
References
- 1.Li W, Sakai T, Nishii T, Nakamura N, Takao M, Yoshikawa H, Sugano N. Distribution of TRAP-positive cells and expression of HIF-1alpha, VEGF, and FGF-2 in the reparative reaction in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27:694–700. doi: 10.1002/jor.20802. [DOI] [PubMed] [Google Scholar]
- 2.Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;74:250–261. doi: 10.5435/00124635-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63:16–28. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 6.Vail TP, Covington DB. The incidence of osteonecrosis. In: Osteonecrosis: Etiology, Diagnosis , Treatment , editors. Rosemont, IL: American Academy of Orthopedic Surgeons;; 1997. pp. 43–49. [Google Scholar]
- 7.Larson AN, McIntosh AL, Trousdale RT, Lewallen DG. Avascular necrosis most common indication for hip arthroplasty in patients with slipped capital femoral epiphysis. J Pediatr Orthop. 2010;30:767–773. doi: 10.1097/BPO.0b013e3181fbe912. [DOI] [PubMed] [Google Scholar]
- 8.Luedtke LM, Flynn JM, Pill SG. A review of avascular necrosis in developmental dysplasia of the hip and contemporary efforts at prevention. Univ Penn Orthop J. 2000;13:22–28. [Google Scholar]
- 9.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92:2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 10.Morse CG, Mican JM, Jones EC, Joe GO, Rick ME, Formentini E, Kovacs JA. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis. 2007;44:739–748. doi: 10.1086/511683. [DOI] [PubMed] [Google Scholar]
- 11.Grose AW, Gardner MJ, Sussmann PS, Helfet DL, Lorich DG. The surgical anatomy of the blood supply to the femoral head: description of the anastomosis between the medial femoral circumflex and inferior gluteal arteries at the hip. J Bone Joint Surg Br. 2008;90:1298–1303. doi: 10.1302/0301-620X.90B10.20983. [DOI] [PubMed] [Google Scholar]
- 12.Jones JP. Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Relat Res. 1993;292:294–308. [PubMed] [Google Scholar]
- 13.Calder JD, Buttery L, Revell PA, Pearse M, Polak JM. Apoptosis--a significant cause of bone cell death in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2004;86:1209–1213. doi: 10.1302/0301-620x.86b8.14834. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 15.Youm YS, Lee SY, Lee SH. Apoptosis in the osteonecrosis of the femoral head. Clin Orthop Surg. 2010;2:250–255. doi: 10.4055/cios.2010.2.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bejar J, Peled E, Boss JH. Vasculature deprivation--induced osteonecrosis of the rat femoral head as a model for therapeutic trials. Theor Biol Med Model. 2005;2:24. doi: 10.1186/1742-4682-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 18.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;344:8–19. [PubMed] [Google Scholar]
- 19.Wang GJ, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59:729–735. [PubMed] [Google Scholar]
- 20.Kannu P, O'Rielly DD, Hyland JC, Kokko LA. Avascular necrosis of the femoral head due to a novel C propeptide mutation in COL2A1. Am J Med Genet A. 2011;155A:1759–1762. doi: 10.1002/ajmg.a.34056. [DOI] [PubMed] [Google Scholar]
- 21.van den Bogert AJ, Read L, Nigg BM. An analysis of hip joint loading during walking, running, and skiing. Med Sci Sports Exerc. 1999;31:131–142. doi: 10.1097/00005768-199901000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg ME. Avascular necrosis: diagnosis, staging, and management. J Musculoskel Med. 1997;14:13–25. [Google Scholar]
- 23.Jones LC, Mont MA, Le TB, Petri M, Hungerford DS, Wang P, Glueck CJ. Procoagulants and osteonecrosis. J Rheumatol. 2003;30:783–791. [PubMed] [Google Scholar]
- 24.Arlet J, Ficat P. [Non-traumatic avascular femur head necrosis. New methods of examination and new concepts] Chir Narzadow Ruchu Ortop Pol. 1977;42:269–276. [PubMed] [Google Scholar]
- 25.Steinberg ME. Diagnostic imaging and the role of stage and lesion size in determining outcome in osteonecrosis of the femoral head. Techniques in Orthopaedics. 2001;16:6–15. [Google Scholar]
- 26.ARCO (Association Research Circulation Osseous): committee on terminology and classification. ARCO News. 1992;4:41–46. [Google Scholar]
- 27.McGrory BJ, York SC, Iorio R, Macaulay W, Pelker RR, Parsley BS, Teeny SM. Current practices of AAHKS members in the treatment of adult osteonecrosis of the femoral head. J Bone Joint Surg Am. 2007;89:1194–1204. doi: 10.2106/JBJS.F.00302. [DOI] [PubMed] [Google Scholar]
- 28.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–178. doi: 10.1097/00003086-199603000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Hirota Y, Hirohata T, Fukuda K, Mori M, Yanagawa H, Ohno Y, Sugioka Y. Association of alcohol intake, cigarette smoking, and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am J Epidemiol. 1993;137:530–538. doi: 10.1093/oxfordjournals.aje.a116706. [DOI] [PubMed] [Google Scholar]
- 30.Castro FP, Barrack RL. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop (Belle Mead NJ) 2000;29:187–194. [PubMed] [Google Scholar]
- 31.Ciombor DM, Aaron RK. Biologically augmented core decompression for the treatment of osteonecrosis of the femoral head. Techniques in Orthopaedics. 2001;16:32–38. [Google Scholar]
- 32.Song WS, Yoo JJ, Kim YM, Kim HJ. Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res. 2007;454:139–146. doi: 10.1097/01.blo.0000229342.96103.73. [DOI] [PubMed] [Google Scholar]
- 33.Gangji V, Toungouz M, Hauzeur JP. Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther. 2005;5:437–442. doi: 10.1517/14712598.5.4.437. [DOI] [PubMed] [Google Scholar]
- 34.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Hauzeur JP, Gangji V. Phases 1-3 clinical trials using adult stem cells in osteonecrosis and nonunion fractures. Stem Cells Int. 2010;2010:410170. doi: 10.4061/2010/410170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brannon JK. Influence of acetabular coverage on hip survival after free vascularized fibular grafting for femoral head osteonecrosis. J Bone Joint Surg Am. 2007;89:448–49; author reply 448-49;. doi: 10.2106/00004623-200702000-00036. [DOI] [PubMed] [Google Scholar]
- 37.Katz MA, Urbaniak JR. Free vascularized fibular grafting of the femoral head for the treatment of osteonecrosis. Techniques in Orthopaedics. 2001;16:44–60. [Google Scholar]
- 38.Malizos KN, Soucacos PN, Beris AE. Osteonecrosis of the femoral head. Hip salvaging with implantation of a vascularized fibular graft. Clin Orthop Relat Res. 1995;314:67–75. [PubMed] [Google Scholar]
- 39.Urbaniak JR, Coogan PG, Gunneson EB, Nunley JA. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting. A long-term follow-up study of one hundred and three hips. J Bone Joint Surg Am. 1995;77:681–694. doi: 10.2106/00004623-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Beris AE, Soucacos PN. Optimizing free fibular grafting in femoral head osteonecrosis: the Ioannina aiming device. Clin Orthop Relat Res. 2001;386:64–70. doi: 10.1097/00003086-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Kim SY, Kim YG, Kim PT, Ihn JC, Cho BC, Koo KH. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005;87:2012–2018. doi: 10.2106/JBJS.D.02593. [DOI] [PubMed] [Google Scholar]
- 42.Atsumi T, Muraki M, Yoshihara S, Kajihara T. Posterior rotational osteotomy for the treatment of femoral head osteonecrosis. Arch Orthop Trauma Surg. 1999;119:388–393. doi: 10.1007/s004020050435. [DOI] [PubMed] [Google Scholar]
- 43.Langlais F, Fourastier J. Rotation osteotomies for osteonecrosis of the femoral head. Clin Orthop Relat Res. 1997;343:110–123. [PubMed] [Google Scholar]
- 44.Sugioka Y. Transtrochanteric anterior rotational osteotomy of the femoral head in the treatment of osteonecrosis affecting the hip: a new osteotomy operation. Clin Orthop Relat Res. 1978;130:191–201. [PubMed] [Google Scholar]
- 45.Sugioka Y, Hotokebuchi T, Tsutsui H. Transtrochanteric anterior rotational osteotomy for idiopathic and steroid-induced necrosis of the femoral head. Indications and long-term results. Clin Orthop Relat Res. 1992;277:111–120. [PubMed] [Google Scholar]
- 46.Sekiya JK, Ruch DS, Hunter DM, Pope TL, Koman LA, Poehling GG, Russell GB. Hip arthroscopy in staging avascular necrosis of the femoral head. J South Orthop Assoc. 2000;9:254–261. [PubMed] [Google Scholar]
- 47.Wang ZG, Wang Y, Liu YJ, Li ZL, Cai X, Wei M. [Clinical evaluation of small diameter decompression and arthroscopy in the treatment of early avascular necrosis of femoral head] Zhonghua YiXue ZaZhi. 2007;87:2041–2044. [PubMed] [Google Scholar]
- 48.Ivankovich DA, Rosenberg AG, Malamis A. Reconstructive options for osteonecrosis of the femoral head. Techniques in Orthopaedics. 2001;16:66–79. [Google Scholar]
- 49.Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, Urbaniak JR. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–355. [PubMed] [Google Scholar]
- 50.Mont MA, Rajadhyaksha AD, Hungerford DS. Outcomes of limited femoral resurfacing arthroplasty compared with total hip arthroplasty for osteonecrosis of the femoral head. J Arthroplasty. 2001;16:134–139. doi: 10.1054/arth.2001.28722. [DOI] [PubMed] [Google Scholar]
- 51.Myers TG, Mihalko WM, Brown TE, Saleh KJ, Cui Q. Outcomes of total hip arthroplasty for osteonecrosis of the hip: systematic review and meta-analysis. Curr Orthop Practice. 2010;21:81–88. [Google Scholar]
- 52.Squire M, Fehring TK, Odum S, Griffin WL, Bohannon Mason J. Failure of femoral surface replacement for femoral head avascular necrosis. J Arthroplasty. 2005;20:108–114. doi: 10.1016/j.arth.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 53.DeSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92:1642–1647. doi: 10.1302/0301-620X.92B12.24654. [DOI] [PubMed] [Google Scholar]
- 54.Xenakis TA, Beris AE, Malizos KK, Koukoubis T, Gelalis J, Soucacos PN. Total hip arthroplasty for avascular necrosis and degenerative osteoarthritis of the hip. Clin Orthop Relat Res. 1997;341:62–68. [PubMed] [Google Scholar]
- 55.Ritter MA, Helphinstine J, Keating EM, Faris PM, Meding JB. Total hip arthroplasty in patients with osteonecrosis. The effect of cement techniques. Clin Orthop Relat Res. 1997;338:94–99. doi: 10.1097/00003086-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Levin D, Norman D, Zinman C, Rubinstein L, Sabo E, Misselevich I, Reis D, Boss JH. Treatment of experimental avascular necrosis of the femoral head with hyperbaric oxygen in rats: histological evaluation of the femoral heads during the early phase of the reparative process. Exp Mol Pathol. 1999;67:99–108. doi: 10.1006/exmp.1999.2273. [DOI] [PubMed] [Google Scholar]
- 57.Peled E, Bejar J, Zinman C, Boss JH, Reis DN, Norman D. Prevention of distortion of vascular deprivation-induced osteonecrosis of the rat femoral head by treatment with alendronate. Arch Orthop Trauma Surg. 2009;129:275–279. doi: 10.1007/s00402-008-0656-0. [DOI] [PubMed] [Google Scholar]
- 58.Vadasz Z, Misselevich I, Norman D, Peled E, Boss JH. Localization of vascular endothelial growth factor during the early reparative phase of the rats' vessels deprivation-induced osteonecrosis of the femoral heads. Exp Mol Pathol. 2004;77:145–148. doi: 10.1016/j.yexmp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Hofstaetter JG, Wang J, Yan J, Glimcher MJ. The effects of alendronate in the treatment of experimental osteonecrosis of the hip in adult rabbits. Osteoarthritis Cartilage. 2009;17:362–370. doi: 10.1016/j.joca.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 60.Conzemius MG, Brown TD, Zhang Y, Robinson RA. A new animal model of femoral head osteonecrosis: one that progresses to human-like mechanical failure. J Orthop Res. 2002;20:303–309. doi: 10.1016/S0736-0266(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 61.Malizos KN, Quarles LD, Seaber AV, Rizk WS, Urbaniak JR. An experimental canine model of osteonecrosis: characterization of the repair process. J Orthop Res. 1993;11:350–357. doi: 10.1002/jor.1100110306. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Boyd K, Kaste SC, Kamdem Kamdem L, Rahija RJ, Relling MV. A mouse model for glucocorticoid-induced osteonecrosis: effect of a steroid holiday. J Orthop Res. 2009;27:169–175. doi: 10.1002/jor.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han N, Yan Z, Guo CA, Shen F, Liu J, Shi Y, Zhang Z. Effects of p-glycoprotein on steroid-induced osteonecrosis of the femoral head. Calcif Tissue Int. 2010;87:246–253. doi: 10.1007/s00223-010-9385-9. [DOI] [PubMed] [Google Scholar]
- 64.Iwakiri K, Oda Y, Kaneshiro Y, Iwaki H, Masada T, Kobayashi A, Asada A, Takaoka K. Effect of simvastatin on steroid-induced osteonecrosis evidenced by the serum lipid level and hepatic cytochrome P4503A in a rabbit model. J Orthop Sci. 2008;13:463–468. doi: 10.1007/s00776-008-1257-z. [DOI] [PubMed] [Google Scholar]
- 65.Kuribayashi M, Fujioka M, Takahashi KA, Arai Y, Ishida M, Goto T, Kubo T. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 2010;81:154–160. doi: 10.3109/17453671003628772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drescher W, Weigert KP, Bünger MH, Ingerslev J, Bünger C, Hansen ES. Femoral head blood flow reduction and hypercoagulability under 24 h megadose steroid treatment in pigs. J Orthop Res. 2004;22:501–508. doi: 10.1016/j.orthres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Terayama H, Ishikawa M, Yasunaga Y, Yamasaki T, Hamaki T, Asahara T, Ochi M. Prevention of osteonecrosis by intravenous administration of human peripheral blood-derived CD34-positive cells in a rat osteonecrosis model. J Tissue Eng Regen Med. 2011;5:32–40. doi: 10.1002/term.285. [DOI] [PubMed] [Google Scholar]
- 68.Wang BL, Sun W, Shi ZC, Lou JN, Zhang NF, Shi SH, Guo WS, Cheng LM, Ye LY, Zhang WJ, et al. Decreased proliferation of mesenchymal stem cells in corticosteroid-induced osteonecrosis of femoral head. Orthopedics. 2008;31:444. doi: 10.3928/01477447-20080501-33. [DOI] [PubMed] [Google Scholar]
- 69.Li ZH, Liao W, Cui XL, Zhao Q, Liu M, Chen YH, Liu TS, Liu NL, Wang F, Yi Y, et al. Intravenous transplantation of allogeneic bone marrow mesenchymal stem cells and its directional migration to the necrotic femoral head. Int J Med Sci. 2011;8:74–83. doi: 10.7150/ijms.8.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res. 2009;27:442–446. doi: 10.1002/jor.20759. [DOI] [PubMed] [Google Scholar]
- 71.Peng J, Wen C, Wang A, Wang Y, Xu W, Zhao B, Zhang L, Lu S, Qin L, Guo Q, et al. Micro-CT-based bone ceramic scaffolding and its performance after seeding with mesenchymal stem cells for repair of load-bearing bone defect in canine femoral head. J Biomed Mater Res B Appl Biomater. 2011;96:316–325. doi: 10.1002/jbm.b.31770. [DOI] [PubMed] [Google Scholar]
- 72.Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Reports. 2011;5:296. doi: 10.1186/1752-1947-5-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pengde K, Fuxing P, Bin S, Jing Y, Jingqiu C. Lovastatin inhibits adipogenesis and prevents osteonecrosis in steroid-treated rabbits. Joint Bone Spine. 2008;75:696–701. doi: 10.1016/j.jbspin.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;386:173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 75.Kang P, Gao H, Pei F, Shen B, Yang J, Zhou Z. Effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Int J Exp Pathol. 2010;91:235–243. doi: 10.1111/j.1365-2613.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Motomura G, Yamamoto T, Miyanishi K, Jingushi S, Iwamoto Y. Combined effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Arthritis Rheum. 2004;50:3387–3391. doi: 10.1002/art.20517. [DOI] [PubMed] [Google Scholar]
- 77.Silverman SL. Bisphosphonate use in conditions other than osteoporosis. Ann N Y Acad Sci. 2011;1218:33–37. doi: 10.1111/j.1749-6632.2010.05769.x. [DOI] [PubMed] [Google Scholar]
- 78.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 79.Agarwala S, Jain D, Joshi VR, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology (Oxford) 2005;44:352–359. doi: 10.1093/rheumatology/keh481. [DOI] [PubMed] [Google Scholar]
- 80.Vandermeer JS, Kamiya N, Aya-ay J, Garces A, Browne R, Kim HK. Local administration of ibandronate and bone morphogenetic protein-2 after ischemic osteonecrosis of the immature femoral head: a combined therapy that stimulates bone formation and decreases femoral head deformity. J Bone Joint Surg Am. 2011;93:905–913. doi: 10.2106/JBJS.J.00716. [DOI] [PubMed] [Google Scholar]
- 81.Biswal S, Hazra S, Yun HH, Hur CY, Shon WY. Transtrochanteric rotational osteotomy for nontraumatic osteonecrosis of the femoral head in young adults. Clin Orthop Relat Res. 2009;467:1529–1537. doi: 10.1007/s11999-008-0696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ha YC, Kim HJ, Kim SY, Kim KC, Lee YK, Koo KH. Effects of age and body mass index on the results of transtrochanteric rotational osteotomy for femoral head osteonecrosis: surgical technique. J Bone Joint Surg Am. 2011;93 Suppl 1:75–84. doi: 10.2106/JBJS.J.01215. [DOI] [PubMed] [Google Scholar]
- 83.Sayeed SA, Johnson AJ, Stroh DA, Gross TP, Mont MA. Hip resurfacing in patients who have osteonecrosis and are 25 years or under. Clin Orthop Relat Res. 2011;469:1582–1588. doi: 10.1007/s11999-010-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Millar NL, Halai M, McKenna R, McGraw IW, Millar LL, Hadidi M. Uncemented ceramic-on-ceramic THA in adults with osteonecrosis of the femoral head. Orthopedics. 2010;33:795. doi: 10.3928/01477447-20100924-13. [DOI] [PubMed] [Google Scholar]
- 85.Korompilias AV, Beris AE, Lykissas MG, Kostas-Agnantis IP, Soucacos PN. Femoral head osteonecrosis: why choose free vascularized fibula grafting. Microsurgery. 2011;31:223–228. doi: 10.1002/micr.20837. [DOI] [PubMed] [Google Scholar]
- 86.Wang YS, Zhang Y, Li JW, Yang GH, Li JF, Yang J, Yang GH. A modified technique of bone grafting pedicled with femoral quadratus for alcohol-induced osteonecrosis of the femoral head. Chin Med J (Engl) 2010;123:2847–2852. [PubMed] [Google Scholar]
- 87.Liu G, Wang J, Yang S, Xu W, Ye S, Xia T. Effect of a porous tantalum rod on early and intermediate stages of necrosis of the femoral head. Biomed Mater. 2010;5:065003. doi: 10.1088/1748-6041/5/6/065003. [DOI] [PubMed] [Google Scholar]
- 88.Wang S, Song X, Sun Y. [Short-term effect of trabecular metal rod implant on early avascular necrosis of the femoral head] Zhongguo Xiufu Chongjian Waike Zazhi. 2009;23:562–565. [PubMed] [Google Scholar]