Abstract
Purpose
There is a paucity of literature describing posterior spinal fusion (PSF) in the Ehlers–Danlos syndrome (EDS) patient. The vast majority of these studies diagnosed EDS clinically. The purpose of this study is to discuss the management and complications of EDS patients with scoliosis treated with PSF at a single institution.
Methods
Clinical and radiographic data are presented describing six patients who had PSF for EDS. The diagnoses were confirmed by a geneticist.
Results
All of the patients in the current cohort underwent posterior fusion only, with no anterior approach. Neuromonitoring was also used in the majority. Half of our patients experienced complications. One patient had a hemoperitoneum that was initially treated nonoperatively but, unfortunately, they expired 1 month after discharge from abdominal bleeding. Another patient suffered neuropathic pain attributed to the type of implant used. A third underwent a total of seven procedures beginning at the age of 3 years in a different era of spinal surgery. The mean major curve, percentage correction, and estimated blood loss of the current cohort are similar to previous studies.
Conclusion
The fragility of the EDS patient population cannot be overlooked. Despite a conservative surgical approach, half of our patients experienced complications. The surgeon choosing to operate on EDS patients must do so with extreme caution.
Keywords: Ehlers–Danlos syndrome, Posterior spinal fusion, Scoliosis
Introduction
Ehlers–Danlos syndrome (EDS) is an inherited disorder of collagen production. Signs include hyperelastic skin, joint hypermobility, and fragile vasculature. Recent advances in genetics allow for more detailed diagnosis and classification of EDS. Currently, EDS is divided into six types based on clinical and genetic features (Table 1) [1]. Scoliosis is frequently seen in patients with EDS. In our experience, bracing is often ineffective, in agreement with McMaster [2]. For children who develop severe curvature, surgery is often recommended.
Table 1.
Current and prior classifications of Ehlers–Danlos syndrome (EDS)
| Current classification | Previous classification |
|---|---|
| Hypermobility | Type 3 |
| Classical | Types 1 and 2 |
| Vascular | Type 4 |
| Kyphoscoliosis | Type 6 |
| Arthrochalasis | Types 7A and 7B |
| Dermatosparaxis | Type 7C |
Caution has been advised in the surgical management of patients with EDS. In particular, the vascular type of EDS has a propensity for severe bleeding complications [3–5]. Limited case series [2, 4, 5] have been reported; however, all but one were without strict diagnostic criteria for EDS [3]. The purpose of this study is to report on the management and complications of six EDS patients who were diagnosed under strict criteria and underwent surgical treatment of scoliosis.
Materials and methods
EDS was diagnosed based on genetic confirmation or clinical diagnosis made by a geneticist. Surgery was done by multiple primary surgeons, with many different instrumentation systems used. Given the variety of surgeons and instrumentation, no uniform post-operative protocol was utilized for these patients. Neuromonitoring was utilized in the majority of cases and is detailed in Table 2. Institutional review board approval was obtained prior to initiating this retrospective chart and radiograph review.
Table 2.
Summary of procedures
| Patient | Age at surgery (years) | Sex | EDS type | Surgical procedure | Neuromonitoring | Blood loss (ml) | Complications |
|---|---|---|---|---|---|---|---|
| 1 | 18.5 | Male | 6 | T5-L4 PSF, pedicle screws, ICBG | SSEP no changes | 2,000 | None |
| 2 | 14.4 | Female | 7 | T3-sacrum Luque–Galveston fixation | SSEP, TcMEP no changes | 2,000 | Hemoperitoneum, death |
| 3 | 3.2 | Male | 6 | PSF-T3-L3, MOE rod | 480 | Progression, inadequate rod length, implant failure | |
| 4.0 | MOE rod replacement | 250 | Implant failure | ||||
| 4.2 | Harrington rod | 150 | None | ||||
| 5.6 | Harrington rod distraction | 30 | Hook dislodgement | ||||
| 5.9 | Hook replacement and repositioning rod | 150 | Infection | ||||
| 6.1 | I+D, ROH | 100 | None | ||||
| 8.6 | CD PSIF T4-L3, ICBG | 1,300 | Small dural tear | ||||
| 4 | 18.4 | Male | 3 | PSF T4-sacrum, Dunn–McCarthy | SSEP no changes | 2,000 | Symptomatic implant |
| 20.9 | ROH-inferior implants | SSEP no changes | 150 | Infection | |||
| 22.1 | I+D, ROH | SSEP, TcMEP no changes | 500 | None | |||
| 5 | 11.0 | Female | 3 | T3-L3 all hook, single TSRH rod, ICBG | SSEP no changes | 1,800 | None |
| 6 | 14.1 | Female | 7 | T3-sacrum, pedicle screws, hooks, iliac screws | SSEP, TcMEP no changes | 1,900 | None |
Case reports
See Tables 2 and 3 for the clinical and radiographic data.
Table 3.
Radiographic data of the case patients
| Patient | Deformity | Initial correction | Final correction | Major curve correction (%) | |||
|---|---|---|---|---|---|---|---|
| T | L | T | L | T | L | ||
| 1 | 30 | 50 | 0 | 9 | 2 | 12 | 76 |
| 2 | 62 | 117 | 34 | 37 | 34 | 37 | 68 |
| 3 | 98 | 43 | 77 | 53 | 76 | 77 | 22 |
| 4 | 39 | 61 | 20 | 34 | 30 | 41 | 33 |
| 5 | 59 | 63 | 23 | 29 | 36 | 38 | 40 |
| 6 | 63 | 4 | 8 | 87 | |||
Case 1
A male aged 16 years 9 months presented to the clinic with a left lumbar and right thoracic deformity. He had previously been treated with a Milwaukee brace. A geneticist diagnosed him with EDS type 6 (kyphoscoliosis type), based on his hyperextensible skin, excessive lower extremity scarring, and scoliosis.
Brace treatment failed and surgical correction was recommended. The patient underwent posterior spinal fusion (PSF) from T5-L4 with a hybrid construct and iliac crest bone graft (ICBG). The estimated blood loss (EBL) was 2,000 ml and there were no complications. At 2 years follow-up, he was asymptomatic with stable implants and was discharged from the pediatric clinic due to his age.
Case 2
A 13-year-old female with multiple medical problems, including congenital bilateral dislocated hips, congenital bilateral dislocated sternoclavicular joints, and perforated esophagus, was evaluated for scoliosis. She had been seen by multiple geneticists and was diagnosed with EDS type 7 (arthrochalasis type). She presented with a severe left thoracolumbar curve.
The patient underwent PSF from T3 to the sacrum with Luque–Galveston fixation. There were no intra-operative complications and the EBL was 2,000 ml. She did well until post-operative day 5, when she had a syncopal episode after a bowel movement. She then developed abdominal pain and distention with a low blood count, requiring multiple transfusions. Computed tomography (CT) demonstrated free fluid in the pelvis and about the spleen. Pediatric surgery was consulted, and a paracentesis was performed to diagnose a hemoperitoneum. Given the patient’s vascular fragility, the low likelihood of finding a source of bleeding, and her hemodynamic stability, the decision was made to observe the patient. Her blood count stabilized and she was discharged on post-operative day 16. About 2–3 weeks later, she presented to an outside facility moribund. Her hemoperitoneum evolved to massive intra-abdominal bleeding from a myriad of peritoneal micro-aneurysms. The general surgeon could not stem the bleeding and she expired days later. Unfortunately, no post-mortem examination was done to verify the cause of death and determine whether there was any implant-related cause for the abdominal hemorrhage.
Case 3
A 9-month-old boy was referred for scoliosis and torticollis. He was also noted to have hypotonia, as well as inguinal and abdominal hernias requiring repair. He was diagnosed with EDS type 6 (kyphoscoliosis type) by a geneticist. The hypotonia eventually resolved but the scoliosis progressed, despite bracing and casting.
Preoperatively, he had significant kyphosis measuring 79° from T2-L1. He underwent PSF from T3-L3 with a single MOE rod at the age of 3 years (Fig. 1). His deformity progressed and the instrumentation eventually failed, requiring replacement with a single Harrington rod at age 4 years. Distraction of this rod was done 17 months later. Less than 3 months postoperatively, hook dislodgement was appreciated, requiring revision surgery. This instrumentation subsequently became infected and was removed. At the age of 8 years 7 months, the coronal and sagittal deformities had worsened, with a kyphosis measuring 97°. He then underwent PSF from T4 to L3 with ICBG, with a mild deformity correction that remained stable through follow-up to age 17 years. Six to seven years later, this patient expired due to a cerebral aneurysm.
Fig. 1.
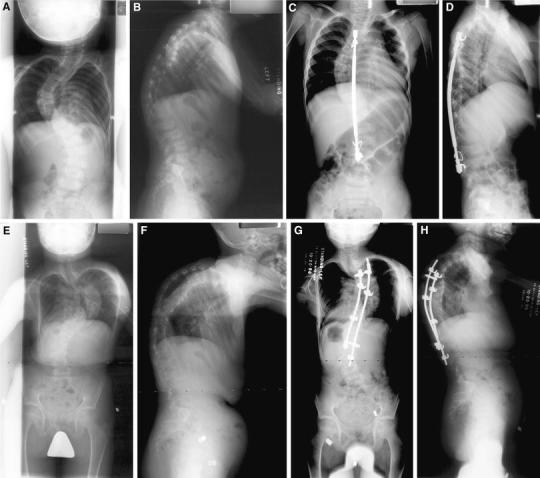
Preoperative anteroposterior (AP) (a) and lateral (b) radiographs of patient 3. Postoperative AP (c) and lateral (d) radiographs after posterior spinal fusion and instrumentation with a single MOE rod. AP (e) and lateral (f) radiographs after the removal of implants due to infection. AP (g) and lateral (h) radiographs postoperatively from final spinal fusion
Case 4
A boy with a complicated medical history including pectus excavatum requiring several surgeries, supraventricular tachycardia (SVT) requiring ablation, joint hypermobility, restrictive lung disease, and a congenital myopathy developed a progressive scoliosis refractory to brace wear. He also had spondylolysis with minimal spondylolisthesis at L5-S1. He was seen by a geneticist who diagnosed this patient with EDS type 3 (hypermobility type).
At 18 years of age, he underwent PSF from T4 to the sacrum with Dunn–McCarthy instrumentation. There were no intra-operative complications. The patient developed dysesthesia in the L5 nerve root distribution that resolved after the removal of S hook instrumentation over the sacrum after failed medical management with gabapentin. We believe that the dysesthesia was directly related to irritation from the implant. Over 1 year later, he developed an infection requiring irrigation and debridement, as well as removal of all of his spinal implants, with resultant clearance of the infection.
Case 5
A 10-year-old female presented to the clinic with a thoracic and lumbar spinal deformity. A geneticist diagnosed her with EDS type 3 (hypermobility type). She was offered surgery at presentation due to evidence of progression based on prior films and underwent PSF from T3-L3. An all-hook construct was used with a single TSRH rod and ICBG (Fig. 2). There were no complications. At the last clinic follow-up over 9 years later, residual lumbar decompensation to the left was noted, with a solid fusion mass.
Fig. 2.
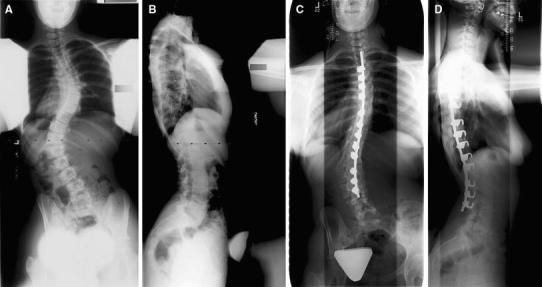
Preoperative AP (a) and lateral (b) radiographs of patient 5. Final AP (c) and lateral (d) radiographs 6 years after spinal fusion
Case 6
A female aged 13 years 6 months with EDS type 7 (arthrochalasis type), diagnosed by a geneticist, presented with a progressive thoracic scoliosis and thoracolumbar kyphosis (71°) that was refractory to brace treatment. She also had a history of joint instability with bilateral hip dislocations.
The patient underwent PSF from T3 to the sacrum with iliac screws, pedicle screws, and hooks. The EBL was 1,900 ml, with no complications. Excellent correction was achieved in both the sagittal and coronal planes. She is currently 5 months out from surgery and asymptomatic.
Discussion
The majority of previous case reports fail to document specific diagnostic criteria for EDS, which often rely on a clinical diagnosis based upon hypermobile joints and elastic skin. Unfortunately, these signs can be found in numerous disorders similar to EDS. Only one study has used stringent diagnostic criteria based on tissue biopsy [3]. Our series used strict diagnosis by a geneticist or genetic testing and excluded several children labeled as having EDS by only the spinal surgeon.
McMaster [2] described five cases of PSF in EDS, with all patients having excessive blood loss and hematoma formation requiring aspiration. There was no mention of neuromonitoring. Vogel and Lubicky [4] described four patients with neurovascular complications, including paraplegia, lower extremity weakness, and avulsion of segmental vessels. The patient with lower extremity weakness underwent a wake-up test, which was normal. One patient with paraplegia had no neuromonitoring, but did have an abnormal wake-up test. The patient with arterial avulsion had normal somatosensory evoked potentials (SSEPs) throughout the procedure and demonstrated no residual neurologic deficit. Akpinar et al. [3] described five patients, with four undergoing anterior and posterior surgery. One vascular complication was noted. No neuromonitoring was performed, and all patients underwent wake-up tests.
The current series of patients is similar to previous cohorts with respect to the mean curve, percentage of curve correction, and EBL (Table 4). The severity of EDS in patients from this cohort could not be compared to previous studies due to the lack of strict diagnostic criteria, as previously discussed. From a surgical standpoint, we were also fairly conservative. All procedures were performed from a posterior approach. Complications from an anterior approach in the EDS patient are well documented [5]. All but one patient in this cohort had neuromonitoring. Three patients were monitored with SSEPs only, and two with both transcranial motor evoked potentials (TcMEPs) and SSEPs. The use of neuromonitoring has been well documented as a means of predicting neurologic injury in idiopathic scoliosis [6].
Table 4.
Summary of previous studies
Despite our precautions, half of our patients experienced complications, some expected, others not. Our most serious complication was intra-abdominal bleeding postoperatively which led to patient death. Infections were noted in two patients, both of which were revisions. Multiple episodes of implant failure were also noted in patient 3; however, these failures were in a growing child operated on in a different era of spine surgery with vastly different equipment. The patient with a neurologic complication underwent PSF with Dunn–McCarthy instrumentation. One recent study found a 14% incidence of severe, neuropathic lower extremity pain with this type of instrumentation [7].
The severity of the problems experienced in our cohort cannot be overlooked. Despite a conservative surgical approach, half of the patients in this study experienced complications. The most severe complications were medical in nature, with additional but less severe instrumentation-related complications. Although instrumentation-related complications could potentially be different in the current era of spinal surgery, no major advances have been made in the medical management of EDS during the time course of this cohort. While the small number of patients in this cohort may not reflect the true incidence of complications, the surgeon choosing to operate on the EDS patient must do so with extreme caution.
Contributor Information
Brien Michael Rabenhorst, Phone: +1-806-7431704, FAX: +1-806-7431919, Email: brien.rabenhorst@ttuhsc.edu.
Sumeet Garg, Phone: +1-720-7776610, Email: garg.sumeet@tchden.org.
J. Anthony Herring, Phone: +1-214-5598471, Email: Tony.Herring@tsrh.org.
References
- 1.Beighton P, De Paepe A, Steinmann B, et al. Ehlers–Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers–Danlos National Foundation (USA) and Ehlers–Danlos Support Group (UK) Am J Med Genet. 1998;77(1):31–37. doi: 10.1002/(SICI)1096-8628(19980428)77:1<31::AID-AJMG8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.McMaster MJ. Spinal deformity in Ehlers–Danlos syndrome. Five patients treated by spinal fusion. J Bone Joint Surg Br. 1994;76(5):773–777. [PubMed] [Google Scholar]
- 3.Akpinar S, Gogus A, Talu U, et al. Surgical management of the spinal deformity in Ehlers–Danlos syndrome type VI. Eur Spine J. 2003;12(2):135–140. doi: 10.1007/s00586-002-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel LC, Lubicky JP. Neurologic and vascular complications of scoliosis surgery in patients with Ehlers–Danlos syndrome. A case report. Spine. 1996;21(21):2508–2514. doi: 10.1097/00007632-199611010-00021. [DOI] [PubMed] [Google Scholar]
- 5.Yang JS, Sponseller PD, Yazici M, et al. Vascular complications from anterior spine surgery in three patients with Ehlers–Danlos syndrome. Spine. 2009;34(4):E153–E157. doi: 10.1097/BRS.0b013e31818d58da. [DOI] [PubMed] [Google Scholar]
- 6.Padberg AM, Wilson-Holden TJ, Lenke LG, et al. Somatosensory- and motor-evoked potential monitoring without a wake-up test during idiopathic scoliosis surgery. An accepted standard of care. Spine. 1998;23(12):1392–1400. doi: 10.1097/00007632-199806150-00018. [DOI] [PubMed] [Google Scholar]
- 7.Walick KS, King JT, Johnston CE, et al. Neuropathic lower extremity pain following Dunn–McCarthy instrumentation. Spine. 2008;33(23):E877–E890. doi: 10.1097/BRS.0b013e3181877b99. [DOI] [PubMed] [Google Scholar]


