Abstract
Any lower limb discrepancy may be equalised by conservative means (insoles, prosthesis and orthosis). However, their long-term acceptance is low in regard to function, costs, expenditure and appearance. Timely epiphysiodesis is the best option in uniplanar deformities with adequate remaining growth and for patients whose predicted final body height is above the 50th percentile. However, many patients present late or with multi-planar deformities, which warrant more sophisticated operative approaches. The history of surgical bone lengthening comprises 100 exciting years of struggling, development and ongoing learning. The initial strategy of acute or rapid incremental distraction had lasted almost half a century until Ilizarov recognised the benefits of biological periosteum-preserving osteotomies and incremental lengthening at slow rates (1 mm/day) at a 4 × 0.25-mm daily rhythm, well appreciated as callotasis. In parallel, ring and wire constructs made complex three-dimensional axial, translational and rotational bone moulding possible. Taylor Spatial Frames—built on hexapod strut-linked platform technology as known from flight simulators—took limb correction to a more reliable, more precise and aesthetical level, all the more that the whole process became web-based. It represents state-of-the-art methodology and technology for complex, multi-plane deformities. Due to the significant risk of secondary malalignment, indications for lengthening by unilateral fixation have shrunken to moderate amounts of length disparity and uni- to bi-planar deformities in patients with still open physes. Mechanical or motorised, minimally invasively placed nails prevent muscle fixation and, therefore, ease rehabilitation, increase patient comfort and potentially shorten the overall time of sick leave and refrain from sports activities. Hence, they offer a valuable alternative for low-grade complexity situations. It remains to be proved if the significantly higher implant costs are compensated by lower treatment costs. Overall, limb lengthening, particularly in combination with multi-planar deformity correction, can still be an arduous endeavour. In any case, wise judgement of the patient’s deformity, medical and biological situation, psychosocial environment, selection of the appropriate method and hardware, as well as meticulous operating technique by an experienced surgeon are the cornerstones of successful outcomes.
Keywords: Leg lengthening, Taylor Spatial Frame, Callotasis, Ilizarov, Motorised nails
Introduction
Leg length discrepancies are frequent: about one-third of the population shows 0.5–1.5-cm disparities, 5 % more than 1.5 cm and about 1/1,000 have been prescribed a shoe lift [1–3].
Despite a lack of biomechanical data supporting a seemingly fundamental human anatomic principle, it is persistent orthopaedic common sense that the pelvis needs to be horizontal and the lumbar spine symmetrically loaded in a bipedal standing position and in the stance phase during gait. Leg length inequality is a correctable risk factor for knee osteoarthritis [4]. Hence, acquired or congenital disparities ought to be restored, though the apodictic and historic [5] 2-cm rule of acceptable leg length discrepancy is more enigma than science in light of the individual anatomic parameters such as pelvic width, absolute leg length, muscle force and proprioceptive capacity. Recent developments in deformity correction allow for a patient- and surgeon-friendly web-based bone restoration of any complex three-dimensional deformity. Nevertheless, there are still considerable biological limitations and challenges. The patient and his psychosocial, medico-economic, logistic and legal environment must be consented, educated and prepared for a potentially long-lasting journey paved with hazards such as delayed consolidation, pseudarthrosis, malalignment, joint contractures and dislocations, pain, infection, prolonged inability to work and refrain from school and sports activities [6–24].
The basic principles of bone generation and moulding—gentle, periosteum-respecting osteotomy followed by incremental 1-mm/day fragment separation and consolidation—are set. All current methods of gradual leg lengthening rely on distraction osteogenesis [25–29]. However, concurring implants and techniques for mechanical bone guidance such as Taylor Spatial Frames (TSFs), traditional Ilizarov ring and wire constructs, various methods for unilateral external fixation and intramedullary mechanical or motor-driven nails offer space for individual application given by the skills and preferences of the surgeon, the affordability, the patient’s needs and wishes, and the medical problem defined as a mixture of bony deformity and the condition of the joint, muscle and soft tissue compound adjoining the segment to be corrected.
We deemed it worthwhile to shine through the current body of knowledge. Not astonishingly in view of myriad patient variables, it is mostly formed by heterogenous case series and well remote from a high level of evidence.
History
The young and rocky history of surgical leg lengthening started only 100 years ago, probably when the German visionary surgeon Bernhard von Langenbeck 1869 claimed that 2–4-cm leg shortening causes significant impairment of function and that it should be equalised surgically by stretching of the bone.1 However, it lasted until 1903, when pioneer Alessandro Codivilla (Rizzoli Institute Bologna/Italy) performed femoral osteotomies in patients with coxa vara. Traction (25–75 kg) was applied by a cast and a transcalcaneal wire [30, 31]. The understanding of the physiology of bone formation and the role of soft tissues in the lengthening process was very limited and opposed to the eagerness to lengthen as much as possible, often “acutely” as a one-stage procedure up to 8 cm. The conditions, however, were adventurous and often torturing for the patients, resulting in nerve lesions, muscle convulsions, pseudarthrosis and malunions, not to speak of the appalling pain at this pre-anaesthetic age or even death on the operation table [32]. Louis Ombrédanne 1913—first gradual (5-mm/day) lengthening on a femur using an external fixator with one pin above and one pin below the osteotomy—achieved an additional 4 cm [33]. Vittorio Putti conjoined the former ideas of external fixation, Z-shaped osteotomy and continuous but slower distractions (2–3-mm daily) for up to 8-cm lengthening, which resembles modern unilateral methods. However, the apparatus and the pin purchase in the bone were not strong enough to promote the method’s breakthrough [34]. Probably the first to recognise and practise the importance of delaying lengthening after the osteotomy was August Bier, who reported on femoral lengthening in 1923 [32]. He failed since he cut the periosteum completely at the osteotomy site. Leroy Abbott from St. Louis further developed the method of callus distraction in the mid-1920s with further refinements by others in the 1930s, for example, stronger fixation, periosteum preservation and a 2-week interval before commencing distraction [32]. A broad array of biological and hardware trials and errors over time such as fascia resection, wide soft tissue dissection to overcome soft tissue resistance or stirrup-shaped irons for pin fixation witness the manifold frustrations, obstacles and challenges in the history of bone lengthening, not to speak of subsequent foot and knee deformities most often preventing the improvement of gait and function, even in the case of successful bone restoration [32]. Promising steps such as Wittmoser’s first ring fixator were not recognised as such [35]. The decisive further methodological and hardware refinement was a genius single man’s pioneering effort: Gavril Ilizarov (1921–1992) was a young general practitioner geographically and medico-socially isolated in southwest Siberia (Dolgovka/Kurgan), faced with a high load of bone tuberculosis cases, desperate World War II veterans and material shortness. He treated countless infected pseudarthroses (in the pre-antibiotic era!), lengthened stumps after amputations, corrected complex post-traumatic deformities and filled bony defects based on the principles of desmoid ossification by segmental transport after percutaneous corticotomy, a latency period of some days, semi-rigid fixation and a defined distraction mode of 4 × 0.25 mm/day. Bone lengthening became possible without bone grafting and was followed by further milestones: in 1951, the development of metal rings and tensioned K-wires [36] for fracture treatment; in the early 1960s, the first successful lengthening of the lower extremity up to 25 cm [36]; distraction–compression; promotion of tissue nutrition and joint mobility by semi-rigid fixation; full weight-bearing and physiotherapy [37]. Since the Moscovitch medical elite mocked him, it was not until 1967 when he gained recognition by successfully treating the former Olympic gold medal winner, high jumper Valeriy Brumel after his motorcycle accident [36]. The isolation of communist Russia hindered the further spread of this revolutionary approach until De Bastiani (Verona, Italy) exposed it to the world in the early 1980s [38]. Unaware of Ilizarov’s progresses, unilateral fixation, fast lengthening (2–4 mm/day) after cutting restraining soft tissues (including periosteum), followed by bone grafting and plate osteosynthesis after the distraction phase (Wagner method) [39, 40] was temporarily popular in the Western world in the 1970s and 1980s but it was problem-prone: premature growth arrests, bad bony regenerate and multiple revision surgeries were common. It was eventually abandoned, as was epiphyseal distraction (acute or sub-acute epiphysiolysis without osteotomy), which was often acutely painful and also provoked physeal arrest [41–45]. In contrast, Ilizarov’s principles have stood the test of time. At the beginning of the 1990s, modern web-based hexapod platform technology2 (1994, Taylor Spatial Frame [TSF]) and motorised, fully implantable, solid intramedullary nails have lifted them to better predictability, more comfort and lower risk [46, 47].
Indications for leg lengthening
Over the last 100 years, indications have shifted from leg length discrepancies and deformities due to poliomyelitis, war wounds, osteomyelitis and malunited fractures to congenital problems such as femoral deficiencies, simple femoral hypoplasia, fibular hemimelia and tibial aplasia, hemihyper- and hemihypotrophy, and a long list of acquired problems, such as post-traumatic growth arrest, post-infectious issues, avascular necrosis, Perthes’ disease, Blount’s disease, skeletal dysplasia, rickets, syndromes, Ollier’s disease, enchondromatosis etc. [48–50]. The composition of causes varies [12, 51–55]. Some series include bilateral lengthening for short stature, which is not the focus of this article [54]. Ring fixators are predominantly used for tibiae [48, 49], and mechanical and motorised nails mainly for femora [51, 55, 56]. As a rule of thumb, a leg length discrepancy of more than 2 cm is regarded as an indication for therapeutic measures, 2–4 cm and enough growth in an individual above the 50th percentile for height an indication for growth modulation (epiphysiodesis) and discrepancies of more than 4 cm an indication for callotasis.
The average amount of lengthening ranges between 2.5 and 5 cm (min. 2 cm to max. 17.4 cm) for Ilizarov ring fixators (IRF) [48, 57, 58] and intramedullary nails [16, 46, 50, 59–64] and around 2.5–3 cm (min. 8 mm to max. 80 mm) for TSF [48, 65]. The latter is mostly indicated for complex three-dimensional problems, where the shortness is not the most prominent issue. Though callotasis and complex deformity correction is predominantly a paediatric orthopaedic field, the age of patients ranges from as young as 2 years up to 70 years (!). Patients treated with ring fixators and unilateral external fixators have an average age of around 12–16 years [48, 49, 65], whereas m`g [50, 53, 59–61] or by solid nails [16, 46, 51–56, 60, 62, 63, 65] constitute young adults around the age of 18–25 years.
Baseline diagnostics
It is of utmost importance that the diagnostic algorithm does not only focus on the bony deformity but creates a bigger picture including the patient’s physical and mental status, as well as the local biology. In summary, this allows to gauge the level of difficulty and risk [50] and to inform and educate the patient by weighing out all the potential hazards: joint instabilities ranging from mild laxity to fixed subluxation or even dislocation, fixed flexion deformities and decreased range of motion of the knee, osteoarthritic changes, poor bone or soft tissue quality, previous infections and general medical issues like smoking, diabetes and regenerate-inhibiting medication, among others. Psychological assessment (intelligence, compliance, social environment) to determine if the patient and family is suitable for the proposed endeavour and contact with other patients might be wise in order to define the expectations [16, 66].
The core process is precise three-dimensional deformity analysis in order to set the goal and choose the type of correction. Only the minority of patients presents with mere leg shortening. Most also present with frontal plane deformities or, to a lesser degree, with sagittal and rotational abnormalities [48]. The deformity is graded as type I (one-dimensional) to type IV (four-dimensional), which allows for the comparison of results of lengthening and axial deformity corrections in the dependence of the complexity [48].
The basic radiographic work-up includes standing patella-forward full-length anteroposterior and lateral radiographs of both legs (orthoradiograms) with blocks placed under the foot of the short leg to level the pelvis. A computed tomography (CT) scan is ordered in case of rotational malalignment to determine femoral anteversion and tibial torsion. Modern imaging systems, e.g. sterEOS®, simultaneously depict the whole erect human skeleton in orthogonal planes with less irradiation and projectional errors and the option for three-dimensional reconstruction [67].
Manual, digitised or software-based deformity analysis includes a malalignment test, centre of rotation analysis (CORA), determination of the mechanical axis and its relation to the centre of the knee and standardised measurements of the joint orientation angles (e.g. mechanical lateral distal femoral angle [mLDFA], medial proximal tibial angle [MPTA]). Deformity analysis and the choice of the site of osteotomy determine the amount of angular and translational correction. A thorough clinical status focuses on joint instability and range of motions, the condition of the soft tissues and includes the neurovascular status. Functional judgement by an experienced physiotherapist, force measurements (isokinetic testing) and gait laboratory are helpful tools in the decision-making process of complex cases.
Alternatives to operative lengthening
Before surgery is considered, the natural history and conservative options should be discussed in cases of isolated leg shortening without concomitant axial deformities. In principle, every discrepancy can be equalised by conservative measures ranging from simple insoles to shoe lifts, orthosis and prosthesis. The outlook of life-long orthotechnical aids, their repeat adaption, the summarising costs which may be larger than for surgical leg lengthening, impaired biomechanics, uncorrected bare-footed gait, limited work and sports capacity and unattractive aesthetics including limited shoe choice, let most patients seriously weigh up the pros and cons compared to callotasis. However, much more appealing in terms of patient satisfaction, cosmesis, risks, costs and surgical expenditure is an epiphysiodesis (growth plate tethering or surgical drilling). Though technically simple, its limitations may consist of predictability and the patient’s reduced final height. Pre-conditions are, therefore, discrepancies of 2–5 cm in individuals with adequate growth left and taller than the 50th percentile for height (women 164 cm, men 176 cm). Since timing is crucial, the prediction of remaining growth and final leg length discrepancy is a condition sine qua non [68, 69]. Acute shortening by bone resection followed by gradual lengthening is a possible strategy for complex fractures, infected non-union, congenital pseudarthrosis, osteomyelitis and bone tumours [70–73].
Osteotomy techniques and biology of callotasis
The fundament of successful iatrogenic new bone formation is an adequate osteotomy technique: prevention of thermal damage, careful bone separation, creation of vascular bone surfaces, respecting the periosteal sleeve and—after a latency period of several days—gradually pulling the two bony segments apart are the biological cornerstones for good callus formation [74].
Preservation of periosteum as the main source of blood supply is critical [46, 75–81]. Its disruption significantly decelerates bone formation [75, 82]. Ilizarov and others firmly recommended corticotomies to preserve medullary blood flow [26, 80, 83]. However, this is difficult to perform and experimental work revealed that complete bone separation (osteotomy) is equally effective [76, 82, 84–87]. Dual-energy X-ray absorptiometry (DEXA) and histomorphometrical studies showed that the periosteum produces up to five-fold more callus than bone marrow during lengthening [74, 75, 88, 89]. Hence, most surgeons nowadays perform percutaneous formal osteotomies varying in technical details depending on personal preferences, site of osteotomy and type of implant used. However, they all adhere to the same above-mentioned principles [25, 59] be exerted by the Gigli saw [86, 90], osteotome [25], oscillating saw [82], low-energy osteotomy or by multiple drill holes and osteotomy completed with osteotomes or manual osteoclasis [51, 55, 63, 77, 90, 91]. Intramedullary saws are used for diaphyseal osteotomies mainly in conjunction with solid nails but are prone to breakage or disassembly [59, 66]. Dome osteotomies are an alternative for mono- or bi-planar deformities and if no fragment translation is required. The completeness of the osteotomy and the correct functioning of the lengthening apparatus (e.g. motor of the nail) should be verified intra-operatively.
The choice of osteotomy site (diaphyseal, metaphyseal, proximal or distal) depends on the localisation of the deformity, the correction strategy, biological issues (e.g. previous surgery, soft tissue condition, tibiofibular synostosis) and the implant used [50]. In case of lengthening by nails, their geometry and the localisation of the telescoping parts need to be considered [62]. The pattern of secondary malalignments during distraction is determined by the muscles crossing nearby and, therefore, is also site-dependent (Fig. 1).
Fig. 1.
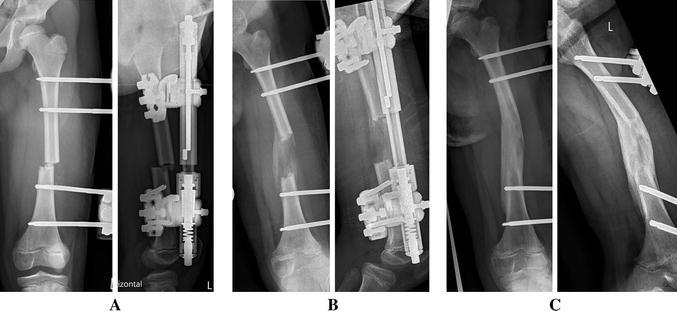
An 8-year-old girl with 4 cm congenital left femoral shortening (Pappas type IX). a Day 1 after diaphyseal drill hole osteotomy. Normal alignment, 50 % lateral translation. b 7.5 weeks after the index procedure, 6 weeks of distraction. Secondary 10° varus deformity. Timely callus formation and ossification. c Four months follow-up prior to hardware removal. Anteroposterior and slight oblique view to prevent bone–fixator overlap in order to provide a free projection of the femur. Three of four cortices are consolidated. Residual varus angulation, full restoration of length
The recommended latency time between surgery and the start of distraction is 3–10 days [8, 10, 13, 28, 92]. After detailed oral and written instructions are given to the patient, distraction is usually effected with classic 1 mm/day (rate) [26] divided into 4 increments/day (rhythm) [8, 28]. The ridge walk between non-union and premature consolidation of the distraction gap requires experienced and watchful adaption of latency period and distraction mode to the local vascularity, angular lengthening, site of osteotomy and patient factors such as age, smoking habits, bone quality, vascular status, aetiology and the stability of the apparatus [19, 93, 94].
Children promote bone more easily and, due to the thick, encircling, well-vascularised muscle envelope, the femur heals faster than the tibia [10, 95]. Drug-promoted bone formation has, hitherto, not found broad clinical application, though bisphosphonates improve bone mineral density, volume and mechanical properties of regenerate bone in immature rabbit models [96, 97]. Others have negative impact, such as non-steroidal anti-inflammatory drugs and chemotherapeutical agents [46, 98].
The metaphyseal area is better vascularised, has a bigger bony surface, therefore, provides better stability and easier bone formation, shows thin cortices which are easier to separate, but the many local muscle insertions require higher distraction loads [8, 99]. Intra-membranous bone formation is the underlying biological process of callotasis [8, 26, 27, 78, 80, 93, 100, 101]. Typically, it evolves in defined radiological and histological zones: central type I collagen with adjacent vascular periosteal and endosteal ingrowth comprising osteoblasts depositing osteoid along the collagen bundles, laterally neighboured by longitudinal columns of mineralised bone stretching out parallel to the distraction forces and eventually bridging transversely [8, 100, 102–104]. Bone appears already around the tenth day of distraction [105]. Parallel to increasing mineralisation, also the load—the force to achieve further distraction—increases [79, 99]. The tissue in the distraction gap contributes to more than two-thirds of the overall load [106]. High loads indicate premature consolidation or incomplete osteotomy and—vice versa—low loads indicate non-union [99]. Load increases after every increment of distraction and then returns to a baseline, which reaches increasingly higher levels [107]. Apart from the new regenerate bone, also soft tissues (skin, fascia, muscle, periosteum) and friction of the implant, e.g. nail versus bone, contribute to the required force [107].
Vascularity is crucial: blood flow is increased at the site of bone formation but also in remote areas of the same bone [8, 79, 102]. At the end of the distraction phase, the regenerate begins to consolidate and bone remodelling occurs until the normal cortico-medullary architecture is reached and full, unprotected weight-bearing is possible. Insufficient stability and/or vascularity (e.g. thermal damage) entail micro-haemorrhages and necrosis with the subsequent formation of (fibro-)cartilage, cystic necrosis and, eventually, non-union [8, 27, 82].
Plain radiography—weekly to bi-weekly during distraction, monthly in the consolidation phase and 3–4 monthly after consolidation—is still the most useful methodology to monitor the fragment alignment, as well as the quantity and quality of bone formation [60]. More than a typical central 4–6-mm-thick radiolucent zone corresponds to an exaggerated rate of distraction and vice versa [38, 92, 108–111]. The assessment of the shape, type and quality of regenerate bone may be helpful to predict the stability [112]. In daily clinical use, the most common definition of consolidation and stability to allow for the removal of hardware is tricortical radiographic consolidation on two orthogonal radiographs [92]. For more specific requirements, quantitative CT and finite element modelling offer predictions of bone stiffness [106].
Numeric parameters—distraction–consolidation time (DCT) and healing index (HI)—aim at quantifying the quality and speed of bone formation. The consolidation time (end of distraction to bone stability which allows hardware removal) is about twice as long as the distraction time in children, but may be three to four times longer in adults [22, 92]. The consolidation index quantifies the time to consolidation per centimetre of distraction gap [59]. The healing time (period between index operation and full weight-bearing without crutches) is a measure for nails [55].
The healing index (DCT/cm) [25, 89] is the most widely used parameter. It amounts usually 1 month/cm in children and 2–3 months/cm in adults [8, 77], plateaus for long distances and increases for very small gaps [92]. It depends on the complexity of correction [22], the bone [21, 25, 92], the level of osteotomy [92], number [22, 29, 92] and form of osteotomy [113], aetiology [21] and age [21, 22, 49, 92] (significant lower for <14 years of age) [49]. Figures regarding the role of the type of fixation are contradictory and should be handled with care, as there is a bias to more complex deformities for ring fixators [49, 114]: Albizzia nails 26–107 days/cm [59] (all femur), ISKD nails 36 days/cm [19, 52], Fitbone nails average 35–42 days/cm (18.8–70.9 days/cm when the femur is longer than the tibia) [46, 54, 56, 63], lengthening over nail 36.9 days/cm [61] and TSF, IRF and unilateral external fixation average 57 days/cm [49, 115].
Effects of bone lengthening on surrounding tissues
Gradual bone lengthening has a negative impact on the surrounding muscles through stretch, impalement by pins and wires, pain and inflammation. The speed of the gain in length during callotasis with a 1-mm/day rate is about four to eight times faster than during the adolescent growth spurt with its temporary muscle shortenings [8]. No wonder that the clinical challenges of muscle distraction and subsequent decrease of adjacent joint range of motion go in parallel with the amount of lengthening. This is reflected by histological changes after lengthening of more than 30 % of its original length [22, 116–119], as the whole muscle from origin to insertion is stretched [81]. The elastic limit of stretched muscles (strength–strain curve) is 10–15 % of the length at rest. Excessive stretch leads to plastic deformation and subsequent contractures, which commonly affect muscles spanning two joints (rectus femoris, hamstrings). There are only few data about the loss of muscle power during the distraction–consolidation process and the speed and amount of recovery thereafter. There is a small residual decrease in muscle strength and power after surgical lengthening without any impact on the activities of daily living [120]. Pre-operative muscle training as a preventive measure and a post-operative intense rehabilitation programme including continuous passive motion, extension splint, strengthening and stretching exercises, as well as proprioceptive training, are mandatory until the pre-operative level is reached [66]. Nerves and vessels adapt in length during the distraction process and recover from temporary degenerative changes within 2 months after the halting of distraction [104]. Excessive gradual (>20–30 %) or acute distraction (>15 %) may both lead to partial or complete loss of nerve potentials [121, 122].
Joint cartilage may be exposed to reactive forces which increase linearly with distraction [123]. In addition, non-weight-bearing and decreased range of motion diminishes nutrition of the cartilage and may support histological changes which appear after 30 % lengthening in animal experiments [124, 125].
Physeal cartilage shows experimental histopathological changes secondary to increased axial load [124]. However, alterations of the growth rate were never observed in a clinical setting [126].
Technical options
The common pathway of all leg lengthening methods is 1-mm/day distraction after an initial latency period. Technical options comprise external unilateral or ring fixation (Ilizarov, TSF), intramedullary solid nails as a mechanical or motorised standalone technology or in combination with an external device during the distraction phase (lengthening over nail). Any external fixation bears the drawback of cumbersome, visible hardware and of pins or wires transgressing soft tissues. The latter may cause pain by inflammation of the pin tracks, impaired range of motion and prolonged rehabilitation by muscle impalement. High expenditure for pin site care to prevent superficial or deep infections is routine: two fingerbreadths of clearance beneath the skin, patient instruction, daily change of gauze wraps, local disinfection, debridement of any necrotic tissues or a short course of oral broad-spectrum antibiotics in case of an inceptive infection is mandatory [22].
Unilateral external fixation
This is best indicated for simple lengthening over a short to medium distance. It is applicable in any age group. Though it avoids the bulk and multi-pin or -wire fixation of ring fixators, it does not prevent pin muscle transfixation, which is mainly an issue in case of femoral lengthening. The appealing ease of surgical application (percutaneous placement of 4–6 pins, mounting of the clamps and bar) contrasts with the cantilever design and eccentric load, which offer less mechanical control than ring constructs [49]. Monolateral fixation is often not able to withstand the muscle forces during excessive lengthenings [127]. Secondary malalignment, premature cessation of lengthening, unilateral premature consolidation and realignment procedures for >5° angulations under anaesthesia are potential sequelae (see Outcomes and complications).
External ring fixation
One can solve any three-dimensional, multi-plane, 6-axes deformity problem including fragment translation and contractures at any age with a custom-made frame. Ring constructs, usually with hybrid wire and screw bone fixation to reduce the number of soft tissue transfixations, provide the greatest versatility and best mechanical control. Adjacent joint instability may be temporarily controlled by bridging constructs. For complex cases with fragment rotation and translation, traditional IRFs (Smith and Nephew, Memphis, TN, USA) require less workspace (minimal frame height) but more planning, surgeon experience and, sometimes, repeat change of constructs, e.g. repositioning of hinges, compared to TSF [128]. In a comparative study between 129 TSFs and 79 IRFs, there were 90 % achievements versus 56 %, respectively, with increasingly diverging results for increasing complexity. Irrespective of the method used, there is an overall decreasing percentage of full achievement with increasing complexity (Fig. 2).
Fig. 2.
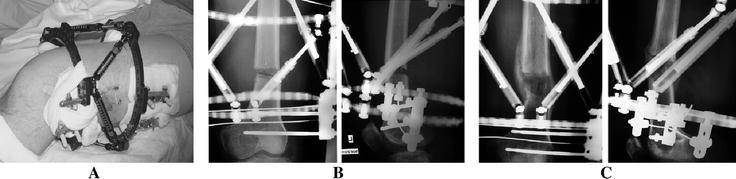
A 24-year-old man with 2-cm shortening, 10° external rotation, valgus and slight extension deformity of his left femur due to partial lateral growth arrest after traumatic epiphysiolysis type Salter-Harris II of the distal femur at the age of 13 years. The centre of rotation analysis (CORA) is, therefore, juxtaarticular at the level of the growth plate. a Taylor Spatial Frame (hexapod) with hybrid fixation by tensioned Ilizarov wires and lateral half-pins. b Three weeks after the index procedure (percutaneous metaphyseal distal femur drill holes osteotomy completed by an osteotome) and 7 days of distraction with a rate of 1 mm per day and a rhythm of 4 × 0.25 mm per day. The osteotomy gap is opening correctly. c 3.5 months follow-up. Timely ossification of the regenerate bone. Since the osteotomy is proximal to the CORA, a slight lateral translation is necessary to compensate for the medial shift of the condylar fragment with varisation. In the lateral view, a minor correction into flexion with slight anterior translation was achieved as planned
Compared to traditional Ilizarov constructs, TSFs (Smith and Nephew, Memphis, TN, USA) and alternative hexapod constructs provide less complications, more patient and surgeon satisfaction and comfort, and—above all—more precision without frame adjustments during the process [48, 65, 129]: two full or partial rings (Stewart platform) linked by six manually adjustable, oblique telescopic struts allow for the free combination of rotation and translation in a space only limited by the length of the struts but without any additional special hardware like hinges. Based on the patient’s deformity, the soft tissue data (definition of structures at risk), the desired result, the architecture (mounting, size) of the frame and the relation between origin (reference fragment, virtual hinge) and corresponding (moving fragment) points, a web-based software algorithm delivers the correction protocol and velocity with mathematic accuracy [48, 130, 131]. Adjustments of strut prescription usually do not necessitate return to the operating room and do not cause extra morbidity for the patient [49]. The lack of small rings and struts for young children and the high costs for a TSF are the only limiting factors.
Intramedullary fixation: general considerations
Solid nails in combination with early removal or complete avoidance of external fixation promise diminution of fixator-associated issues such as infections and contractures, as well as better tolerance and comfort [50, 132–134]. This particularly holds true during the unpredictable waiting time from the end of distraction until full consolidation. A nail protects the regenerate and provides enough rotational and axial stability to then allow return to normal activities. Diminishing knee stiffness issues by preserving the soft tissue envelope and the risk of refracture after hardware removal are further benefits [60]. Ilizarov’s core recommendation is preservation of the endosteum by cortical osteotomies [83]. Some authors hypothesise that reaming may compromise endosteal blood supply and, thus, affect the quality of the regenerate [135, 136]. However, there is evidence that the periosteal blood supply may increase after intramedullary nailing, which is particularly important for effective distraction osteogenesis [76, 137]. This is confirmed by clinical observation in that reaming does not inhibit bone formation in the distraction gap—in contrary—that revascularisation after reaming often provides abundant new bone formation supported by better stability, earlier functional loading and less osteopaenic changes than with external fixation [16, 50, 59]. Correction of angular and rotatory deformities at the time of corticotomy is possible but limited in comparison to ring fixators [50, 56], usually to 6–9° [51]. The amount of lengthening depends on the nail design. Excessive lengthening is possible with two consecutive implantations of nails.
Lengthening over nail (LON, monorail method)
A different strategy for the distraction and consolidation phases aims at reducing the total fixator time by simultaneously placing an intramedullary solid nail at the time of osteotomy and by removing the external device at the end of distraction [50, 60, 138]. This concept was introduced in 1956 by Bost and Larsen [11]. LON is technically more demanding than the placement of a ring or unilateral fixator: after reaming, the nail is temporarily placed, then the pins and wires beneath and distally beyond the nail, followed by nail removal, placement of the external fixator, osteotomy and definitive nail insertion. After the distraction phase, the nail is locked to stabilise the gained length and axis before removal of the fixator. In the earliest cases, the nails were not solid enough to stabilise through the consolidation phase but fulfilled the minimal goal of stable alignment through the distraction phase. Premature removal of the fixator may lead to fracture, delayed union, pseudarthrosis, loss of length or malalignment [50, 139]. New-generation solid, locked nails unify both guidance of and stability for the regenerate [60]. The main concern of combining external fixation with solid nailing is deep infection. The risk ranges between 5 and 15 % [50, 60, 132]. The time with external fixation is significantly reduced, e.g. down to an average of 20–26 days/cm lengthening, which was much less than the healing index of 37 cm, which did not differ between methods [60, 61], or 4 months total time for a LON group compared to 7.5 months for the IRF group in a study with matched groups [50]. Blood loss and costs were higher in the LON group. However, only hospital charges and implant costs were taken into account, but not the overall treatment costs including physiotherapy, outpatient visits or unfitness for work. The type and severity of complications was comparable between the groups except superficial pin tract infections and refractures (more with IRF) [50, 61].
Intramedullary lengthening nails
As opposed to LON, the nail is exposed to full load during distraction and needs to withstand the eccentric and cyclic loading during weight-bearing during the consolidation phase. Full weight-bearing without crutches is allowed when radiographs show newly formed bone bridging two cortices. After Ilizarov’s fundamental appreciation of regenerate biology and guided three-dimensional bone moulding, the development of expanding intramedullary nails formed the next milestone. They apply to more simple deformities after the end of growth and require appropriate medullary canal sizes and bone lengths. At the end of the 1970s and the beginning of the 1980s, the first intramedullary lengthening devices appeared: in 1975, a hydraulic pressure system and a nail to lengthen the femur [140] and in 1977, an extension nail with external driver passed through the soft tissues in a canine experiment [141]. Bliskunov used the patient’s own energy source (movement between the femur and iliac wing) to generate length. All these open-source devices with external components directly linked to the nail and medullary canal failed due to infections and unbearable pain [141, 142].
The first fully implantable solution was presented in 1978 [143]. In the late 1980s, clinically applied mechanical devices were Albizzia [1, 16, 88], then soon after ISKD nails [62] and, in the early 1990s, the so-far unique motor-driven implant, the Fitbone nail [46, 47]. The driving unit for gradual controlled distraction is the main challenge: enough force—at least 700 N—and a non-invasive activation are the basic requirements [144]. The potential advantages are many: fewer scars, improved aesthetics, better body image and psychological well being, no irritation by pins and wires, reduced pain, uncommon infections, secondary axial deviation avoided, less joint stiffness, higher activity level during lengthening consolidation [62], faster rehabilitation [6], less risk of neurovascular compromise due to wire or screw insertion, and improvement in the ability to work during and after treatment [59, 62, 145, 146]. Deep vein thrombosis has been reported in 3–20 % [55, 56, 60]. However, the risk of pulmonary embolism is minimal since the osteotomy is performed before nail insertion. Nevertheless, there are also some limitations with nailing: the axial deformities need to be addressed at the time of nail insertion. Deformity correction is possible either directly with the nail or in combination with a simultaneous second osteotomy and plating as effected with Fitbone nails [16, 64, 147]. The amount of possible correction depends on its localisation, soft tissue conditions, medullary canal size, the amount of translation required, available nail diameters, types and lengths. The osteotomy level is dependent on the geometry of the nail. If it is too far away from the CORA, the amount of fragment translation needed for anatomic restoration may prevent nailing. Retrograde planning accounts for any translational and angular changes and uncovers limitations for nailing [64]. The concern that lengthening along the anatomical femoral axis [6] causes lateral shift of the mechanical axis and, thus, produces a valgus deformity does not hold true [50, 60, 63]. Since intramedullary nailing precludes secondary axial adaptions during the lengthening process, as it is possible with external devices, precise operative technique based on a meticulous pre-operative strategy is a condition sine qua non for full achievement. The intra-operative use of a grid plate with radio-opaque straight lines, which is placed underneath the patient on a radiolucent table, is helpful to assess the relation between the line between the mechanical axis and the middle of the knee joint [63]. In soft metaphyseal bone, it may be difficult to maintain the nail position for perfect alignment. Anteroposterior placement of bicortical so-called poller screws in close proximity to the implant block its position [148]. Rotational control with Steinmann pins is recommended [56]. An additional osteotomy or accurate choice of entry point and reaming may compensate for the physiologic antecurvatum of the femur [46].
Long lengthenings up to 20 cm have been reported with IRF [57, 58]. This exceeds the maximal lift of any available nail. Subsequent change of nails, simultaneous femoral and tibial lengthening or combination with contralateral epiphysiodesis are possible strategies [32, 64]. Previous infections and open growth plates are relative contraindications for nailing [16, 149]. Femoral nails can be inserted in a retrograde, through-the-knee technique (straight nail), antegrade through the fossa piriformis (straight nail) or over the greater trochanter (curved nail). Avascular necrosis of the femoral head are potential hazards of antegrade straight femoral nailing [150]. The reliability of the device (blockage, breakage, running back), difficulties upon hardware removal, more precision in planning and demanding operative technique, higher operation time and learning curve, accurate measurement and monitoring of lengthening are potential challenges.
The clinically most widely used, purely mechanically activated first-generation device was the Albizzia nail (GEN = gradual elongation nail, DePuy, Villeurbanne, France), a pure femoral steel implant named after a rapidly growing, flowering tree [1, 59, 66]. It consisted of two telescopic tubes (outer threaded, inner rod) and came in 11–15-mm diameters and 24–32-cm lengths, which provided 6–10 cm of elongation but no shortening mechanism. Special, shorter custom-made nails were possible. Patients themselves lengthened through torque activation of a ratcheted distraction mechanism by inter-fragmentary alternating internal and external rotation (15 rotations equalled 1 mm) of more than 20°. In consecutive case series, significant pain with ratcheting (twist and shout nail) occurred in 5/24 patients, 13/41 needed some ratchetings under general anaesthesia and 4/24 had to stop lengthening [16, 59]. To the authors’ current knowledge, the product is no longer commercially available. Actually, there are two commercial implants available: the mechanically driven ISKD nail (developed in Orlando/Florida) and the motorised Fitbone nail (Munich/Germany).
The intramedullary skeletal kinetic distractor (ISKD, Orthofix Inc., McKinney, TX, USA) [62] comes as straight humeral, femoral and curved tibial titanium alloy nail of 10.5–14.5 mm in diameter and 50–80 mm of distraction. It bears significantly less complications than the Albizzia nail. It transforms the physiologic forces of as little as 3° rotation during normal gait (or manually up to 9° with fewer oscillations than with walking) by an inherent ratchet mechanism (roller clutches and threaded rod) into a one-way, irreversible distraction. The distraction rate is supervised by an external hand-held magnet-based sensing device [62]. Premature or delayed consolidations reflect difficulties to control the distraction rate and rhythm [19, 151]. Patient compliance is important since lengthening depends on the activity level and tolerance of torsional movements [51]. For slow starters, even manipulation under general anaesthesia may become necessary [55]. In reality, failure to obtain the desired end length occurs in up to 18 % and secondary implant failure (blockage, break) in up to 36 % [55]. The reliability for tibial lengthening (45 %) is much lower than for femora (90 %) [55]. About half of the difficulties are directly implant-related [55]. In a comparative study, the distraction control with ISKD was difficult and unexpected surgery more frequent (6/12 vs. 1/22), but the regenerate was better than in the LON group [53].
The Fitbone (Wittenstein Intens, Igersheim, Germany) is a motorised steel nail [46, 47] (Fig. 3). Its fully implanted, hermetically enclosed electromagnetic motor delivers torque (1,800 N peak force, which is twice the force required to distract femora [144, 152]) which is converted to axial movement by a gear and spindle mechanism. The patient activates and controls the system by a transmitter (induction current) over a subcutaneous receiver system (reception antenna). There are two types of nails available: the Fitbone SAA (Slide Active Actuator) straight nail for antegrade femoral placement has a slide hole, an external diameter of 13 mm and is available in lengths between 260 and 520 mm. Lengthening of up to 85 mm and bone transport of up to 200 mm are possible. The Fitbone TAA (Telescope Active Actuator) nail is a telescopic version with a diameter of 11 mm in the shaft and 12 mm near the joint. There is a straight 24.5-cm-long version for retrograde insertion into the femur and a curved 22.5-cm-long version with Herzog angulation for anterograde placement into the tibia. Lengthenings of up to 80 mm in the femur and 60 mm in the tibia may be achieved. Custom-made nails, e.g. for stump lengthening after tumour resections, are possible. The company’s restricted centre of excellence licensing programme confines its use to one institution per country. A high implant reliability and success rate has been reported in clinical series [20, 46, 54, 56, 63, 64, 141, 147].
Fig. 3.
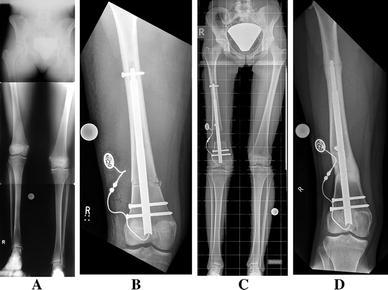
a Pre-operative long leg standing radiograph of a 14-year-old girl with an idiopathic femoral leg length discrepancy of 5 cm. b Post-operative radiographic control after the implantation of a Fitbone TAA (Telescope Active Actuator) femoral nail. c Long standing radiograph at the end of distraction after 38 days (1.18 mm/day), displaying a straight leg axis and the correction of length on the operated side of 4.5 cm. d Complete remodelled femur (1 year follow-up) after a consolidation period of 5 months
Outcomes and complications
Simplistically spoken, the success of leg lengthening is a result within 5 mm of the desired length [12]. However, a comprehensive assessment of outcomes also includes the range of motion of adjacent joints, the degree of limp, joint angles, pain and the performance in daily life, work and sports activities [50].
The retrospective character, heterogeneity and relative small size of the reported series and the lack of standardised assessment and reporting put expressiveness at a low evidence level. Nevertheless, one can still extract some helpful facts, figures and recommendations which may be supportive for patient and family counselling and the manifold decision-makings a surgeon is confronted with before, during and after the lengthening. Since the amount and rate of surgical bone lengthening is not a physiological process, some sorts of difficulties are prevalent with all methods. Most do not affect the final outcome and do not require operative interventions (Grade I, problems), but some do (Grade II, obstacles) or are severe and result in minor or major permanent sequelae (Grade III), despite unplanned surgery [13, 22]. Results and complication rates are, above all, dependent on the surgeon’s experience [13, 119] and the length of distraction [13, 119, 153]. There seems not to be a difference between femoral and tibial lengthenings, not between congenital and acquired problems but higher age, severity of deformity, unilateral fixation and amount of acute correction may have some additional negative impact [13, 154]. The figures for complications according to Paley [22] are biased, since IRF and TSF are commonly used for more complex cases: they range from 46 to 72 % for external fixation [13, 17, 22], up to 60 % for LON [60], are around 29 % [16, 49] for Albizzia nails, 31–50 % [51, 55] for ISKD and 12.5–27 % [56, 63] for Fitbone nails.
Pain is the most common complaint, usually the most during the distraction phase and then decreasing gradually, but often persists throughout the whole distraction and consolidation process [24]. The first healing response occurs in week 2–3, often accompanied by loss of appetite and depression, triggered by mental stress and unknown outcome [15, 24]. Pain is initially caused by stretching of the periosteum, muscle spasms, contractions due to wire or pin transfixation, and later also due to pin- and wire-related soft tissue and bone inflammation. Peaks during the night, distraction and exercises are common. Strong pain may even cause interruption or cessation of lengthening [59, 66] or at least inhibit joint motion, weight-bearing and functional loading of the regenerate.
Nerve palsies are reported to occur in up to 2.5 % of patients and mostly affect the deep peroneal nerve. They may result from excessive femoral lengthening, pre-existing scarring or from a compartment syndrome with tibial lengthening. Slowing down or stopping the lengthening process leads to the recovery of neurapraxia; immediate fasciotomy is the surgical answer to a tight compartment [51, 59, 65, 146].
Implant failures predominantly concern intramedullary nails, which are more fragile than single-block trauma nails. Their construction is much more sophisticated and the overall experience much lower than for IRF and TSF. Underlying causes may be manufacturing defects, e.g. false assembly, surgeon-related, e.g. hammering during nail insertion, forceful manipulation, no over-reaming, biologically related situations, e.g. non-union, or patient-related during vulnerable periods of lengthening, e.g. weight-bearing during lengthening with subsequent blockage. Prevention, early clinical and radiographic recognition and, eventually, timely change of implant, method or stoppage of the process are crucial [12]. Over-reaming (1.5–2 mm) to expand the intramedullary canal for the passage of the nail is preventive and best done with straight rotary reamers in increments of 0.5 mm. It defines the perfect alignment according to the pre-operative planning [54, 90]. A dummy nail confirms an easy insertion. The correct functioning of the lengthening mechanism should be checked intra-operatively [52].
Breakage, blockage and runaways are nail-specific incidences. Breakages at the site of lengthening or stress risers (interlocking holes, slot, change in diameter, “welded” junction) [12] were reported for Albizzia (1/41) [16] and ISKD nails (2/20) [62], (4/69) [55] but not in recent Fitbone series [46, 54, 63] of, in total, 57 nails. However, the latter may also exhibit technical problems like broken wire and motor failure, blocked motor and running backs in up to 10 % [46, 54, 56, 63]. Nail blockage also occurred in Albizzia series [16] and ISKD nails [51]. It is the main underlying cause of premature consolidation, which is about 12 % for ISKD [51, 53, 151], compared to below 5 % for all the other implants [16, 50, 53]. Runaway nails unintentionally lengthen more than 1.5 mm/day, a problem encountered in around 10 % of ISKDs [19, 51, 52, 55]; one patient even underwent accidental 3 cm lengthening during manipulation under anaesthesia [51] and another expanded to 4 cm unintended length during the first 2 weeks [52]. Bolt loosening [63] and problems with locking screws [66] are obstacles without permanent consequences. Patella baja is a rare event after retrograde femoral nailing [155] and does not necessarily cause anterior knee pain [56].
Poor regenerate formation is rare in young individuals: fast distraction (e.g. runaway nail), age >30 years, smoking, gain >4 cm, osteotomy at the same site of previous trauma or surgery and acute correction of associated deformities may be associated factors [19, 53, 63]. Partial or complete bony defects have been reported in up to 22 % for ISKD nails [19, 51], mostly due to inadvertent higher distraction rates (1.5 vs 1.1 mm). Pseudarthrosis is a non-event in most series [53, 60, 62]. It was prevalent in 3–8 % after Albizzia nailing [16, 59]. After the end of lengthening and after hardware removal, there is a linear increase of tensile forces. In animal experiments, mechanical stiffness only reached 50 % of normal bone 6 weeks after removal of the external fixator [102]. One of the most striking advantages of lengthening with solid intramedullary nails is the ease of both patient and doctor in terms of deciding whether a callus is already strong enough to avoid a refracture. The patients start to increasingly weight-bear at the end of the distraction phase anyway and the callus continuously builds up until nail removal, usually 1–1.5 years after the index procedure [16, 46, 50, 52, 62]. In case of external fixation, bazar-like negotiations about the right time for frame removal may come up if the healing index is high and the time with the external fixator in place exceeds the patient’s expectations. This urges early hardware removal with the inherent risk of fracturing a premature callus. Refracture after hardware removal may occur as osteoporotic stress fracture through regenerate bone, pins or a screw hole. The rate for unilateral and ring fixator ranges from 0 to 37 % [21, 24, 50, 65, 156]. Dynamisation of the frame, e.g. replacement of TSF struts with loosened Ilizarov rods or strut removal first to check if the new bone tolerates full load, may help as a preventive measure.
Infection rates naturally differ considerably between external and intramedullary devices. For fixators, up to 96 % [7] and 100–280 % (1–2.8 infections/patient) [13, 22] are reported if superficial inflammation around the pin tracts are included. Half-pins or wires make no difference [49, 157]. In our own experience, 28–45 % [22, 65] for superficial and up to 23 % [14, 158] for deep infections with surgical revision (debridement, change or removal of pins or wires) seem more realistic, with family instruction and supervision regarding thorough daily pin care being provided. Although superficial infections may be a source of pain, of decreased range of motion and of delayed rehabilitation, they usually respond well to local care and a short course of antibiotics.
Most studies on intramedullary nails report a 0 % incidence [20, 46, 52, 54, 59, 62, 66], except one late infected Albizzia nail after primary lengthening with an external fixator [16]. Deep infection with subsequent debridement by reaming of the medullary canal and IV antibiotics is a major concern if external fixation is combined with solid intramedullary nailing (LON). The rate is 3–12 % [50, 60]. A history of open fractures prior to lengthening should be taken as a warning. Care must be taken in order to prevent direct contact of the external fixation pins with the nail.
The core outcome parameter is deformity correction, which does not necessarily correspond to perfectly anatomic axis but, rather, to meeting the preset goal. Intended over- or undercorrection [48, 65], depending on the individual patient’s pre-conditions and expectations, has to be taken into account. The final result may be rated as I (no deformity), II (minor, <5°), III moderate (6–10°), IV (>10°) [48]. The average values for TSF, IRF and monolateral fixators are similar, but TSF corrections show much less variance and monolateral fixation requires more secondary manipulations. In addition, the achievements of TSFs are much less dependent on the complexity, whilst the IRF is increasingly becoming inaccurate with more complex cases, 90 versus 56 %, respectively [48, 49]. Intramedullary nailing (LON, Albizzia, ISKD and Fitbone) leads to accurate corrections in most series [16, 46, 54, 60, 62]. However, direct comparison to ring fixators is not justified, since the complexity of the cases is lower.
A good range of joint motion prevents muscular atrophy, diminishes joint reaction forces, avoids cartilage damage, decreases pain, prevents adhesions of muscles to the bony regenerate, increases blood supply to the bone and induces bone formation [6, 18, 50, 159, 160]. In addition, it keeps the patients in a positive state of mind and supports maintaining stamina towards lengthening. Perfect length and alignment are not enough for good long-term results. Full range of motion, muscle strength and proprioceptive capacities are as important. It is easier to encompass motion than to fight it back. Accordingly, preventive measures are of utmost importance and should begin pre-operatively with muscle strengthening and stretching training programmes monitored with isokinetic testing machines [6, 16]. They also include the minimisation of soft tissue trauma and muscle tethering, passive mobilisation through the full range of motion at the end of the index procedure, post-operative positioning in 90° hip and knee flexion for a couple of days, adequate analgesia, a structured physiotherapy protocol including knee mobilisation strategies, continuous passive motion, extension splints and early weight-bearing [9]. Cycling provides—as soon as hip and knee motion allows for it—more flexion/extension cycles than any physiotherapist could provide [6]. Stretching stimulates the production of actin and myosin filaments [161].
Muscle contracture, arthrofibrosis and damage to cartilage are the underlying issues for temporary or permanent restrictions. Maximal knee flexion can reduce to 30–40° during the distraction period and is more of a problem than extension [6, 9, 18, 156, 160, 162].
There are more temporary stiffness and more pin-related problems in circular fixators due to the mechanical interaction between the device and the muscle [49]. Knee flexion at the end of distraction was worse with ring fixators (37°+, −15°) [18] compared to a Fitbone series (65°+, −14°) [46]. Also, the regaining of motion during consolidation is better with nails [52–54, 56, 59, 60, 62, 63]—independent of the type of nail [6]—compared to femoral lengthening with external fixators [15, 18]. The return to full range of motion after metal removal may take 1–2 years after external fixation [18]. Nails provide better values during distraction–consolidation and a speedier return to full functioning, but comparable range of motion at the latest follow-up [6, 16, 18, 50]. The regaining of knee range of motion in case of retrograde femoral insertion is slower compared to anterograde placement [52].
Careful planning, analysis of joint stability and spanning of the knee joint with the fixator in case of pre-existing instabilities, as well as intensive physiotherapy and splinting, are mandatory [18, 23, 163, 164]. During the distraction phase, mindful regular radiographic observation of the adjacent joints is recommended, since the prevalence of decentration is around 3–7 % [21, 156]. Halt of distraction, soft tissue release or protective secondary bridging of the affected joint should be considered.
Fibular migration and distal tibiofibular subluxation during tibial lengthening is prevented by the excision of a 1-cm bony segment from the fibular shaft and a syndesmotic screw [54]. In case of mismatch of the relative lengths of tibia and fibula, isolated tibial lengthening has to be weight out against acute or continuous fibular shortening. The latter may be beneficial as a first step to prevent high articular pressure in complex deformities.
Conclusions
Our ancestors’ end-of-the-nineteenth-century vision of surgical bone lengthening has become true: a combination of basic knowledge on the biology of bone remodelling and soft tissue adaption to stretching, the benefits of modern implants and correction systems, a skilled, experienced and cautious surgeon, a health system commensurate to patient needs and a compliant patient provide relatively safe and predictable corrections, even in complex situations. The Taylor Spatial Frame (TSF) hexapod technology constitutes the benchmark for complex, multi-plane deformities. For cases of lower complexity, motorised intramedullary nails are valuable alternatives after and unilateral fixators during growth. For minor to moderate isolated leg length discrepancies during growth, timely epiphysiodesis is the method of choice in tall enough individuals. In any case, conservative measures should be discussed. However, for most patients, they are a less than valuable option.
Visionary directions of research and development may focus on programmable apparatus providing continuous biological correction equipped with enough intelligence to react to biological and biomechanical feedback from bone, soft tissues and implants. The stiffness of the implant may variably adapt to the state of the bony regenerate, thus, building an optimal environment for maturation. Shortening of the consolidation phase by the promotion of bone formation probably bears the highest potential to diminish complications and lower the overall treatment time and costs. The latter includes an increasing economic burden for sophisticated implants hopefully supercompensated by diminishing expenditures for rehabilitation and working incapacities. Future studies should, therefore, focus on the overall treatment cost and standardised outcome measures, which will allow for fair benchmarking [50, 63].
Acknowledgments
Conflict of interest
None.
Footnotes
Berliner Klinische Wochenschrift 1869: An den unteren Extremitäten bedingen 2–4 cm betragende Verkürzungen schon erhebliche Funktionsstörungen und es wäre gewiss wichtig, sie in operativer Weise auszugleichen. Von grosser Bedeutung wird, so glaube ich, für die chirurgische Praxis die Tatsache sein, dass das Längenwachstum der Knochen durch Dehnung gesteigert werden kann.
Contributor Information
Carol C. Hasler, Phone: +41-61-7042803, FAX: +41-61-7041213, Email: carol.hasler@bluewin.ch
Andreas H. Krieg, Phone: +41-61-7042806, FAX: +41-61-7041213, Email: andreas.krieg@ukbb.ch
References
- 1.Guichet JM, Spivak JM, Trouilloud P, et al. Lower limb-length discrepancy. An epidemiologic study. Clin Orthop Relat Res. 1991;272:235–241. [PubMed] [Google Scholar]
- 2.Gross RH. Leg length discrepancy: how much is too much? Orthopedics. 1978;1:307–310. doi: 10.3928/0147-7447-19780701-08. [DOI] [PubMed] [Google Scholar]
- 3.Hellsing AL. Leg length inequality. A prospective study of young men during their military service. Ups J Med Sci. 1988;93:245–253. doi: 10.3109/03009738809178550. [DOI] [PubMed] [Google Scholar]
- 4.Harvey WF, Yang M, Cooke TD, et al. Association of leg-length inequality with knee osteoarthritis: a cohort study. Ann Intern Med. 2010;152:287–295. doi: 10.1059/0003-4819-152-5-201003020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyre-Brook AL. Bone-shortening for inequality of leg lengths. Br Med J. 1951;1:222–225. doi: 10.1136/bmj.1.4700.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acharya A, Guichet JM. Effect on knee motion of gradual intramedullary femoral lengthening. Acta Orthop Belg. 2006;72:569–577. [PubMed] [Google Scholar]
- 7.Antoci V, Ono CM, Antoci V, Jr, et al. Pin-tract infection during limb lengthening using external fixation. Am J Orthop (Belle Mead NJ) 2008;37:E150–E154. [PubMed] [Google Scholar]
- 8.Aronson J. Experimental and clinical experience with distraction osteogenesis. Cleft Palate Craniofac J. 1994;31:473–481. doi: 10.1597/1545-1569_1994_031_0473_eacewd_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 9.Barker KL, Simpson AH, Lamb SE. Loss of knee range of motion in leg lengthening. J Orthop Sports Phys Ther. 2001;31:238–244. doi: 10.2519/jospt.2001.31.5.238. [DOI] [PubMed] [Google Scholar]
- 10.Bonnard C, Favard L, Sollogoub I, et al. Limb lengthening in children using the Ilizarov method. Clin Orthop Relat Res. 1993;293:83–88. [PubMed] [Google Scholar]
- 11.Bost FC, Larsen LJ. Experiences with lengthening of the femur over an intramedullary rod. J Bone Joint Surg Am. 1956;38-A:567–584. [PubMed] [Google Scholar]
- 12.Burghardt RD, Herzenberg JE, Specht SC, et al. Mechanical failure of the Intramedullary Skeletal Kinetic Distractor in limb lengthening. J Bone Joint Surg Br. 2011;93:639–643. doi: 10.1302/0301-620X.93B5.25986. [DOI] [PubMed] [Google Scholar]
- 13.Dahl MT, Gulli B, Berg T. Complications of limb lengthening. A learning curve. Clin Orthop Relat Res. 1994;301:10–18. [PubMed] [Google Scholar]
- 14.Eldridge JC, Bell DF. Problems with substantial limb lengthening. Orthop Clin North Am. 1991;22:625–631. [PubMed] [Google Scholar]
- 15.García-Cimbrelo E, Olsen B, Ruiz-Yagüe M, et al. Ilizarov technique. Results and difficulties. Clin Orthop Relat Res. 1992;283:116–123. [PubMed] [Google Scholar]
- 16.Guichet JM, Deromedis B, Donnan LT, et al. Gradual femoral lengthening with the Albizzia intramedullary nail. J Bone Joint Surg Am. 2003;85-A:838–848. doi: 10.2106/00004623-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Guidera KJ, Hess WF, Highhouse KP, et al. Extremity lengthening: results and complications with the Orthofix system. J Pediatr Orthop. 1991;11:90–94. [PubMed] [Google Scholar]
- 18.Herzenberg JE, Scheufele LL, Paley D, et al. Knee range of motion in isolated femoral lengthening. Clin Orthop Relat Res. 1994;301:49–54. [PubMed] [Google Scholar]
- 19.Kenawey M, Krettek C, Liodakis E, et al. Insufficient bone regenerate after intramedullary femoral lengthening: risk factors and classification system. Clin Orthop Relat Res. 2011;469:264–273. doi: 10.1007/s11999-010-1332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieg AH, Speth BM, Foster BK. Leg lengthening with a motorized nail in adolescents: an alternative to external fixators? Clin Orthop Relat Res. 2008;466:189–197. doi: 10.1007/s11999-007-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noonan KJ, Leyes M, Forriol F, et al. Distraction osteogenesis of the lower extremity with use of monolateral external fixation. A study of two hundred and sixty-one femora and tibiae. J Bone Joint Surg Am. 1998;80:793–806. doi: 10.2106/00004623-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990;250:81–104. [PubMed] [Google Scholar]
- 23.Suzuki S, Kasahara Y, Seto Y, et al. Dislocation and subluxation during femoral lengthening. J Pediatr Orthop. 1994;14:343–346. doi: 10.1097/01241398-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Young N, Bell DF, Anthony A. Pediatric pain patterns during Ilizarov treatment of limb length discrepancy and angular deformity. J Pediatr Orthop. 1994;14:352–357. doi: 10.1097/01241398-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 25.De Bastiani G, Aldegheri R, Renzi-Brivio L, et al. Limb lengthening by callus distraction (callotasis) J Pediatr Orthop. 1987;7:129–134. doi: 10.1097/01241398-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285. [PubMed] [Google Scholar]
- 27.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- 28.Paley D. Current techniques of limb lengthening. J Pediatr Orthop. 1988;8:73–92. doi: 10.1097/01241398-198801000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Aronson J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J Bone Joint Surg Am. 1997;79:1243–1258. doi: 10.2106/00004623-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Codivilla A. On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. 1904. Clin Orthop Relat Res. 1994;301:4–9. [PubMed] [Google Scholar]
- 31.Codivilla A. On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. Am J Orthop Surg. 1905;2:353–369. [Google Scholar]
- 32.Wiedemann M. Callus distraction: a new method? A historical review of limb lengthening. Clin Orthop Relat Res. 1996;327:291–304. [PubMed] [Google Scholar]
- 33.Ombrédanne L. Allongement d’un fémur sur un membre trop court. Bull Mém Soc Chir Paris. 1913;39:1177–1180. [Google Scholar]
- 34.Putti V. The operative lengthening of the femur. JAMA. 1921;77:934–935. [PubMed] [Google Scholar]
- 35.Wittmoser R. Zur Druckosteosynthese. Langenbecks Arch Klin Chir. 1953;276:229–231. [PubMed] [Google Scholar]
- 36.Golyakhovsky V. Gavriel A. Ilizarov: “The magician from Kurgan”. Bull Hosp Jt Dis Orthop Inst. 1988;48:12–16. [PubMed] [Google Scholar]
- 37.Paterson D. Leg-lengthening procedures. A historical review. Clin Orthop Relat Res. 1990;250:27–33. [PubMed] [Google Scholar]
- 38.Kenwright J, White SH. A historical review of limb lengthening and bone transport. Injury. 1993;24(Suppl 2):S9–S19. doi: 10.1016/0020-1383(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 39.Wagner H. Surgical leg prolongation. Chirurg. 1971;42:260–266. [PubMed] [Google Scholar]
- 40.Wagner H. Operative lengthening of the femur. Clin Orthop Relat Res. 1978;136:125–142. [PubMed] [Google Scholar]
- 41.Ring PA. Experimental bone lengthening by epiphysial distraction. Br J Surg. 1958;46:169–173. doi: 10.1002/bjs.18004619617. [DOI] [PubMed] [Google Scholar]
- 42.Fishbane BM, Riley LH., Jr Continuous trans-physeal traction: a simple method of bone lengthening. Johns Hopkins Med J. 1976;138:79–81. [PubMed] [Google Scholar]
- 43.Monticelli G, Spinelli R. Distraction epiphysiolysis as a method of limb lengthening. III. Clinical applications. Clin Orthop Relat Res. 1981;154:274–285. [PubMed] [Google Scholar]
- 44.Monticelli G, Spinelli R. Distraction epiphysiolysis as a method of limb lengthening. I. Experimental study. Clin Orthop Relat Res. 1981;154:254–261. [PubMed] [Google Scholar]
- 45.Monticelli G, Spinelli R, Bonucci E. Distraction epiphysiolysis as a method of limb lengthening. II. Morphologic investigations. Clin Orthop Relat Res. 1981;154:262–273. [PubMed] [Google Scholar]
- 46.Baumgart R, Betz A, Schweiberer L. A fully implantable motorized intramedullary nail for limb lengthening and bone transport. Clin Orthop Relat Res. 1997;343:135–143. [PubMed] [Google Scholar]
- 47.Betz A, Baumgart R, Schweiberer L. First fully implantable intramedullary system for callus distraction—intramedullary nail with programmable drive for leg lengthening and segment displacement. Principles and initial clinical results. Chirurg. 1990;61:605–609. [PubMed] [Google Scholar]
- 48.Manner HM, Huebl M, Radler C, et al. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1:55–61. doi: 10.1007/s11832-006-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dammerer D, Kirschbichler L, Donnan G, et al. Clinical value of the Taylor Spatial Frame: a comparison with the Ilizarov and Orthofix fixators. J Child Orthop. 2011;5:343–349. doi: 10.1007/s11832-011-0361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paley D, Herzenberg JE, Paremain G, et al. Femoral lengthening over an intramedullary nail. A matched-case comparison with Ilizarov femoral lengthening. J Bone Joint Surg Am. 1997;79:1464–1480. doi: 10.2106/00004623-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Kenawey M, Krettek C, Liodakis E, et al. Leg lengthening using intramedullay skeletal kinetic distractor: results of 57 consecutive applications. Injury. 2011;42:150–155. doi: 10.1016/j.injury.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Leidinger B, Winkelmann W, Roedl R. Limb lengthening with a fully implantable mechanical distraction intramedullary nail. Z Orthop Ihre Grenzgeb. 2006;144:419–426. doi: 10.1055/s-2006-942169. [DOI] [PubMed] [Google Scholar]
- 53.Mahboubian S, Seah M, Fragomen AT et al (2011) Femoral lengthening with lengthening over a nail has fewer complications than intramedullary skeletal kinetic distraction. Clin Orthop Relat Res (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 54.Singh S, Lahiri A, Iqbal M. The results of limb lengthening by callus distraction using an extending intramedullary nail (Fitbone) in non-traumatic disorders. J Bone Joint Surg Br. 2006;88:938–942. doi: 10.1302/0301-620X.88B7.17618. [DOI] [PubMed] [Google Scholar]
- 55.Schiedel FM, Pip S, Wacker S, et al. Intramedullary limb lengthening with the Intramedullary Skeletal Kinetic Distractor in the lower limb. J Bone Joint Surg Br. 2011;93:788–792. doi: 10.1302/0301-620X.93B6.25581. [DOI] [PubMed] [Google Scholar]
- 56.Lenze U, Hasler CC, Krieg AH. Intramedullary motorized nail for equalization of posttraumatic leg length discrepancies. Unfallchirurg. 2011;114:604–610. doi: 10.1007/s00113-010-1820-x. [DOI] [PubMed] [Google Scholar]
- 57.Ilizarov GA. Basic principles of transosseous compression and distraction osteosynthesis. Ortop Travmatol Protez. 1971;32:7–15. [PubMed] [Google Scholar]
- 58.Ilizarov GA, Deviatov AA, Trokhova VG. Surgical lengthening of the shortened lower extremities. Vestn Khir Im I I Grek. 1972;108:100–103. [PubMed] [Google Scholar]
- 59.García-Cimbrelo E, Curto de la Mano A, García-Rey E, et al. The intramedullary elongation nail for femoral lengthening. J Bone Joint Surg Br. 2002;84:971–977. doi: 10.1302/0301-620x.84b7.12984. [DOI] [PubMed] [Google Scholar]
- 60.Simpson AH, Cole AS, Kenwright J. Leg lengthening over an intramedullary nail. J Bone Joint Surg Br. 1999;81:1041–1045. doi: 10.1302/0301-620x.81b6.9359. [DOI] [PubMed] [Google Scholar]
- 61.Jasiewicz B, Kacki W, Tesiorowski M, et al. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73:177–183. [PubMed] [Google Scholar]
- 62.Cole JD, Justin D, Kasparis T, et al. The intramedullary skeletal kinetic distractor (ISKD): first clinical results of a new intramedullary nail for lengthening of the femur and tibia. Injury. 2001;32(Suppl 4):SD129–SD139. doi: 10.1016/s0020-1383(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 63.Krieg AH, Lenze U, Speth BM, et al. Intramedullary leg lengthening with a motorized nail. Acta Orthop. 2011;82:344–350. doi: 10.3109/17453674.2011.584209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baumgart R, Bürklein D, Hinterwimmer S, et al. The management of leg-length discrepancy in Ollier’s disease with a fully implantable lengthening nail. J Bone Joint Surg Br. 2005;87:1000–1004. doi: 10.1302/0301-620X.87B7.16365. [DOI] [PubMed] [Google Scholar]
- 65.Eidelman M, Bialik V, Katzman A. Correction of deformities in children using the Taylor spatial frame. J Pediatr Orthop B. 2006;15:387–395. doi: 10.1097/01.bpb.0000228380.27239.8a. [DOI] [PubMed] [Google Scholar]
- 66.Guichet JM. Leg lengthening and correction of deformity using the femoral Albizzia nail. Orthopade. 1999;28:1066–1077. doi: 10.1007/s001320050432. [DOI] [PubMed] [Google Scholar]
- 67.Dubousset J, Charpak G, Skalli W, et al. EOS stereo-radiography system: whole-body simultaneous anteroposterior and lateral radiographs with very low radiation dose. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:141–143. doi: 10.1016/s0035-1040(07)92729-4. [DOI] [PubMed] [Google Scholar]
- 68.Dewaele J, Fabry G. The timing of epiphysiodesis. A comparative study between the use of the method of Anderson and Green and the Moseley chart. Acta Orthop Belg. 1992;58:43–47. [PubMed] [Google Scholar]
- 69.Paley D, Bhave A, Herzenberg JE, et al. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82-A:1432–1446. doi: 10.2106/00004623-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi M, Kawasaki Y, Matsui Y, et al. Fragmental bone transport in conjunction with acute shortening followed by gradual lengthening for a failed infected nonunion of the tibia. J Orthop Sci. 2010;15:420–424. doi: 10.1007/s00776-009-1423-y. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X, Liu T, Li Z, et al. Reconstruction with callus distraction for nonunion with bone loss and leg shortening caused by suppurative osteomyelitis of the femur. J Bone Joint Surg Br. 2007;89:1509–1514. doi: 10.1302/0301-620X.89B11.19239. [DOI] [PubMed] [Google Scholar]
- 72.Yokoyama K. Acute shortening and re-lengthening in the management of bone and soft-tissue loss in complicated fractures of the tibia. J Bone Joint Surg Br. 2007;89:846. doi: 10.1302/0301-620X.89B8.19833. [DOI] [PubMed] [Google Scholar]
- 73.Said GZ, el-Sherif EK. Resection-shortening-distraction for malignant bone tumours. A report of two cases. J Bone Joint Surg Br. 1995;77:185–188. [PubMed] [Google Scholar]
- 74.Aldegheri R. Femoral callotasis. J Pediatr Orthop B. 1997;6:42–47. doi: 10.1097/01202412-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Guichet JM, Braillon P, Bodenreider O, et al. Periosteum and bone marrow in bone lengthening: a DEXA quantitative evaluation in rabbits. Acta Orthop Scand. 1998;69:527–531. doi: 10.3109/17453679808997792. [DOI] [PubMed] [Google Scholar]
- 76.Kojimoto H, Yasui N, Goto T, et al. Bone lengthening in rabbits by callus distraction. The role of periosteum and endosteum. J Bone Joint Surg Br. 1988;70:543–549. doi: 10.1302/0301-620X.70B4.3403595. [DOI] [PubMed] [Google Scholar]
- 77.Aronson J, Shen X. Experimental healing of distraction osteogenesis comparing metaphyseal with diaphyseal sites. Clin Orthop Relat Res. 1994;301:25–30. [PubMed] [Google Scholar]
- 78.Aronson J, Harrison BH, Stewart CL, et al. The histology of distraction osteogenesis using different external fixators. Clin Orthop Relat Res. 1989;241:106–116. [PubMed] [Google Scholar]
- 79.Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994;301:124–131. [PubMed] [Google Scholar]
- 80.Delloye C, Delefortrie G, Coutelier L, et al. Bone regenerate formation in cortical bone during distraction lengthening. An experimental study. Clin Orthop Relat Res. 1990;250:34–42. [PubMed] [Google Scholar]
- 81.Yasui N, Kojimoto H, Sasaki K, et al. Factors affecting callus distraction in limb lengthening. Clin Orthop Relat Res. 1993;293:55–60. [PubMed] [Google Scholar]
- 82.Frierson M, Ibrahim K, Boles M, et al. Distraction osteogenesis. A comparison of corticotomy techniques. Clin Orthop Relat Res. 1994;301:19–24. [PubMed] [Google Scholar]
- 83.Ilizarov GA. Clinical application of the tension–stress effect for limb lengthening. Clin Orthop Relat Res. 1990;250:8–26. [PubMed] [Google Scholar]
- 84.Brutscher R, Rahn BA, Rüter A, et al. The role of corticotomy and osteotomy in the treatment of bone defects using the Ilizarov technique. J Orthop Trauma. 1993;7:261–269. doi: 10.1097/00005131-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 85.Steen H, Fjeld TO, Miller JA, et al. Biomechanical factors in the metaphyseal- and diaphyseal-lengthening osteotomy. An experimental and theoretic analysis in the ovine tibia. Clin Orthop Relat Res. 1990;259:282–294. [PubMed] [Google Scholar]
- 86.Paley D, Tetsworth K. Percutaneous osteotomies. Osteotome and Gigli saw techniques. Orthop Clin North Am. 1991;22:613–624. [PubMed] [Google Scholar]
- 87.Price CT, Cole JD. Limb lengthening by callotasis for children and adolescents. Early experience. Clin Orthop Relat Res. 1990;250:105–111. [PubMed] [Google Scholar]
- 88.Guichet JM, Grammont PM, Trouilloud P. A nail for progressive lengthening. An animal experiment with a 2-year follow-up. Chirurgie. 1992;118:405–410. [PubMed] [Google Scholar]
- 89.Aldegheri R, Renzi-Brivio L, Agostini S. The callotasis method of limb lengthening. Clin Orthop Relat Res. 1989;241:137–145. [PubMed] [Google Scholar]
- 90.Hankemeier S, Gösling T, Pape HC, et al. Limb lengthening with the Intramedullary Skeletal Kinetic Distractor (ISKD) Oper Orthop Traumatol. 2005;17:79–101. doi: 10.1007/s00064-005-1123-5. [DOI] [PubMed] [Google Scholar]
- 91.Cattaneo R, Villa A, Catagni MA, et al. Lengthening of the humerus using the Ilizarov technique. Description of the method and report of 43 cases. Clin Orthop Relat Res. 1990;250:117–124. [PubMed] [Google Scholar]
- 92.Fischgrund J, Paley D, Suter C. Variables affecting time to bone healing during limb lengthening. Clin Orthop Relat Res. 1994;301:31–37. [PubMed] [Google Scholar]
- 93.Ganey TM, Klotch DW, Sasse J, et al. Basement membrane of blood vessels during distraction osteogenesis. Clin Orthop Relat Res. 1994;301:132–138. [PubMed] [Google Scholar]
- 94.Herzenberg JE, Waanders NA. Calculating rate and duration of distraction for deformity correction with the Ilizarov technique. Orthop Clin North Am. 1991;22:601–611. [PubMed] [Google Scholar]
- 95.Price CT, Mann JW. Experience with the Orthofix device for limb lengthening. Orthop Clin North Am. 1991;22:651–661. [PubMed] [Google Scholar]
- 96.Little DG, Cornell MS, Hile MS, et al. Effect of pamidronate on distraction osteogenesis and fixator-related osteoporosis. Injury. 2001;32(Suppl 4):SD14–SD20. doi: 10.1016/s0020-1383(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi M, Yukata K, Matsui Y, et al. Bisphosphonate modulates morphological and mechanical properties in distraction osteogenesis through inhibition of bone resorption. Bone. 2006;39:573–581. doi: 10.1016/j.bone.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 98.Beck A, Salem K, Krischak G, et al. Nonsteroidal anti-inflammatory drugs (NSAIDs) in the perioperative phase in traumatology and orthopedics effects on bone healing. Oper Orthop Traumatol. 2005;17:569–578. doi: 10.1007/s00064-005-1152-0. [DOI] [PubMed] [Google Scholar]
- 99.Aronson J, Harp JH. Mechanical forces as predictors of healing during tibial lengthening by distraction osteogenesis. Clin Orthop Relat Res. 1994;301:73–79. [PubMed] [Google Scholar]
- 100.Aronson J, Good B, Stewart C, et al. Preliminary studies of mineralization during distraction osteogenesis. Clin Orthop Relat Res. 1990;250:43–49. [PubMed] [Google Scholar]
- 101.Karaharju EO, Aalto K, Kahri A, et al. Distraction bone healing. Clin Orthop Relat Res. 1993;297:38–43. [PubMed] [Google Scholar]
- 102.Orbay JL, Frankel VH, Finkle JE, et al. Canine leg lengthening by the Ilizarov technique. A biomechanical, radiologic, and morphologic study. Clin Orthop Relat Res. 1992;278:265–273. [PubMed] [Google Scholar]
- 103.Shearer JR, Roach HI, Parsons SW. Histology of a lengthened human tibia. J Bone Joint Surg Br. 1992;74:39–44. doi: 10.1302/0301-620X.74B1.1732262. [DOI] [PubMed] [Google Scholar]
- 104.Ippolito E, Peretti G, Bellocci M, et al. Histology and ultrastructure of arteries, veins, and peripheral nerves during limb lengthening. Clin Orthop Relat Res. 1994;308:54–62. [PubMed] [Google Scholar]
- 105.Eyres KS, Bell MJ, Kanis JA. New bone formation during leg lengthening. Evaluated by dual energy X-ray absorptiometry. J Bone Joint Surg Br. 1993;75:96–106. doi: 10.1302/0301-620X.75B1.8421047. [DOI] [PubMed] [Google Scholar]
- 106.Harp JH, Aronson J, Hollis M. Noninvasive determination of bone stiffness for distraction osteogenesis by quantitative computed tomography scans. Clin Orthop Relat Res. 1994;301:42–48. [PubMed] [Google Scholar]
- 107.Brunner UH, Cordey J, Schweiberer L, et al. Force required for bone segment transport in the treatment of large bone defects using medullary nail fixation. Clin Orthop Relat Res. 1994;301:147–155. [PubMed] [Google Scholar]
- 108.Blane CE, Herzenberg JE, DiPietro MA. Radiographic imaging for Ilizarov limb lengthening in children. Pediatr Radiol. 1991;21:117–120. doi: 10.1007/BF02015621. [DOI] [PubMed] [Google Scholar]
- 109.Hughes TH, Maffulli N, Green V, et al. Imaging in bone lengthening. A review. Clin Orthop Relat Res. 1994;308:50–53. [PubMed] [Google Scholar]
- 110.Minty I, Maffulli N, Hughes TH, et al. Radiographic features of limb lengthening in children. Acta Radiol. 1994;35:555–559. [PubMed] [Google Scholar]
- 111.Walker CW, Aronson J, Kaplan PA, et al. Radiologic evaluation of limb-lengthening procedures. AJR Am J Roentgenol. 1991;156:353–358. doi: 10.2214/ajr.156.2.1898813. [DOI] [PubMed] [Google Scholar]
- 112.Li R, Saleh M, Yang L, et al. Radiographic classification of osteogenesis during bone distraction. J Orthop Res. 2006;24:339–347. doi: 10.1002/jor.20026. [DOI] [PubMed] [Google Scholar]
- 113.Sakurakichi K, Tsuchiya H, Kabata T, et al. Correction of juxtaarticular deformities in children using the Ilizarov apparatus. J Orthop Sci. 2005;10:360–366. doi: 10.1007/s00776-005-0908-6. [DOI] [PubMed] [Google Scholar]
- 114.Aldegheri R. Distraction osteogenesis for lengthening of the tibia in patients who have limb-length discrepancy or short stature. J Bone Joint Surg Am. 1999;81:624–634. doi: 10.2106/00004623-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 115.Paley D, Herzenberg JE, Tetsworth K, et al. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25:425–465. [PubMed] [Google Scholar]
- 116.Kawamura B, Hosono S, Takahashi T, et al. Limb lengtening by means of subcutaneous osteotomy. Experimental and clinical studies. J Bone Joint Surg Am. 1968;50:851–878. [PubMed] [Google Scholar]
- 117.Lee DY, Choi IH, Chung CY, et al. Effect of tibial lengthening on the gastrocnemius muscle. A histopathologic and morphometric study in rabbits. Acta Orthop Scand. 1993;64:688–692. doi: 10.3109/17453679308994599. [DOI] [PubMed] [Google Scholar]
- 118.Matano T, Tamai K, Kurokawa T. Adaptation of skeletal muscle in limb lengthening: a light diffraction study on the sarcomere length in situ. J Orthop Res. 1994;12:193–196. doi: 10.1002/jor.1100120207. [DOI] [PubMed] [Google Scholar]
- 119.Velazquez RJ, Bell DF, Armstrong PF, et al. Complications of use of the Ilizarov technique in the correction of limb deformities in children. J Bone Joint Surg Am. 1993;75:1148–1156. doi: 10.2106/00004623-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 120.Barker KL, Lamb SE, Simpson HR, et al. Recovery of muscle strength and power after limb-lengthening surgery. Arch Phys Med Rehabil. 2010;91:384–388. doi: 10.1016/j.apmr.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 121.Lee DY, Han TR, Choi IH, et al. Changes in somatosensory-evoked potentials in limb lengthening. An experimental study on rabbits’ tibiae. Clin Orthop Relat Res. 1992;285:273–279. [PubMed] [Google Scholar]
- 122.Strong M, Hruska J, Czyrny J, et al. Nerve palsy during femoral lengthening: MRI, electrical, and histologic findings in the central and peripheral nervous systems—a canine model. J Pediatr Orthop. 1994;14:347–351. doi: 10.1097/01241398-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 123.Olney BW, Jayaraman G. Joint reaction forces during femoral lengthening. Clin Orthop Relat Res. 1994;301:64–67. [PubMed] [Google Scholar]
- 124.Lee DY, Chung CY, Choi IH. Longitudinal growth of the rabbit tibia after callotasis. J Bone Joint Surg Br. 1993;75:898–903. doi: 10.1302/0301-620X.75B6.8245079. [DOI] [PubMed] [Google Scholar]
- 125.Stanitski DF. Treatment of deformity secondary to metabolic bone disease with the Ilizarov technique. Clin Orthop Relat Res. 1994;301:38–41. [PubMed] [Google Scholar]
- 126.Hope PG, Crawfurd EJ, Catterall A. Bone growth following lengthening for congenital shortening of the lower limb. J Pediatr Orthop. 1994;14:339–342. doi: 10.1097/01241398-199405000-00012. [DOI] [PubMed] [Google Scholar]
- 127.Grill F. Correction of complicated extremity deformities by external fixation. Clin Orthop Relat Res. 1989;241:166–176. [PubMed] [Google Scholar]
- 128.Rödl R, Leidinger B, Böhm A, et al. Correction of deformities with conventional and hexapod frames—comparison of methods. Z Orthop Ihre Grenzgeb. 2003;141:92–98. doi: 10.1055/s-2003-37296. [DOI] [PubMed] [Google Scholar]
- 129.Seide K, Wolter D, Kortmann HR. Fracture reduction and deformity correction with the hexapod Ilizarov fixator. Clin Orthop Relat Res. 1999;363:186–195. [PubMed] [Google Scholar]
- 130.Fadel M, Hosny G. The Taylor spatial frame for deformity correction in the lower limbs. Int Orthop. 2005;29:125–129. doi: 10.1007/s00264-004-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sluga M, Pfeiffer M, Kotz R, et al. Lower limb deformities in children: two-stage correction using the Taylor spatial frame. J Pediatr Orthop B. 2003;12:123–128. doi: 10.1097/01.bpb.0000049578.53117.03. [DOI] [PubMed] [Google Scholar]
- 132.Oedekoven G, Jansen D, Raschke M, et al. The monorail system—bone segment transport over unreamed interlocking nails. Chirurg. 1996;67:1069–1079. doi: 10.1007/s001040050106. [DOI] [PubMed] [Google Scholar]
- 133.Lee WH, Huang SC. Femoral lengthening: callotasis with Ilizarov external fixator alone and with intramedullary locking nail. J Formos Med Assoc. 1997;96:98–102. [PubMed] [Google Scholar]
- 134.Raschke M, Oedekoven G, Ficke J, et al. The monorail method for segment bone transport. Injury. 1993;24(Suppl 2):S54–S61. doi: 10.1016/0020-1383(93)90020-7. [DOI] [PubMed] [Google Scholar]
- 135.Klein MP, Rahn BA, Frigg R, et al. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109:314–316. doi: 10.1007/BF00636168. [DOI] [PubMed] [Google Scholar]
- 136.Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77:490–493. [PubMed] [Google Scholar]
- 137.Trueta J, Buhr AJ. The vascular contribution to osteogenesis. V. The vasculature supplying the epiphysial cartilage in rachitic rats. J Bone Joint Surg Br. 1963;45:572–581. [PubMed] [Google Scholar]
- 138.Lin CC, Huang SC, Liu TK, et al. Limb lengthening over an intramedullary nail. An animal study and clinical report. Clin Orthop Relat Res. 1996;330:208–216. doi: 10.1097/00003086-199609000-00028. [DOI] [PubMed] [Google Scholar]
- 139.Kristiansen LP, Steen H. Lengthening of the tibia over an intramedullary nail, using the Ilizarov external fixator. Major complications and slow consolidation in 9 lengthenings. Acta Orthop Scand. 1999;70:271–274. doi: 10.3109/17453679908997806. [DOI] [PubMed] [Google Scholar]
- 140.Götz J, Schellmann WD. Continuous lengthening of the femur with intramedullary stabilisation (author’s transl) Arch Orthop Unfallchir. 1975;82:305–310. doi: 10.1007/BF00418926. [DOI] [PubMed] [Google Scholar]
- 141.Baumann F, Harms J. The extension nail. A new method for lengthening of the femur and tibia (author’s transl) Arch Orthop Unfallchir. 1977;90:139–146. doi: 10.1007/BF00414987. [DOI] [PubMed] [Google Scholar]
- 142.Bliskunov AI. Intramedullary distraction of the femur (preliminary report) Ortop Travmatol Protez. 1983;10:59–62. [PubMed] [Google Scholar]
- 143.Witt AN, Jäger M, Bruns H, et al. An implantable femur distractor for operative leg lengthening (author’s transl) Arch Orthop Trauma Surg. 1978;92:291–296. doi: 10.1007/BF02341812. [DOI] [PubMed] [Google Scholar]
- 144.Younger AS, Mackenzie WG, Morrison JB. Femoral forces during limb lengthening in children. Clin Orthop Relat Res. 1994;301:55–63. [PubMed] [Google Scholar]
- 145.Pettine KA, Chao EY, Kelly PJ. Analysis of the external fixator pin–bone interface. Clin Orthop Relat Res. 1993;293:18–27. [PubMed] [Google Scholar]
- 146.Young NL, Davis RJ, Bell DF, et al. Electromyographic and nerve conduction changes after tibial lengthening by the Ilizarov method. J Pediatr Orthop. 1993;13:473–477. doi: 10.1097/01241398-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 147.Krieg AH, Lenze U, Hasler CC, et al. Ilizarov hip reconstruction without external fixation: a new technique. J Child Orthop. 2010;4:259–266. doi: 10.1007/s11832-010-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krettek C, Stephan C, Schandelmaier P, et al. The use of Poller screws as blocking screws in stabilising tibial fractures treated with small diameter intramedullary nails. J Bone Joint Surg Br. 1999;81:963–968. doi: 10.1302/0301-620x.81b6.10000. [DOI] [PubMed] [Google Scholar]
- 149.Neel MD, Heck R, Britton L, et al. Use of a smooth press-fit stem preserves physeal growth after tumor resection. Clin Orthop Relat Res. 2004;426:125–128. doi: 10.1097/01.blo.0000141386.97866.bb. [DOI] [PubMed] [Google Scholar]
- 150.Raney EM, Ogden JA, Grogan DP. Premature greater trochanteric epiphysiodesis secondary to intramedullary femoral rodding. J Pediatr Orthop. 1993;13:516–520. doi: 10.1097/01241398-199307000-00018. [DOI] [PubMed] [Google Scholar]
- 151.Potaczek T, Kacki W, Jasiewicz B, et al. Femur lengthening with a telescopic intramedullary nail ISKD—method presentation and early clinical results. Chir Narzadow Ruchu Ortop Pol. 2008;73:10–14. [PubMed] [Google Scholar]
- 152.Wolfson N, Hearn TC, Thomason JJ, et al. Force and stiffness changes during Ilizarov leg lengthening. Clin Orthop Relat Res. 1990;250:58–60. [PubMed] [Google Scholar]
- 153.Hardy JM, Tadlaoui A, Wirotius JM, et al. The Sequoia circular fixator for limb lengthening. Orthop Clin North Am. 1991;22:663–675. [PubMed] [Google Scholar]
- 154.Donnan LT, Saleh M, Rigby AS. Acute correction of lower limb deformity and simultaneous lengthening with a monolateral fixator. J Bone Joint Surg Br. 2003;85:254–260. doi: 10.1302/0301-620x.85b2.12645. [DOI] [PubMed] [Google Scholar]
- 155.Krieg JC, Mirza A. Case report: Patella baja after retrograde femoral nail insertion. Clin Orthop Relat Res. 2009;467:566–571. doi: 10.1007/s11999-008-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stanitski DF, Bullard M, Armstrong P, et al. Results of femoral lengthening using the Ilizarov technique. J Pediatr Orthop. 1995;15:224–231. [PubMed] [Google Scholar]
- 157.Gordon JE, Kelly-Hahn J, Carpenter CJ, et al. Pin site care during external fixation in children: results of a nihilistic approach. J Pediatr Orthop. 2000;20:163–165. [PubMed] [Google Scholar]
- 158.Green SA. Complications of external skeletal fixation. Clin Orthop Relat Res. 1983;180:109–116. [PubMed] [Google Scholar]
- 159.Haapala J, Arokoski JP, Hyttinen MM, et al. Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop Relat Res. 1999;362:218–229. [PubMed] [Google Scholar]
- 160.Stanitski DF. The effect of limb lengthening on articular cartilage. An experimental study. Clin Orthop Relat Res. 1994;301:68–72. [PubMed] [Google Scholar]
- 161.Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194(Pt 3):323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Simpson H, Barker K. Effect on knee flexion of a modification to the surgical technique of pin placement during femoral lengthening. J Pediatr Orthop B. 2002;11:307–312. doi: 10.1097/00009957-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 163.Coglianese DB, Herzenberg JE, Goulet JA. Physical therapy management of patients undergoing limb lengthening by distraction osteogenesis. J Orthop Sports Phys Ther. 1993;17:124–132. doi: 10.2519/jospt.1993.17.3.124. [DOI] [PubMed] [Google Scholar]
- 164.Green SA. Postoperative management during limb lengthening. Orthop Clin North Am. 1991;22:723–734. [PubMed] [Google Scholar]


