Abstract
Interactions between particles are dependent on the physicochemical characteristics of the interacting particles but it is also important to consider the manufacturing process. Blending active pharmaceutical ingredient (API) with carrier is a critical stage that determines the blend homogeneity and is the first step towards obtaining the final quality of the powder blend. The aim of this work was to study parameters that influence the interactions between API and carrier in adhesive mixtures used in DPI and their effect on API dispersion. The study was done with fluticasone propionate blended with lactose ‘Lactohale 200’. The study was based on the influence of the operating conditions (speed, mixing time, resting steps during mixing), the size of the carrier and the storage conditions on the blend properties and on the API dispersion. The quality of the blends was examined by analysing the API content uniformity. Adhesion characteristics were evaluated by submitting mixtures to a sieving action by air depression with the Alpine air-jet sieve. Aerodynamic evaluation of fine particle fraction (FPF) was obtained using a Twin Stage Impinger; the FPF being defined as the mass percentage of API below 6.4 μm. For good dispersion and therefore good homogeneity of the API in the carrier particles, speed and powder blending time have to be sufficient, but not too long to prevent the appearance of static electricity, which is not favourable to homogeneity and stability. The FPF increases with the decrease in the carrier size. The storage conditions have also to be taken into consideration. Higher humidity favours the adhesion of API on the carrier and decreases the FPF.
KEY WORDS: adhesion, DPI performance, fluticasone propionate, operating parameters
INTRODUCTION
Particle interactions are of great importance in dry powder inhaler formulation where the dispersion of active pharmaceutical ingredient (API) particles from carrier particles is critical for lung deposition. Interactions between particles are dependent on the physicochemical characteristics of the interacting particles. On the one hand, the adhesion force must be strong enough to maintain the blend homogeneity during manufacturing process. On the other hand, it must first be overcome by inhalation flow to allow detachment in order to effectively deposit fine API into the lung. Various studies have extensively investigated the factors influencing the interaction forces, such as carrier surface roughness (1,2), carrier size (3,4), particle morphology (5–7), carrier material (8,9), environmental humidity (10,11), presence of excipient fines (12,13) and other formulation characteristics.
The manufacturing process is also important to consider. Blending API with carrier is a critical stage that determines the blend homogeneity and is the first step to obtain the final quality (14,15). Various blending options are available to the formulator. These include: low-energy tumbling blending; high-energy blending, with paddles or impeller blades actively cutting the powder bed and redistributing powder within the blending vessel (16). The blending process employed can influence the dispersion behaviour of the final formulation. In case of inhalation products, individual API particles will have aerodynamic diameters within the respirable range, whereas agglomerates will likely be larger than micron-sized. Thus, these factors influence the lung dose of inhalation products. In the mixing of cohesive powder, coarse particles act as nuclei, which are coated with a single layer of fine particles (17). If fine particles are in excess, it is assumed that they would agglomerate in a similar way around a nucleus of a fine particle. The kinetics of mixing process of micronized cohesive API with carrier in tumbling mixer was studied (18) and compared with the dissolution of final product in 995 mL of phosphate buffer, pH 5 (19) and 5 mL of 1% (w/v) sodium lauryl sulphate solution. An increase in mixing time led to an increase in dispersion. An initial fast de-agglomeration, indicated by an increase in dissolution, an increase in content uniformity and a decrease in particle size, was followed by substantially slower de-aggregation of remaining agglomerates and smaller aggregates. Better mixing time and mixing speed can accelerate de-agglomeration kinetics (18,20). However, precautions must be taken because the adhesive tendency of API particles in a model API–carrier system increases with blending time. Under the influence of shear forces within a mixer, API-containing particles would progressively move to positions of greatest stability (21). The presence of fine particles in the formulation can improve the dispersion of API in an interactive mixture. Interestingly, different mixing sequences were also shown to result in different lung deposition profiles of API (22,23). The addition of fine particles of lactose was also shown to reduce the electrostatic charge of coarse lactose particles (24).
During the mixing process, friction between particles or with the blender surface induce electrostatic charges on the particle surface (through tribo-electrification), which can affect greatly the quality of blend, such as homogeneity and segregation tendency (25,26). The decay rate of acquired surface electrostatic charges depends on the environmental humidity and the nature of materials in contact with the blend (polymer, glass…) (27). Thus, the mixing time followed by a rest time during mixing could influence the blend quality. Despite significant importance of mixing parameters, there is a limited understanding of how blending process affects the aerodynamic performance of inhalation products.
High relative humidity also plays a major role in DPI performance since the adsorption of water on the surface of a powder has a significant impact on the capillary forces, solid bridge formation and electrostatic forces. High relative humidity may increase inter-particulate forces due to increased capillary interactions resulting in the formation of larger agglomerates that are less breakable. And, lactose monohydrate may dissolve and then recrystallize resulting in solid bridges producing stronger agglomerates that do not disperse (28). An increase in relative humidity in the range 0–86% produces a corresponding decrease in charge and adhesion values for API, lactose and DPI formulation during tribo-electrification in association with mixing vessel surfaces (29).
The aim of the present investigation was to study parameters which predominantly influence the interactions between API and carrier particles in adhesive mixtures used in dry powder inhalers and their effect on API dispersion. Influences of the operating conditions (speed, mixing time, rest time during mixing), size of the carrier and storage conditions on the blend properties and on the API dispersion were studied.
MATERIAL AND METHODS
Materials
‘Lactohale 200’ (Friesland Foods Domo, Zwolle, The Netherlands) and micronized fluticasone propionate (FP) supplied by APTAR PHARMA (Le Vaudreuil, France) were used as supplied. The powder formulation was manually weighed and filled in the hard gelatine capsules (size 2; Capsugel, Colmar, France). The DPI chosen for performance testing was the Inhalator Ingelheim (Boehringer Ingelheim, Ingelheim and Rhein, Germany).
The grade of ‘Lactohale 200’ used had a volume mean diameter of 62.8 μm, a volume median diameter of 54.3 μm, 10% of particles were below 6.1 μm and 90% of particles were below 139.7 μm (size distribution obtained by laser scattering Mastersizer S). From the initial grade of ‘Lactohale 200’, three granulometric fractions were prepared and used as carrier (blends 11–13). These fractions (32–40 μm, 40–63 μm and 63–125 μm) were obtained after passage through an Alpine air-jet sieve used with an airflow that produced a pressure drop of 4 kPa.
Blending Lactose with Fluticasone Propionate
‘Lactohale 200’, used as the carrier, was mixed with 2.5% (w/w) of FP in a batch size of 100 g using a Turbula tumbling mixer (Bachofen Maschinenfabrik, Basel, Switzerland). The different blends manufactured and tested are listed in Table I.
Table I.
Operating Conditions of the Blends
| Blend | Carrier | Mixing speed (rpm) | Mixing time (min) |
|---|---|---|---|
| 1 | Lactohale 200 | 54 | 60 |
| 2 | Lactohale 200 | 74 | 60 |
| 3 | Lactohale 200 | 90 | 60 |
| 4 | Lactohale 200 | 100 | 60 |
| 5 | Lactohale 200 | 90 | 180 |
| 6 | Lactohale 200 | 90 | 120 |
| 7 | Lactohale 200 | 90 | 120 + 30 + 30 |
| 8 | Lactohale 200 | 90 | 60 + 30 |
| 9 | Lactohale 200 | 90 | 90 + 30 |
| 10 | Lactohale 200 | 90 | 120 + 30 |
| 11 | Lactohale 200, 32–40 μm fraction | 90 | 180 |
| 12 | Lactohale 200, 40–63 μm fraction | 90 | 180 |
| 13 | Lactohale 200, 63–125 μm fraction | 90 | 180 |
rpm rotation per minute
A 24-h rest time was observed for blends 7 to 10 between the different mixing times. All blends were analysed 24 h after the end of mixing to limit the electrostatic effects.
Flow Properties
The powder flow was tested using the angle of repose method described in the European Pharmacopoeia (30). The angle of repose is the constant, solid angle (relative to the horizontal base) assumed naturally by a cone-shaped pile of powder. The angle of repose was determined by allowing an excess quantity of material (25 g) positioned above a fixed diameter base to “drain” from a funnel (8 mm diameter) fixed at 6 cm above the base.
Angles of repose of less than 30° indicated excellent flow characteristics; values beyond 45° indicated poor powder flowability. Each measurement was done in triplicate.
Preparation of the Capsules
The lactose/API blends were filled into hard gelatine capsules manually so that each capsule contained 20 mg of blend (500 μg of FP).
Measurement of API Content and Content Uniformity
The API content of the blends was assessed by analysing the quantity of API in aliquots of 20 mg sampled powder which was the amount of powder in each capsule. Ten aliquots were taken randomly from each powder blend and assayed using HPLC (ProStar 230, Varian, Paris, France) with a Pursuit C18 column (150 × 4.6 mm, 5 μm) according to an internal validated method of APTAR Pharma. The API was detected spectrophotometrically at a wavelength of 236 nm. From the ten results of API content in the samples, the average API content in blend and the mean recovery related to the nominal dose were calculated. The coefficient of variation was the metric used to assess content uniformity of each blend.
Evaluation of Adhesion Characteristics
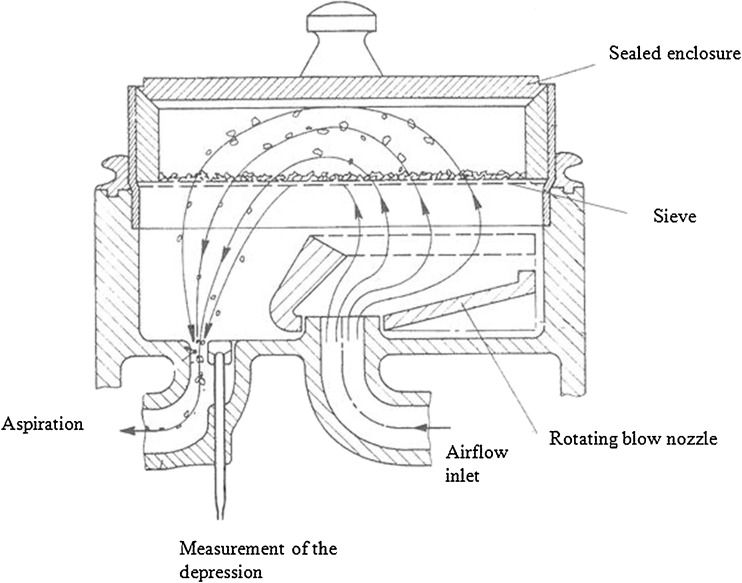
A simple method was developed to assess the adhesion of respirable sized API to carrier particles. This procedure was based on the aspiration principle and considered the whole blend as it is used in dry powder inhalers. Adhesion characteristics were evaluated by submitting the mixtures to a sieving action by air depression with the Alpine air-jet sieve (Fig. 1). In detail, 30 g of blend were put on the 32 μm sieve section of the Alpine air-jet apparatus, in a sealed enclosure. Particles were submitted on the one hand to an airflow released by a blow nozzle rotating under the sieve and, on the other hand, to aspiration through the sieve. The API particles that were detached from the carrier and suspended in air were carried by aspiration through the sieve. Three samples of 20 mg were removed from the powder bed after sieving for different lengths of time: 5, 30, 60, and 150 s. The percentage of remaining API associated with each sample was compared to the initial API mass, which was an indicator of the quantity of API that adhered to the carrier. Indeed, if the API particles had been completely separated from the carrier, they would have been carried away through the sieve by aspiration. Temperature and relative humidity were controlled at 20°C (±1°C) and 40–45% respectively for all experiments.
Fig. 1.
Experimental setup for the air-jet sieving
Aerodynamic Evaluation of Fine Particle Dose and Emitted Dose
In vitro deposition of API from dry powder formulations was determined using a twin stage impinger (TSI, Apparatus A, (31)). The TSI was assembled and loaded with 7 ml and 30 ml of solvent in the upper and lower stages respectively. Each deposition experiment involved the aerosolization at 60 l/min via an Inhalator Ingelheim of five capsules each containing 20 mg of blend (total mass = 500 μg FP). The different parts of the TSI were rinsed and the amount of API deposited in the upper and lower stage was determined.
For each blend, the assays were performed in triplicate and the following parameters were used to characterize the deposition profiles of the API:
The emitted dose (ED), which is the sum of API collected at upper and lower stages, divided by the number of capsules tested,
The fine particle dose (FPD) < 6.4 μm aerodynamic diameter at a flow rate of 60 L/min, defined as the mass of API deposited in the lower stage of the TSI, divided by the number of capsules tested,
The percentage emission calculated as the ratio of ED to the average content and
The fine particle fraction (FPF) calculated as the ratio of FPD to the emitted dose.
Temperature and relative humidity were controlled at 20°C (±1°C) and 40–45% for all experiments.
A one-way ANOVA F test was applied to the results obtained with TSI in order to determine the influence of the operating conditions on the DPI performance.
Stability of the Blends
To study the influence of the storage conditions, capsules containing the blends were stored in glass bottle placed in different conditions of temperature and humidity, that is to say: 20°C/12% RH, 20°C/40% RH, 20°C/65% RH and 40°C/75% RH. Content uniformity and aerodynamic evaluation of fine particle dose were both determined after 1 and 3 months. The performance of these blends was also assessed after storage of the capsules in either open or sealed glass containers after 1 and 3 months.
RESULTS AND DISCUSSION
Influence of Mixing Speed
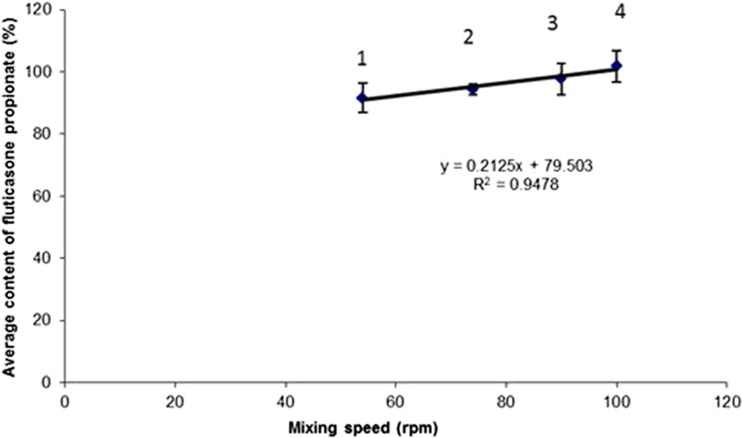
The mixing time was fixed at 60 min in order to study the influence of mixing speed. The comparison of blends 1, 2, 3, and 4 showed that increasing the speed of mixing increased the average FP content (probably because of a better de-aggregation and dispersion of the API) and the uniformity (Fig. 2). There was a linear relationship (R2 = 0.9478) between these two parameters. An increase in mixing speed favoured the dispersion of the API particles in the blend. The forces involved during the collisions between particles exceeded the cohesion forces between the FP particles. This finding is in agreement with Sebti et al. who indicated that the achievement of a homogeneous mixture from a composition containing a low-dose, cohesive active API and coarse excipients required the use of “powerful” shear mixers that broke down the aggregates so that the adhesive forces (API–carrier interaction) exceeded the cohesive forces (API–API interaction) between the fine particles (32).
Fig. 2.
Influence of mixing speed on average fluticasone content in blend (mixing time = 60 min). The numbers indicated on the figure refer to the blend number
Influence of Mixing Time
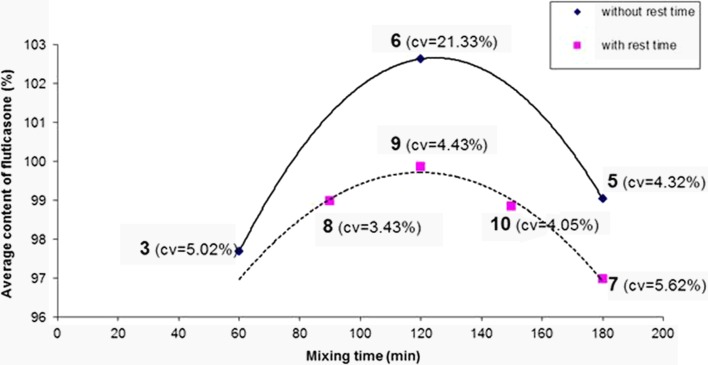
The mixing speed was fixed at 90 rpm in order to evaluate the influence of mixing time. The results are presented on Fig. 3.
Fig. 3.
Influence of mixing time on average fluticasone content and blend uniformity. The numbers indicated on the figure refer to the blend number
Mean API contents of the blends were poorly correlated with mixing time (ANOVA, p value = 0.678). However, a mixing time of 120 min without rest time (blend 6) gave the lowest homogeneity with a very high coefficient of variation (21.33%); this blend significantly differed from other blends (f test, p value < 0.05). The other blends were not significantly different (f test, p value > 0.05).
A pause during the mixing operation seemed to improve the content uniformity (dash line) since the coefficients of variation were lower. This behaviour was clearly observed when comparing blends 6 (without rest) and 9 (with rest; f test, p value < 0.05).This could be explained by a decrease of static electricity. Indeed, it was found that the total adhesion forces between drug and excipient particles decreased with the electrostatic charge decay during storage (33). During mixing, particles collided and rubbed against each other, and against the interior surfaces of the blender which favoured tribo-electrification. During rest time, particle charge acquisition decreased leading to a decrease of the cohesion forces and a better repartition of the API particles. But too long mixing time and rest time did not appear to have improved the blend uniformity. Thus, blend 5 (180 min. without rest) and blend 7 (180 min., two rest times) were not significantly different (f test, p value > 0.05).
When the API content was close to 100% (500 μg), lowest coefficients of variation were obtained for blends 5, 8, 9 and 10 and were comparable to each other.
Influence of Carrier Particle Size
Table II presents the aerodynamic performance of the different blends. Blends 11, 12, 13 carried out with granulometric fractions of ‘Lactohale 200’, containing no particles finer than 32 μm, had API FPF much lower than those of the blends containing unfractionated ‘Lactohale 200’. The decrease in the aerodynamic performance was probably due to the absence of fine particles of lactose. Two theories exist about the role of the fine particles of lactose. The first one supposes the occupation of the high-energy sites on the carrier by the lactose fine particles leaving low-energy sites available for the API, thus enabling greater API detachment (22,34). In the second theory also called redistribution theory, aggregates of API and lactose fine particles termed multiplets are formed enabling greater API detachment (22,34–36). At the present time, there is no preferential theory to explain the influence of lactose fine particles.
Table II.
Aerodynamic Performance of Blends
| Blend | Emitted dose (%) | Fine particle fraction (%) |
|---|---|---|
| 2 | 84.8 (±5.3) | 37.6 (±0.8) |
| 4 | 81.8 (±3.1) | 43.0 (±11.2) |
| 5 | 86.2 (±2.5) | 32.0 (±2.3) |
| 7 | 79.5 (±1.8) | 40.3 (±0.2) |
| 8 | 79.2 (±3.3) | 37.0 (±1.9) |
| 9 | 80.4 (±1.2) | 37.9 (±2.0) |
| 10 | 83.4 (±1.5) | 38.0 (±3.9) |
| 11 | 75.3 (±3.5) | 21.1 (±0.9) |
| 12 | 73.6 (±2.3) | 16.1 (±0.3) |
| 13 | 77.9 (±3.4) | 11.4 (±0.4) |
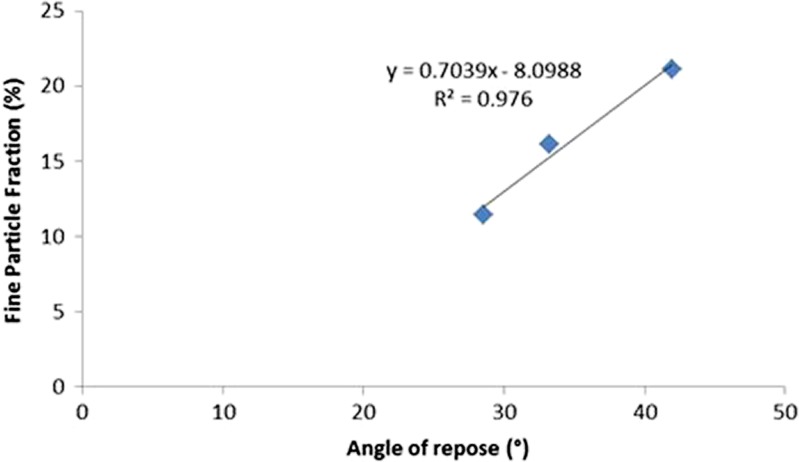
The absence of fine particles of lactose influenced the flowability evaluated by the angle of repose. The angles of repose were respectively 41.95 (±1.67), 33.22 (±1.52) and 28.52 (±1.07) for the blends 11, 12 and 13. The flowability decreased when the size decreased. Interestingly, we observed a linear relationship when plotting the FPF against the angle of repose (Fig. 4).
Fig. 4.
Relation between fine particle fraction and angle of repose of fractions of Lactohale 200
Higher values of FPF were obtained when the flowability was lower that is to say when the carrier size was lower. This observation is interesting because comparing angles of repose of different potential carriers could be used to select the most promising carrier for FP.
The influence of the size of the carrier on the drug adhesion was studied.
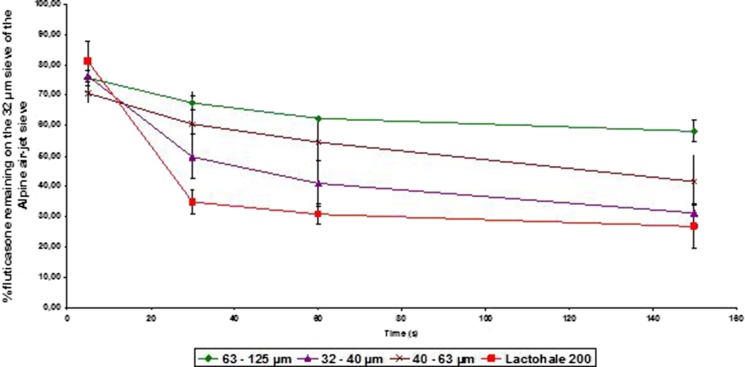
During the evaluation of adhesion characteristics, API-containing particles were rapidly carried away in the airflow of the Alpine air-jet sieve, when evaluated with this apparatus. The evolution of the mass percentage of FP remaining attached to the carrier particles as a function of operating time for this sieve was both dependent on test duration and blend size (Fig. 5). These data demonstrated the time-dependent nature of the API detachment process, with not all of the FP becoming separated from the carrier particles. With the 63–125 μm lactose fraction, adhesion of fluticasone seemed to be higher.
Fig. 5.
Percentage of fluticasone propionate remaining fixed onto the carrier in relation to the functioning time of the air-jet sieve
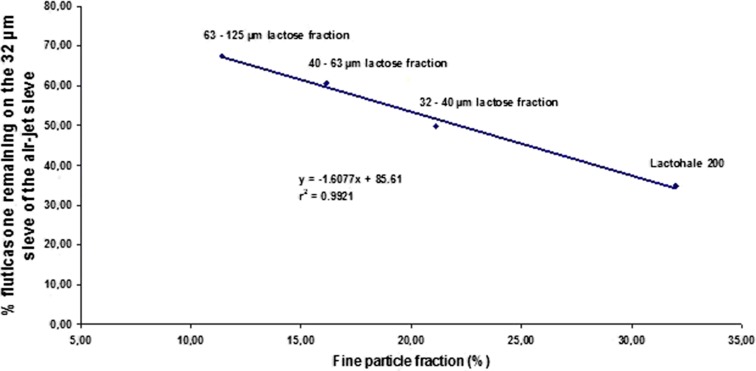
The results of API separation from the carrier by sieving with the Alpine air-jet sieve were compared with those obtained from in vitro impaction studies with the TSI. Figure 6 represents the relationship between fine particle fraction and the percentage of FP remaining on the 32 μm sieve of the air-jet sieve after 30 s. The relationship between FPF and the mass percentage of carrier-attached FP remaining on the 32 μm sieve was linear (r2 of 0.9921). The FPF increased when the carrier size decreased (37). This could be explained by the fact that surface forces between API and carrier particles were relatively high thus preventing liberation of API from the carrier. The highest FPF was obtained with the ‘Lactohale 200’ which possesses fine particles of lactose (10% of the particles are below 6.1 μm). These fine particles of lactose could be fixed on the higher energy sites of the ‘Lactohale 200’ which limited the adhesion of the fine particles of API and made their dispersion easier.
Fig. 6.
Relation between the fine particle fraction and the percentage of fluticasone remaining on the 32 μm sieve of the air-jet sieve after 30 s
Stability of the Formulations
Table III presents the influence of the storage time on the aerodynamic performance of blend 2. After 3 months, the FPF were not significantly different between the 20°C/12% RH, 20°C/40% RH and 20°C/65% RH conditions (ANOVA, p > 0.05) but they were lower in the case of the 40°C/75% RH conditions. This finding is in agreement with the work of Berard et al. (38), who observed that an increase in relative humidity increased the adhesion force between drug and carrier.
Table III.
Influence of Storage Time on Aerodynamic Performance of Blend 2
| Emitted dose (%) | Fine particle fraction (%) | |||
|---|---|---|---|---|
| After 1 month | After 3 months | After 1 month | After 3 months | |
| 20°C/12% RH | 82.6 (±1.9) | 79.0 (±1.0) | 34.3 (±0.9) | 32.3 (±1.4) |
| 20°C/40% RH | 84.9 (±2.0) | 82.2 (±6.5) | 35.1 (±2.1) | 29.4 (±4.1) |
| 20°C/65% RH | 85.0 (±1.3) | 79.6 (±2.1) | 38.9 (±3.8) | 31 (±0.2) |
| 40°C/75% RH | 84.1 (±2.8) | 79.6 (±1.1) | 32.1 (±3.8) | 26.9 (±0.2) |
To study the influence of the storage conditions, the aerodynamic performance was compared when the capsules were stored in sealed or open glass bottles, under different conditions of temperature and humidity (Table IV). Variations of the emitted doses were very low. A two-way ANOVA test showed that no significant difference was found between the different conditions (p > 0.05). However, FPF decreased significantly (p < 0.05) when the relative humidity exceeded 65%, particularly with the opened bottles. The incidence of the opening only appeared when the humidity was higher than 65%. The humidity could strengthen the adhesion between the drug and the carrier and therefore decrease the dispersibility of the drug. This finding is in agreement with the work of Berard et al. (38), who observed that an increase in relative humidity increased the adhesion force between drug and carrier.
Table IV.
Influence of Storage Conditions on Aerodynamic Performance of Blend 10
| Emitted dose (%) | Fine particle fraction (%) | |||
|---|---|---|---|---|
| Sealed bottle | Open bottle | Sealed bottle | Open bottle | |
| 20°C/12% RH | 80.6 (±1.0) | 82.5 (±2.2) | 33.0 (±5.3) | 37.3 (±3.2) |
| 20°C/40% RH | 81.5 (±2.4) | 82.0 (±2.3) | 36.6 (±3.4) | 37.1 (±3.1) |
| 20°C/65% RH | 82.3 (±0.8) | 83.3 (±1.1) | 38.3 (±2.0) | 30.7 (±2.2) |
| 40°C/75% RH | 80.2 (±0.3) | 78.3 (±1.3) | 29.9 (±1.5) | 17.7 (±3.9) |
CONCLUSION
The speed, the mixing time as well as the rest time between several phases of mixing influenced the blend homogeneity but also the adhesion characteristics and therefore the API detachment from the carrier, hence product performance. Speed and blending time have to be sufficient for good dispersion of API particle within powder blend, therefore good homogeneity of the API among the carrier particles, but not too high to prevent an excessive adhesion of the API on the carrier. Indeed, under extensive blending, API particles would progressively move to positions of greatest stability from an energetic standpoint, although an increased tribo-electrification of the particles may also contribute to the increased adhesion (39). The FPF of FP increased with the decrease in the carrier size. Finally, the storage conditions have also to be taken into consideration, since higher relative humidity in excess of 65% favoured the adhesion of FP on the carrier and decreased the FPF.
REFERENCES
- 1.Young PM, Roberts D, Chiou H, et al. Composite carriers improve the aerosolisation efficiency of drugs for respiratory delivery. J Aerosol Sci. 2008;39(1):82–93. doi: 10.1016/j.jaerosci.2007.10.003. [DOI] [Google Scholar]
- 2.Flament M-P, Leterme P, Gayot A. The influence of carrier roughness on adhesion, content uniformity and the in vitro deposition of terbutaline sulphate from dry powder inhalers. Int J Pharm. 2004;275(1–2):201–209. doi: 10.1016/j.ijpharm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Kassem NM, Ganderton D. Dry Powder Inhalers. In: Advances in Pharmaceutical Sciences. Vol 6. London: Academic; 1992:165–191.
- 4.Islam N, Stewart P, Larson I, Hartley P. Effect of carrier size on the dispersion of salmeterol xinafoate from interactive mixtures. J Pharm Sci. 2004;93(4):1030–1038. doi: 10.1002/jps.10583. [DOI] [PubMed] [Google Scholar]
- 5.Vanbever R, Mintzes JD, Wang J, et al. Formulation and physical characterization of large porous particles for inhalation. Pharm Res. 1999;16(11):1735–1742. doi: 10.1023/A:1018910200420. [DOI] [PubMed] [Google Scholar]
- 6.Hassan MS, Lau R. Inhalation performance of pollen-shape carrier in dry powder formulation with different drug mixing ratios: comparison with lactose carrier. Int J Pharm. 2010;386(1–2):6–14. doi: 10.1016/j.ijpharm.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Zeng XM, Martin GP, Marriott C, Pritchard J. The influence of carrier morphology on drug delivery by dry powder inhalers. Int J Pharm. 2000;200(1):93–106. doi: 10.1016/S0378-5173(00)00347-1. [DOI] [PubMed] [Google Scholar]
- 8.Steckel H, Bolzen N. Alternative sugars as potential carriers for dry powder inhalations. Int J Pharm. 2004;270(1–2):297–306. doi: 10.1016/j.ijpharm.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Saint-Lorant G, Leterme P, Gayot A, Flament MP. Influence of carrier on the performance of dry powder inhalers. Int J Pharm. 2007;334(1–2):85–91. doi: 10.1016/j.ijpharm.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Jashnani RN, Byron PR. Dry powder aerosol generation in different environments: performance comparisons of albuterol, albuterol sulfate, albuterol adipate and albuterol stearate. Int J Pharm. 1996;130(1):13–24. doi: 10.1016/0378-5173(95)04211-3. [DOI] [Google Scholar]
- 11.Jashnani RN, Byron PR, Dalby RN. Testing of dry powder aerosol formulations in different environmental conditions. Int J Pharm. 1995;113(1):123–130. doi: 10.1016/0378-5173(94)00197-D. [DOI] [Google Scholar]
- 12.Jones MD, Price R. The influence of fine excipient particles on the performance of carrier-based dry powder inhalation formulations. Pharm Res. 2006;23(8):1665–1674. doi: 10.1007/s11095-006-9012-7. [DOI] [PubMed] [Google Scholar]
- 13.Zeng XM, Martin GP, Tee S-K, Marriott C. The role of fine particle lactose on the dispersion and deaggregation of salbutamol sulphate in an air stream in vitro. Int J Pharm. 1998;176(1):99–110. doi: 10.1016/S0378-5173(98)00300-7. [DOI] [Google Scholar]
- 14.Food & Drug Administration. “Guidance for industry metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products”. 1998.
- 15.Bridson RH, Robbins PT, Chen Y, Westerman D, Gillham CR, Roche TC, et al. The effects of high shear blending on alpha-lactose monohydrate. Int J Pharm. 2007;339:84–94. doi: 10.1016/j.ijpharm.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Price R. Low and high shear blending. In: Lactose as a carrier for inhalation products. Parma, Italy; 2010.
- 17.Harnby N. Mixing in the process industries. Oxford: Butterworth-Heinemann; 1997. The mixing of cohesive powders; pp. 79–98. [Google Scholar]
- 18.Kale K, Hapgood K, Stewart P. Drug agglomeration and dissolution—what is the influence of powder mixing? Eur J Pharm Biopharm. 2009;72(1):156–164. doi: 10.1016/j.ejpb.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 19.British Pharmacopoeia. Appendix I D “Buffer solutions”: Phosphate buffer solution pH 5. 2005. A 145.
- 20.de Villiers MM. Description of the kinetics of the deagglomeration of drug particle agglomerates during powder mixing. Int J Pharm. 1997;151(1):1–6. doi: 10.1016/S0378-5173(97)04893-X. [DOI] [Google Scholar]
- 21.Kulvanich P, Stewart PJ. The effect of blending time on particle adhesion in a model interactive system. J Pharm Pharmacol. 1987;39(9):732–733. doi: 10.1111/j.2042-7158.1987.tb06978.x. [DOI] [PubMed] [Google Scholar]
- 22.Zeng XM, Martin GP, Tee S-K, Ghoush AA, Marriott C. Effects of particle size and adding sequence of fine lactose on the deposition of salbutamol sulphate from a dry powder formulation. Int J Pharm. 1999;182(2):133–144. doi: 10.1016/S0378-5173(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 23.Zeng XM, Pandhal KH, Martin GP. The influence of lactose carrier on the content homogeneity and dispersibility of beclomethasone dipropionate from dry powder aerosols. Int J Pharm. 2000;197(1–2):41–52. doi: 10.1016/S0378-5173(99)00400-7. [DOI] [PubMed] [Google Scholar]
- 24.Bennett FS, Carter PA, Rowley G, Dandiker Y. Modification of electrostatic charge on inhaled carrier lactose particles by addition of fine particles. Drug Dev Ind Pharm. 1999;25(1):99–103. doi: 10.1081/DDC-100102148. [DOI] [PubMed] [Google Scholar]
- 25.Staniforth JN. Performance-modifying influences in dry powder inhalation systems. Aerosol Sci Tech. 1995;22:346–353. doi: 10.1080/02786829408959752. [DOI] [Google Scholar]
- 26.Staniforth JN. Advances in powder mixing and segregation in relation to pharmaceutical processing. Int J Pharm Tech Prod Mafr. 1987;3(Supplement):1–12. [Google Scholar]
- 27.Carter PA, Rowley G, Fletcher EJ, Stylianopoulos V. Measurement of electrostatic charge decay in pharmaceutical powders and polymer materials used in dry powder inhaler devices. Drug Dev Ind Pharm. 1998;24(11):1083–1088. doi: 10.3109/03639049809089953. [DOI] [PubMed] [Google Scholar]
- 28.Das S, Larson I, Young P, Stewart P. Agglomerate properties and dispersibility changes of salmeterol xinafoate from powders for inhalation after storage at high relative humidity. Eur J Pharm Sci. 2009;37(3–4):442–450. doi: 10.1016/j.ejps.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Elajnaf A, Carter P, Rowley G. Electrostatic characterisation of inhaled powders: effect of contact surface and relative humidity. Eur J Pharm Sci. 2006;29(5):375–384. doi: 10.1016/j.ejps.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 30.European Pharmacopoeia. “2.9.36. Powder flow”. 2010. p. 5107.
- 31.European Pharmacopoeia. “2.9.18. Preparations for inhalation- aerodynamic evaluation of fine particles”. 2009. p. 306.
- 32.Sebti T, Vanderbist F, Amighi K. Evaluation of the content homogeneity and dispersion properties of fluticasone DPI compositions. J Drug del Sci Tech. 2007;17(3):223–229. [Google Scholar]
- 33.Pu Y, Mazumder M, Cooney C. Effects of electrostatic charging on pharmaceutical powder blending homogeneity. J Pharm Sci. 2009;98(7):2412–2421. doi: 10.1002/jps.21595. [DOI] [PubMed] [Google Scholar]
- 34.Larhrib H, Zeng XM, Martin GP, Marriott C, Pritchard J. The use of different grades of lactose as a carrier for aerosolised salbutamol. Int J Pharm. 1999;191:1–14. doi: 10.1016/S0378-5173(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 35.Adi H, Larson I, Chiou H, Young P, Traini D, Stewart P. Agglomerate strength and dispersion of salmeterol xinafoate from powder mixtures for inhalation. Pharm Res. 2006;23(11):2556–2565. doi: 10.1007/s11095-006-9082-6. [DOI] [PubMed] [Google Scholar]
- 36.Lucas P, Clarke MJ, Anderson K, Tobyn MJ, Staniforth JN. The role of fine particle excipients in pharmaceutical dry powder aerosols. In: Dalby RN, Byron PR, Farr SJ, editors. Proceedings of respiratory drug delivery VI. Buffalo Grove: Interpharm; 1998. pp. 243–250. [Google Scholar]
- 37.Steckel H, Muller BW. In vitro evaluation of dry powder inhalers II: influence of carrier particle size and concentration on in vitro deposition. Int J Pharm. 1997;154:31–37. doi: 10.1016/S0378-5173(97)00115-4. [DOI] [Google Scholar]
- 38.Berard V, Lesniewska E, Andrès C, Pertuy D, Laroche C, Pourcelot Y. Dry powder inhaler: influence of humidity on topology and adhesion studied by AFM. Int J Pharm. 2002;232:213–224. doi: 10.1016/S0378-5173(01)00913-9. [DOI] [PubMed] [Google Scholar]
- 39.Zeng XM, Martin GP, Marriott C. Particulate interactions in dry powder formulations for inhalation. London: Taylor & Francis; 2001. Particulate interactions in dry powder aerosols; p. 162. [Google Scholar]