Abstract
Due to the fact that the number of new poorly soluble active pharmaceutical ingredients is increasing, it is important to investigate the possibilities of improvement of their solubility in order to obtain a final pharmaceutical formulation with enhanced bioavailability. One of the strategies to increase drug solubility is the inclusion of the APIs in cyclodextrins. The aim of this study was to investigate the possibility of aripiprazole solubility improvement by inclusion in (2-hydroxy)propyl-β-cyclodextrin (HPBCD) and simultaneous manipulation of pH of the medium and addition of polyvinylpyrrolidone. Aripiprazole–HPBCD complexes were prepared by spray drying aqueous drug–HPBCD solutions, and their properties were compared with those prepared by solvent-drop co-grinding and physical mixing. The obtained powders were characterized by thermoanalytical methods (TGA and DSC), FTIR spectroscopy, their dissolution properties were assessed, while the binding of aripiprazole into the cavity of HPBCD was studied by molecular docking simulations. The solubilization capacity was found to be dependent on pH as well as the buffer solution’s ionic composition. The presence of PVP in the formulation could affect the solubilization capacity significantly, but further experimentation is required before its effect is fully understood. On the basis of solubility studies, the drug/HPBCD stoichiometry was found to be 1:3. The spray-dried products were free of crystalline aripiprazole, they possessed higher solubility and dissolution rate, and were stable enough over a prolonged period of storage. Spray drying of cyclodextrin solutions proved to be an appropriate and efficient technique for the preparation of highly soluble inclusion compounds of aripiprazole and HPBCD.
KEY WORDS: complexation, cyclodextrin, dissolution, physical characterization, spray drying
INTRODUCTION
It is a well-established fact that the number of new poorly soluble active pharmaceutical ingredients (APIs) is increasing (1). Therefore, it is important to investigate the possibility of improvement of their solubility in order to obtain a final pharmaceutical formulation with enhanced bioavailability. One of the strategies to increase drug solubility is the inclusion of the APIs in cyclodextrins, which offers numerous additional advantages, such as improvement of the formulation’s stability, safety, organoleptic properties, etc. (2). Due to these advantages, there are many examples of drugs formulated as cyclodextrin complexes on the market, such as piroxicam, nimesulide, omeprazole, and itraconazole (3,4). Usually desirable characteristics of a drug substance suitable for complexation with cyclodextrin are: a skeleton of more than five atoms (C, P, S, N) in the drug molecule, melting point temperature of the substance below 250 °C, solubility in water less than 10 mg/mL, molecular structure with less than five condensed rings, and molecular weight between 100 and 400 (5). Even when the drug possesses all the aforementioned favorable properties, in certain cases, further enhancement of the solubilizing power of cyclodextrins is desirable in order to reduce their necessary amount in a drug formulation for several reasons including toxicity, cost, and dosage, and it can be achieved by the selection of an appropriate cyclodextrin type, pH adjustment of the medium, and addition of different additives such as water-soluble polymers in the formulation (4,6).
A typical example of an API whose solubility has been enhanced by complexation with cyclodextrins is aripiprazole, a novel API with antipsychotic action, available on the market in the form of tablets, orally disintegrating tablets, oral syrup, and injectable solution. Since it is practically insoluble in water, formulation of dosage forms containing aripiprazole suitable for oral and parenteral administration is an appealing solution to its low bioavailability. Aripiprazole’s potential to be a good drug candidate for complexation with cyclodextrins has been recognized, and there is already a product containing an aripiprazole-beta-cyclodextrin derivative complex, marketed by Otsuka Pharmaceutical Company (Tokyo, Japan) under the brand name Abilify® in the form of intramuscularly injectable solution. However, due to its high molecular weight (448.4), incorporation in cyclodextrins is a challenging task and has not been thoroughly investigated yet. Therefore, the present study aims to elucidate the potential of further enhancing the solubility of aripiprazole using (2-hydroxy)propyl-β-cyclodextrin (HPBCD), which is known for its good aqueous solubility and low toxicity (3), and suitable manipulation of factors that influence the drug–cyclodextrin interaction, such as the pH of the medium and addition of polyvinylpyrrolidone (PVP). Aripiprazole–cyclodextrin complexes were prepared by spray drying aqueous solubilized drug solutions, and their properties were compared with those prepared by solvent-drop co-grinding and physical mixing. The obtained powders were characterized by thermoanalytical methods such as thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), Fourier transform infrared (FTIR) spectroscopy, and their dissolution properties were assessed in order to evaluate the extent of drug solubility and dissolution rate enhancement.
MATERIALS AND METHODS
Materials
In this study, the following materials were used: aripiprazole (Neuland Laboratories, India); (2-hydroxy)propyl-β-cyclodextrin, DS ~ 5.6 (Kleptose HP, Roquette, France); Polyvinylpyrrolidone MW 50,000 (BASF, Germany). All other ingredients were of analytical grade.
Methods
Water Content Determination
Cyclodextrins are known to form hydrates; therefore, they are likely to contain certain amounts of water depending on hydration state, and for the preparation of aqueous solutions of predefined molar concentration, this should be considered for measurement of the appropriate amounts of HPBCD. For the initial characterization of cyclodextrin to be used in the study, semi-micro determination of water was conducted by Karl Fischer titrimetry, which is based upon the quantitative reaction of water with sulfur dioxide and iodine in a suitable anhydrous medium in the presence of a base with sufficient buffering capacity (Ph. Eur. 2.5.12 Method A), by using the Karl Fischer titrator (835 Titrando, Metrohm, Switzerland).
For comparison purposes, loss on drying was also determined for cyclodextrin by a gravimetric method for samples of 2.5 g, using a Halogen moisture analyzer (Mettler-Toledo HR 83, Greifensee, Switzerland) at a temperature of 105 °C, and measurement time until the mean weight loss drops below 1 mg per 50 s.
Differential Scanning Calorimetry
DSC measurements of powder samples were carried out using a Shimadzu DSC50 Differential Scanning Calorimeter (Shimadzu Corporation, Tokyo, Japan). The thermal behavior was studied by heating the samples (2–5 mg) in a sealed aluminum pan from 30 to 300 °C, at a rate of 10 °C/min, using nitrogen as purge gas (flow rate 25 cm3/min). An empty pan was used as a reference.
Thermogravimetric Analysis
TGA measurements of powder samples were carried out using a Shimadzu TGA50 Thermogravimetric Analyzer (Shimadzu Corporation, Tokyo, Japan). The weight loss was monitored during heating the samples (2–5 mg) in an aluminum pan from 30 to 120 °C, at a rate of 10 °C/min, and under a nitrogen flow of 25 cm3/min.
Fourier Transform Infrared Spectroscopy
Attenuated total reflectance (ATR) FTIR spectra were obtained on a Shimadzu IR Prestige 21 spectrometer (Shimadzu Europa GmbH, Duisburg, Germany), with a horizontal Golden Gate MkII single-reflection ATR system (Specac, Kent, UK) equipped with a heated diamond top plate, ZnSe lenses and a 4000 series temperature controller. Spectra were recorded between 4,000 and 500 cm−1 at a resolution of 4 cm−1. A number of 32 scans were added for each spectrum.
Phase Solubility Studies
The solubility of aripiprazole in the presence of cyclodextrin was determined in different aqueous solution media in order to elucidate the effect of pH and ionic composition. Specifically, excess amounts of aripiprazole were added in distilled water, and two different buffers, both of pH = 3.0, a citrate (Ph. Eur. 4008000) and a phosphate buffer (Ph. Eur. 4010000), with increasing concentrations of HPBCD (0–320 mM). Suspensions were stirred on a magnetic stirrer at room temperature for 48 h. All suspensions were filtered through a 0.45-μm membrane filter, suitably diluted and analyzed spectrophotometrically at 217.5 nm on a Camspec M330 UV spectrophotometer (Camspec Inc., Cambridge, UK).
Preparation of Solid Systems
Solid drug–cyclodextrin systems were prepared by spray drying the final clear solution obtained after filtration of suspension from the phase solubility studies, on a Büchi B-191 mini spray drier (Büchi Laboratoriums-Technik AG, Flawil, Switzerland) under the following conditions: inlet temperature 120 °C, outlet temperature 70 °C, pump set to 15 %, aspirator 100 %, and atomization 550 l/h.
Powder samples were also prepared by solvent-drop co-grinding technique, with a cyclodextrin (CD)/drug molar ratio of 16:1 (as obtained in phase solubility studies in pure aqueous solutions of HPBCD). Cyclodextrin was wetted with few droplets of methanol in a ceramic mortar. The required amount of aripiprazole was then slowly added, and the powders were mixed with ceramic pestle for about 15 min. During this process, an appropriate quantity of methanol was added in order to maintain a suitable consistency of mixture. The final product was then dried in an oven at 40 °C for about 2 h and then allowed to equilibrate at room temperature for 24 h.
Two different physical mixtures, a mixture consisting of aripiprazole and cyclodextrin, and another consisting of aripiprazole, cyclodextrin, and PVP, were prepared by blending the powders in a PE bag simulating the mixing in the container mixer, and used as reference.
Dissolution Study
Dissolution studies were conducted in Dissolution Tester DT800 (Erweka, Heusenstamm, Germany) at 37 ± 0.5 °C, in a 900-ml pH 1.2 medium (hydrochloric acid), using a USP Apparatus II (paddle), at two different agitation rates: (a) 60 rpm [www.fda.gov (Dissolution method database)] and (b) 50 rpm (standard agitation speed, more discriminating condition). For these studies, the solid formulations (pure drug, spray-dried complex with HPBCD, and physical mixture with HPBCD) were filled into hard gelatin capsules (size #0). Fill weight of each capsule corresponded to approximately 10–11 mg of drug.
At the preset time intervals, aliquots were withdrawn from the dissolution medium, filtered through a 0.45-μm membrane filter, transferred to the spectrophotometer (8453 UV–visible spectrophotometer, Agilent, Santa Clara, USA), and assayed for aripiprazole at 250 nm. The test was repeated three times, and mean values and relative standard deviation were calculated.
Molecular Docking Simulations
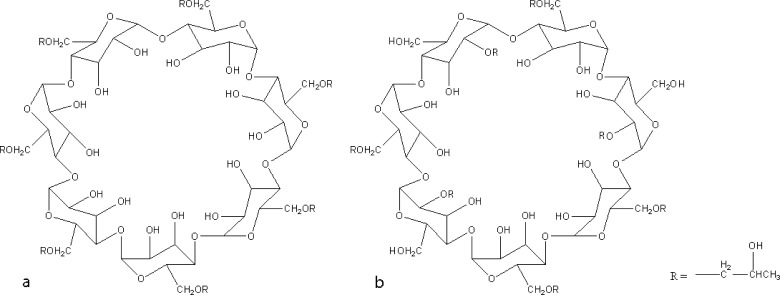
Docking simulations were performed using the AutoDock Vina software program that uses a sophisticated gradient local optimization algorithm, and automates grid map generation and result clustering procedures (7). The starting coordinates of aripiprazole were taken from the crystal structure of the commercial form A (CSD Ref No 285534), CHARMm force field parameters (8) and Gasteiger charges were assigned to the ligand atoms using the VEGA ZZ molecular modeling software (9), and energy minimization of aripiprazole molecule was performed using the NAMD molecular dynamics suite (10). Subsequently, non-polar hydrogen atoms were merged, and rotatable bonds were defined. Since it is impractical to simulate all possible isomers of substituted cyclodextrins, two representative isomers of (2-hydroxy)propyl-β-cyclodextrin were chosen for the simulation on the basis of the declared molecular substitution (M.S.) of the commercial product (Kleptose HP, with M.S. of 0.99; Fig. 1): isomer (a), with seven (2-hydroxy)propyl substituents placed on the O6 oxygens since this is the most reactive site (11), and a randomly substituted isomer (b), with four (2-hydroxy)propyl substituents placed on the O6 oxygens, and three on the next most reactive site, the O2 oxygens (11). Non-polar hydrogen atoms were merged, Kollman united atom type charges, and solvation parameters were added with the aid of the AutoDock tools program (12).
Fig. 1.
(2-Hydroxy)propyl-β-cyclodextrin isomers used for the docking simulations: isomer a, with substitution only at the O6 position, and isomer b, having substituents at the O6 and O2 positions
RESULTS AND DISCUSSION
Physical Characterization
Water Content Determination
The initial water content of the cyclodextrins, determined by the two different methods, was very similar (expressed as the mean of three determinations): by the Karl Fischer method, water content was found to be 4.38 % (standard deviation, 0.15 %), and by loss on drying (gravimetric method), it was found to be 4.27 % (standard deviation, 0.14 %). Results obtained by Karl Fischer method were considered in the calculation of appropriate amounts of HPBCD.
Thermal Analysis
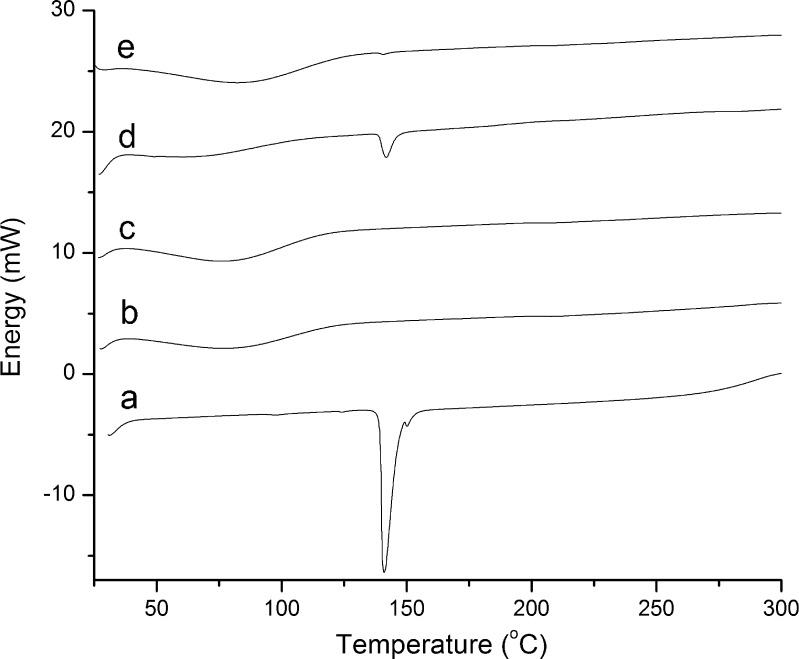
From the differential scanning calorimetry thermograms (Fig. 2), it is seen that aripiprazole has a melting endotherm in the range of 138–150 °C with a maximum at 141 °C, indicating that the raw material belongs to the crystalline form III (13), with a small secondary melting endotherm at 150 °C that can be attributed to the existence of a very small amount of form I.
Fig. 2.
Representative DSC thermograms of aripiprazole a, HPBCD b, aripiprazole–HPBCD inclusion compound produced by spray drying at 160 mM HPBCD concentration c, physical mixture of aripiprazole with HPBCD d, and aripiprazole–HPBCD inclusion compound produced by solvent-drop co-grinding e
The absence of a melting endotherm of the drug in the spray-dried product indicates that the total aripiprazole amount is included in the cavity of the cyclodextrins in a non-crystalline state (Fig. 2c). In the physical mixture, however (Fig. 2d), the melting endotherm of aripiprazole is still present, confirming the existence of crystalline drug and therefore the inability to form an inclusion compound by physical mixing. Another interesting feature of the thermograms is the broad endotherm spanning from room temperature to 100 °C, with a maximum in the range of 79–80 °C that probably corresponds to the loss of strongly bound water from the cyclodextrin hydration shell. No effect of the cyclodextrin molar concentration in the spray drying solution was observed in the thermograms, indicating that the amounts of cyclodextrins used in the present study were suitable and sufficient to fully include the drug in a molecular state.
Samples prepared by solvent-drop co-grinding of aripiprazole with cyclodextrin used in this study (Fig. 2e) exhibit a small melting endotherm at 141 °C, indicating the presence of a small amount of crystalline aripiprazole that has not been included in the cavity of the cyclodextrin. Although the solvent-drop co-grinding method appears as an attractive solvent and energy-saving alternative, more experimental work is required for the optimization of its parameters, which is beyond the scope of the present study.
Thermogravimetric analysis of the samples shows the following mass loss: 6.0 w/w, 5.9 w/w, and 5.5 % w/w for the HPBCD, aripiprazole–HPBCD spray-dried complex, and aripiprazole–HPBCD physical mixture, respectively (standard deviation, <1.5 %). The mass loss is occurring almost from room temperature and until ~90 °C, which supports the view that there is loss of bound water in this temperature range. The mass loss observed in the thermograms is very close to the water content determined by Karl Fischer titrimetry, with the spray-dried products showing a slight increase in water content, probably resulting from further hydration of the cyclodextrins in solution. The water of hydration is strongly bound, and it cannot be removed, despite the high temperature applied during the spray drying process.
FTIR Spectroscopy
Figure 3 illustrates ATR-FTIR spectra of aripiprazole, cyclodextrin, spray-dried product, and corresponding physical mixture. Aripiprazole (Fig. 3a) shows characteristic reflectance bands of polymorph III (13), in agreement with the melting range observed in the DSC thermograms. Specifically, the NH stretching vibration appears at 3,190 cm−1, CH2 stretching is seen at 2,945 cm−1, while the carbonyl stretching vibration is located at 1,684 cm−1, and the O–Ar bond stretch shows multiple peaks at 1,291, 1,275, and 1,239 cm−1.
Fig. 3.
Representative ATR-FTIR spectra of aripiprazole a, HPBCD b, aripiprazole–HPBCD inclusion compound produced by spray drying at 160 mM HPBCD concentration c, and corresponding physical mixture of aripiprazole with HPBCD d
The FTIR spectrum of pure cyclodextrin treated by spray drying without the presence of drug in the solution (Fig. 3b) shows a broad reflectance band in the range of 3,100–3,600 cm−1 with a maximum of ~3,340 cm−1, which is attributed to the OH stretching vibrations, indicating a strong association of the hydroxyl groups via hydrogen bonds. However, the existence of lower intensity but sharp peaks in the range of 3,600–3,700 cm−1 indicates the presence of free OH groups that possibly belong to adsorbed water that is not fully involved in hydrogen bonds.
Regarding the inclusion complex prepared by spray drying (Fig. 3c), the drug can be identified in the CH, CO, and in the fingerprint region; however, the intensity of aripiprazole’s peaks in the inclusion compounds is rather low, in contrast to the spectra of physical mixtures (Fig. 3d), where the drug shows clearly higher intensity peaks, indicating weaker or no interaction between the drug and the cyclodextrin when physically mixed.
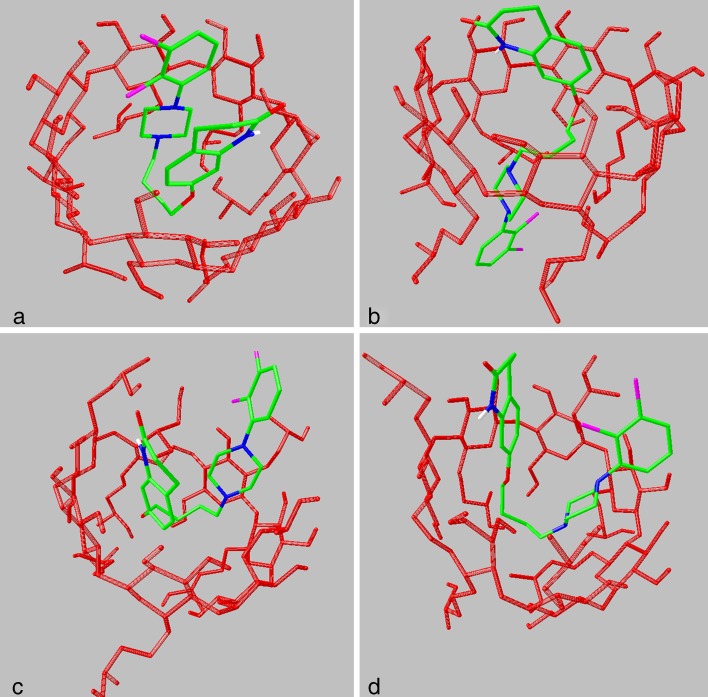
Molecular Docking Simulations
Molecular docking simulations showed that aripiprazole can form stable complexes acquiring various conformations. Figure 4a, b illustrates representative complexes of aripiprazole with HPBCD isomer (a), while Fig. 4c, d illustrates representative complexes of aripiprazole with HPBCD isomer (b) (apolar hydrogens have been removed for clarity). The most stable complex is formed when aripiprazole acquires a bent conformation (Fig. 4a), although it is possible to form complexes having a linear conformation (Fig. 4b). The highest estimated binding affinity is equal to −7.6 kcal/mol and corresponds to a complex with HPBCD isomer (a) (Fig. 4a). The existence of a hydrogen bond between the N–H hydrogen of aripiprazole and a neighboring O–H oxygen of HPBCD with an energy of −0.54 kcal/mol (calculated using CHARMm force field parameters) indicates the importance of hydrogen bonding interactions in the stabilization of the complexes. The estimated binding affinities for complexes in which aripiprazole has a linear conformation (Fig. 4b) are generally lower.
Fig. 4.
Representative docked conformations of aripiprazole in different HPBCD isomers
The location of the (2-hydroxy)propyl substituents also seems to affect binding. The highest calculated binding affinity for isomer (b) that has some (2-hydroxy)propyl substituents randomly placed in the O2 primary hydroxyl groups (Fig. 4c, d) was −6.4 kcal/mol, and the energy of the hydrogen bond between the aripiprazole N–H and a neighboring hydroxyl group of HPBCD was calculated to be −0.36 kcal/mol. Weaker binding of aripiprazole in HPBCD isomer (b) can be attributed to steric hindrance and prevention of approach into the hydrophobic cavity. Considering that the commercial products are usually mixtures of isomers consisting mainly of O6-substituted HPBCD (isomer (a), Fig. 1), with some amount of the O2- and O3-substituted HPBCD, the different binding affinities could further complicate the interpretation of the results of phase solubility studies.
Phase Solubility Studies
Effects of pH, Ionic Composition, and Addition of PVP
Τhe amount of cyclodextrin needed to achieve maximum drug solubility can be determined by phase solubility studies. Aripiprazole is a drug with basic character, and it shows a pH-dependent solubility that decreases with increasing pH. Therefore, in order to elucidate the effect of the pH and ionic composition of the dissolution medium on the solubility of aripiprazole in the presence of HPBCD, the following media were employed in the phase solubility studies: pure (distilled) water, pH 3.0 citrate buffer solution, and pH 3.0 phosphate buffer solution. Additionally, the potential for further solubility improvement by addition of small amounts of PVP was also studied since it is known that PVP could have some influence on the solubilizing effect of HPBCD (14). Besides, PVP is known to act as a nucleation inhibitor (3,15), so it might contribute to the stability of the samples. The results are summarized in Fig. 5 and Tables I and II.
Fig. 5.
Phase solubility diagrams for aripiprazole and HPBCD in different media
Table I.
Aripiprazole Solubility Results that Show the Influence of pH and Ionic Composition of the Medium on Drug Solubility, With and Without the Presence of HPBCD
| Medium | Aripiprazole solubility (mM) |
|---|---|
| Pure water | 0.009 |
| Citrate buffer pH 3 | 1.576 |
| Phosphate buffer pH 3 | 2.420 |
| 160 mM HPBCD solution in pure water | 1.508 |
| 160 mM HPBCD solution in citrate buffer pH 3 | 149.884 |
| 160 mM HPBCD solution in phosphate buffer pH 3 | 15.687 |
Table II.
Aripiprazole Solubility Results that Show the Influence of PVP Addition on Drug Solubility, With and Without the Presence of HPBCD
| Medium | Aripiprazole solubility (mM) |
|---|---|
| Pure water | 0.009 |
| Citrate buffer pH 3 | 1.576 |
| Citrate buffer pH 3, 0.2% w/w PVP | 0.934 |
| 260 mM HPBCD solution in pure water | 9.729 |
| 260 mM HPBCD solution in pure water, PVP/CD 0.005a | 10.961 |
| 260 mM HPBCD solution in pure water, PVP/CD 0.0025b | 7.788 |
| 260 mM HPBCD solution in citrate buffer pH 3 | 172.502 |
| 260 mM HPBCD solution in citrate buffer pH 3, PVP/CD 0.005a | 155.032 |
| 260 mM HPBCD solution in citrate buffer pH 3, PVP/CD 0.0025b | 184.317 |
aPVP concentration 0.2 % w/w
bPVP concentration 0.1 % w/w
It is seen that while the solubility in pure aqueous solutions of CD is low, a significant improvement can be achieved in citrate buffer pH 3 (Table I). Li et al. (16) described the importance of the combined effects of pH and complexation on solubility and the importance of the ionized species in contributing the overall complexation of drugs. It is often assumed that the unionized species are primarily responsible for the formation of the complex and the subsequent increase in solubility. However, ionization of a drug can also result in enhanced complexation, and the solubility of the ionized drug complex can sometimes exceed the solubility of the unionized drug complex.
It can also be observed that the solubility of aripiprazole is dependent not only on the pH of the medium but also on the type of buffer present. Comparing the drug solubility in pH 3.0 phosphate buffer (inorganic salt) and pH 3.0 citrate buffer (hydroxycarboxylic acid), in the absence of HPBCD there is no big difference in drug solubility. However, in the presence of HPBCD, choice of buffer has a significant effect on the drug solubility. This phenomenon was also described by Buchanan et al. (17) for the antifungal drugs and HPBCD. By observing the St/So ratio (St representing the drug solubility in the presence of HPBCD and So intrinsic solubility in the same medium without HPBCD), the calculated value for pure aqueous medium is ca 167.6, in phosphate buffer ca 6.5, and in citrate buffer ca 95.1 It is clear that cyclodextrin plays an important role in the solubilization of the aripiprazole, together with the ionic composition of the buffer, rather than the pH itself. The solubility of the corresponding complexes can be much higher than what could be expected by increasing the total drug solubility through only appropriate pH adjustment. This is possibly due to the specific interaction of the hydroxy acid groups with the hydrogen bond system of the host and/or the modification of the hydrogen bonding network of the surrounding water molecules (6). The drugs can be strongly bound inside the CD cavity even though they are in the ionized form because their basic center (and hence the charge) can be located quite far away from the molecule’s hydrophobic part that is included in the CD cavity, so the complexation ability is not significantly compromised. Moreover, the hydroxy acid can interact with the external hydrogen bonding system of CDs by a mechanism that involves the binding of the hydrophobic part of the drug and simultaneous formation of a strong ion pair. Redenti et al. (6) indicated a number of requirements for exploiting the properties of the multi-component complexation technology, among which are drug solubility less than 0.1 mg/mL, presence of an amino group with a fairly basic character (pKa > 5.0), etc.
Regarding the effect of PVP, it is seen in Fig. 5 that addition of PVP (mass ratio of PVP/CD 0.005) causes a decrease of solubility, which is in agreement with previous studies (18), and it can be attributed to the competition of polymer and drug for complexation with CD. However, optimizing the concentration of PVP could contribute to solubility improvement, as suggested by solubility results obtained CD solutions of the same concentration but with different concentration of PVP (different PVP/CD mass ratio; Table II).
Based on results obtained in phase solubility studies, it could be concluded that the improvement of aripiprazole’s solubility was most significant in citrate buffer with pH 3.0. In this medium, complexation of aripiprazole by HPBCD was the most efficient at the 160-mM CD concentration, as shown by the smallest CD/dissolved drug mass ratio obtained.
Analysis of Solubility Curves
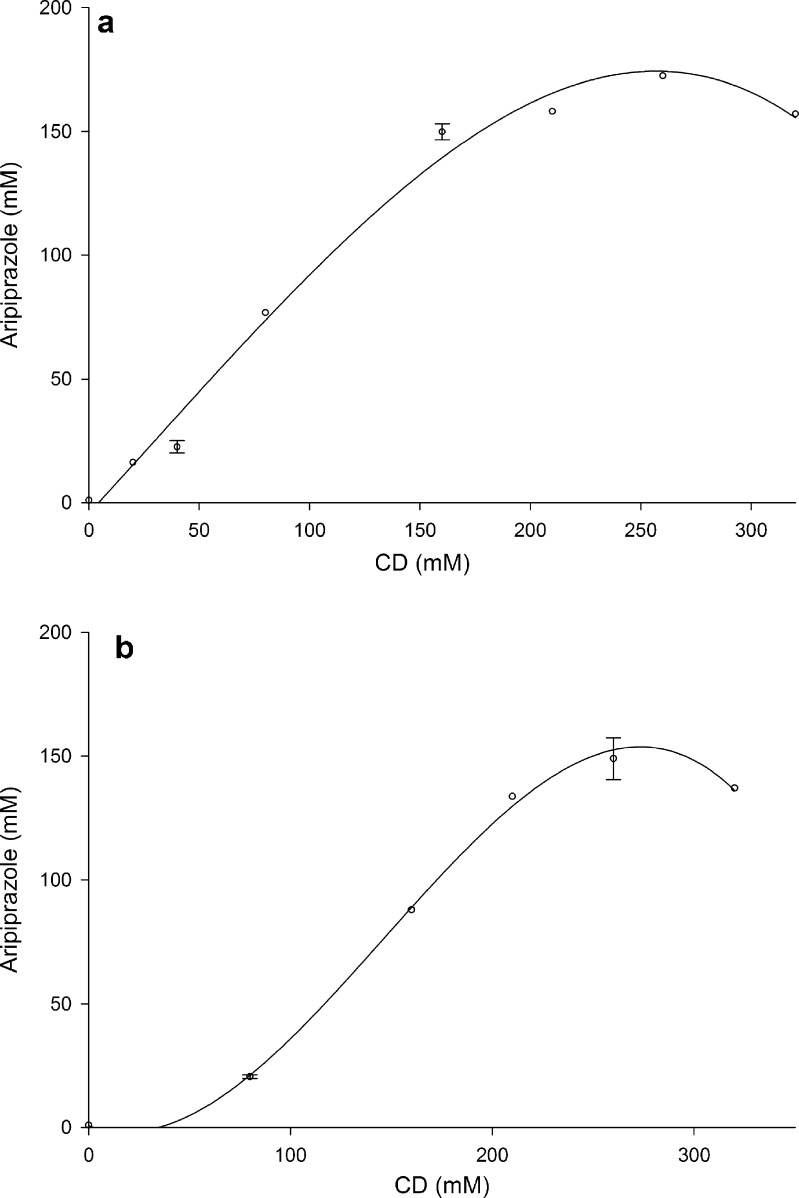
In general, based on the shape of the generated phase solubility relationships, several types of behaviors can be identified (19). From the shapes of the dissolution curves obtained in different media (Fig. 5), and taking into account the variety of conformations that aripiprazole can assume to form complexes with HPBCD of varying stability, as suggested by docking simulations, it can be assumed that there are complex mechanisms involved in aripiprazole solubilization. In this study, aripiprazole solubility was examined in a wider range of cyclodextrin concentration, and depending on concentration range observed, several different A profiles can be recognized. In so-called A systems, the apparent solubility of the substrate increases as a function of CD concentration.
In an aqueous medium containing only aripiprazole and HPBCD, an AP type curve could be observed, suggesting formation of higher-order complexes at higher CD concentration (Fig. 6). As the stoichiometry of the system can be probed by curve fitting of the diagram with linear, quadratic, or a cubic model, for this isotherm the cubic function showed the best fit (R2 = 0.996), suggesting a 1:3 complex formation (D(CD)3).
Fig. 6.
Phase solubility diagram for aripiprazole in HPBCD solution in water
Phase solubility diagrams in citrate buffer medium with and without addition of PVP (Fig. 7) showed profiles that can be classified as AN subtype because at the higher CD concentrations a negative deviation from linearity exists, meaning that CD is less effective at higher concentrations. Best fit was obtained for a cubic function (R2 = 0.996 with PVP, R2 = 0.987 without PVP) suggesting the formation of a 1:3 complex (D(CD)3).
Fig. 7.
Phase solubility diagrams for media with the most significant solubility improvement: a citrate buffer pH 3; b citrate buffer pH 3 with PVP
Similar curvature shapes of the phase solubility profiles were reported by Buchanan et al. (17) for the antifungal drugs and HPBCD in some buffers. AN profiles were explained by bulk changes imparted to the solvent by the solubilizer at various concentrations and/or self-association of the solubilizer (HPBCD) at high concentrations.
Solubility profile could be characterized as a normal profile with a negative deviation at low CD concentrations (A−L), with y-intercept at the negative part of the axis. This behavior was reported by Loftsson et al. (19) and explained by self-association of poorly soluble drug molecules and drug/cyclodextrin complexes, non-inclusion complexation, and excipient interactions. It is possible that the observed solubility increase is a cooperative effect of several different solubilizing processes and interactions. The various anomalies observed in the solubility curves, such as the significant deviation of the experimental intrinsic solubility from that estimated from the y-intercept, or even the negative solubility value suggest the possibility of formation of multi-component complexes and simultaneous formation of inclusion and non-inclusion complexes, or self-association phenomena (3,19,20).
Long-Term Stability of Aripiprazole–Cyclodextrin Inclusion Compounds
The long-term stability of aripiprazole–cyclodextrin inclusion compounds upon storage in ambient conditions was examined after 6 and 12 months by differential scanning calorimetry and thermogravimetric analysis (results not shown). No sign of aripiprazole crystallization could be identified in the thermograms (complete absence of endotherm peaks corresponding to the melting of aripiprazole), which suggests that the inclusion compounds are stable, and no spontaneous crystallization of the dug occurs over a prolonged period of storage. Additionally, examination of the water content by TGA showed no significant change, further supporting the view that the cyclodextrin complexes are stable.
Dissolution Study
For the assessment of drug dissolution rate improvement, dissolution studies were conducted. Figure 8 shows the percent of aripiprazole dissolved vs time for an aripiprazole–HPBCD complex, in comparison to the corresponding physical mixture and pure aripiprazole, at different agitation rates. It is seen that more than 80 % of aripiprazole was dissolved from the complex with HPBCD already after 10 min regardless of agitation rate, which was not the case for the other samples. The presence of cyclodextrin in physical mixture with aripiprazole also contributed to some dissolution improvement in vitro. However, by observing the relative standard deviation values (represented by error bars on Fig. 8), it was concluded that only from the spray-dried drug–CD complex was the dissolution very rapid and uniform, which is a significant characteristic of the dissolution profile. Therefore, the results obtained in this study suggest that the complexation of aripiprazole with cyclodextrins could be used as a formulation strategy for solubility and dissolution rate enhancement.
Fig. 8.
Dissolution rate of aripiprazole from different samples, in 0.1 M HCl, agitation speed: a 60 rpm and b 50 rpm
Having in mind pH-dependent aripiprazole solubility, it would be interesting to further examine the dissolution of aripiprazole from complexes with HPBCD in dissolution media with different pH. As it was reported in a study by Nacsa et al. (21), there is a possibility that the solubility of a drug can become independent of the pH through the application of CD for drug complexation. Since the difference between the profiles was more pronounced at the lower agitation speed, which was expected, this dissolution condition should be used for future dissolution studies, as being more discriminative.
CONCLUSIONS
Spray drying of cyclodextrin solutions proved to be an efficient technique for the preparation of highly soluble inclusion compounds of aripiprazole and HPBCD. The spray-dried products were free of crystalline aripiprazole, they possessed higher solubility and dissolution rate, and proved to be stable enough over a prolonged period of storage.
The solubilization capacity was found to be dependent on pH as well as the buffer solution’s ionic composition, with maximum solubilization being achieved in citrate buffer with a pH value of 3. On the basis of solubility studies, the drug/cyclodextrin stoichiometry was found to be 1:3.
The presence of PVP in the spray drying solution can affect the solubilization capacity significantly, but further experimentation is required before its effect is fully understood.
Docking simulations confirmed aripiprazole’s ability to form stable inclusion complexes with HPBCD and also suggested complicated interactions owing to the flexibility of the drug’s backbone.
ACKNOWLEDGMENTS
This work was supported by the Project TR 34007, Ministry of Education and Science, Republic of Serbia.
REFERENCES
- 1.Dubin CH. Formulation strategies for poorly soluble drugs. Drug Del Technol. 2006;6(6):34–38. [Google Scholar]
- 2.Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS Pharm Sci Tech. 2005;6(2):E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Del Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Szejtli J. Past, present, and future of cyclodextrin research. Pure Appl Chem. 2004;76(10):1825–1845. doi: 10.1351/pac200476101825. [DOI] [Google Scholar]
- 5.Vyas A, Saraf S, Saraf S. Cyclodextrin based novel drug delivery systems. J Incl Phenom Macrocycl Chem. 2008;62:23–42. doi: 10.1007/s10847-008-9456-y. [DOI] [Google Scholar]
- 6.Redenti E, Szente L, Szejtli J. Mini-review drug/cyclodextrin/hydroxy acid multicomponent systems, properties and pharmaceutical applications. J Pharm Sci. 2000;89(1):1–8. doi: 10.1002/(SICI)1520-6017(200001)89:1<1::AID-JPS1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Momany FA, Rone R. Validation of the general purpose QUANTA ®3.2/CHARMm® force field. J Comput Chem. 1992;13:888–900. doi: 10.1002/jcc.540130714. [DOI] [Google Scholar]
- 9.Pedretti A, Villa L, Vistoli G. VEGA: a versatile program to convert, handle and visualize molecular structure on Windows-based PCs. J Mol Graph. 2002;21:47–49. doi: 10.1016/S1093-3263(02)00123-7. [DOI] [PubMed] [Google Scholar]
- 10.Phillips J, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gramera RE, Caimi RJ. Cyclodextrin polyethers and their production. US Patent 3,459,731. August 5, 1969.
- 12.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- 13.Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ. Conformational polymorphism in aripiprazole: preparation, stability and structure of five modifications. J Pharm Sci. 2009;98:2010–2026. doi: 10.1002/jps.21574. [DOI] [PubMed] [Google Scholar]
- 14.Mura P, Faucci MT, Bettinetti GP. The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropyl β-cyclodextrin. Eur J Pharm Sci. 2001;13:187–194. doi: 10.1016/S0928-0987(01)00093-8. [DOI] [PubMed] [Google Scholar]
- 15.Raghavan SL, Trividic A, Davis AF, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 2001;212:213–221. doi: 10.1016/S0378-5173(00)00610-4. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Tabibi SE, Yalkowski SH. Combined effect of complexation and pH on solubilization. J Pharm Sci. 1998;87(12):1535–1537. doi: 10.1021/js9801889. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan CM, Buchanan NL, Edgar KJ, Ramsey MG. Solubility and dissolution studies of antifungal drug:hydroxybutenyl-β-cyclodextrin complexes. Cellulose. 2007;14:35–47. doi: 10.1007/s10570-006-9076-x. [DOI] [Google Scholar]
- 18.Quan P, Liu DF, Li R, Zhang Q, Qian Y, Xu QW. The effects of water-soluble polymers on hydroxypropyl-β-cyclodextrin solubilization of oleanolic acid and ursolic acid. J Incl Phenom Macrocycl Chem. 2009;63:181–188. doi: 10.1007/s10847-008-9505-6. [DOI] [Google Scholar]
- 19.Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302:18–28. doi: 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 20.Messner M, Kurkov SV, Jansook P, Loftsson T. Self-assembled cyclodextrin aggregates and nanoparticles, review article. Int J Pharm. 2010;387(1–2):199–208. doi: 10.1016/j.ijpharm.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Nacsa A, Berkesi O, Szabo-Revesz P, Aigner Z. Achievement of pH-independence of poorly-soluble, ionizable loratadine by inclusion complex formation with dimethyl-β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2009;64:249–254. doi: 10.1007/s10847-009-9558-1. [DOI] [Google Scholar]