Abstract
Liposome-encapsulated polyplex system represents a promising delivery system for oligonucleotide-based therapeutics such as siRNA and asODN. Here, we report a novel method to prepare liposome-encapsulated cationic polymer/oligonucleotide polyplexes based on the reverse-phase evaporation following organic extraction of the polyplexes. The polyplexes of polyethylenimine and oligonucleotide were first formed in aqueous buffer at an N/P ratio of 6. The overall positively charged polyplexes were then mixed with the anionic phospholipids in overall organic media. The overall organic environment and electrostatic interaction between anionic phospholipids and positively charged polyplexes resulted in inverted micelle-like particles with the polyplexes in the core. After phase separation, the hydrophobic particles were recovered in organic phase. Reverse-phase evaporation of the organic solvent in the presence of hydrophilic polymer-grafted lipids resulted in a stable aqueous dispersion of hydrophilic lipid-coated particles with the polyplex in the core. Transmission electron microscopy visualization revealed spherical structures with heavily stained polyplex cores surrounded by lightly stained lipid coats. The lipid-coated polyplex particles showed colloidal stability, complete protection of the loaded oligonucleotide molecules from enzymatic degradation, and high loading efficiency of more than 80%. Thus, this technique represents an alternative method to prepare lipid-coated polyplex particles as a delivery system of oligonucleotide therapeutics.
KEY WORDS: encapsulation, liposome, oligonucleotide, polyethylenimine, polyplexes
INTRODUCTION
Overcoming the poor in vivo stability of conventional liposomal systems, sterically stabilized liposomes by polyethylene glycol (PEG), i.e., PEG-stabilized liposomes, have been shown to be promising delivery systems for small molecular weight drugs as well as macromolecular therapeutics due to dramatic improvement in pharmacokinetics. The PEG-stabilized liposomes have also been successfully applied for in vivo delivery of various nucleic acid-based therapeutics such as plasmid DNA (1,2) or oligonucleotides (3).
However, it has been a challenge to encapsulate nucleic acid-based therapeutics, as with other macromolecular therapeutics, within liposomal systems. A variety of approaches have been proposed to prepare liposomal systems loaded with nucleic acid-based therapeutics, but a majority of them still suffer from low encapsulation efficiency or other complications (4,5). Most of the procedures employed cationic lipids such as DOTAP to facilitate encapsulation of negatively charged nucleic acid-based therapeutics or complicated multi-step procedures such as detergent dialysis (2) and extraction into an organic phase followed by reverse evaporation (3). Condensation of nucleic acid-based therapeutics using a polycationic polymer followed by encapsulation into liposomes has also been reported. “Liposome-entrapped polycation-condensed DNA particles” were prepared by first condensing plasmid DNA with poly-lysine and then entrapping the complexes into folate-targeted anionic liposomes for tumor-specific oligonucleotide transfer (6). Plasmid DNA also was condensed with polyethylenimine (PEI) and entrapped within preformed anionic liposomes (2,7,8). Preformed PEI/DNA polyplexes were also encapsulated in PEG-stabilized liposomes that resulted in a so-called pre-condensed stable plasmid lipid particle (9).
In an effort to improve the poor in vivo stability and pharmacokinetic behavior of the polyplex systems between polyethylenimine and oligonucleotide, we previously reported a procedure to prepare liposome-encapsulated polyethylenimine/oligonucleotide polyplexes within PEG-stabilized liposomes by rehydrating anionic lipid film in aqueous buffer containing preformed polyethylenimine/oligonucleotide polyplexes (10). As an extension of our previous works to construct in vivo applicable delivery systems for oligonucleotide therapeutics, we here report an alternative procedure to encapsulate the polyplexes into PEG-stabilized liposomes. This work is quite similar to the previous study in a sense that both leads to the same final constructs. However, this work describes a novel procedure for the same construct with process simplicity and encapsulation efficiency comparable to the previous study. Low molecular weight polyethylenimine (PEI) (11,12) with MW 2.7 kDa and 20-bp double-stranded oligodeoxynucleotide (ODN) containing the NF-κB cis-element (13) were used to form overall positively charged polyplexes. The polyplexes in aqueous media were completely transferred into organic phase by anionic phospholipids in the form of inverted micelle-like particles. The hydrophobic micelle-like particles in the organic media were subject to reverse-phase evaporation. During the evaporation, neutral phospholipids, including 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (PEG-PE), were added to coat the hydrophobic micelle-like particles in the form of the outer leaflet, resulting in sterically stabilized hydrophilic particle with the polyplex core enveloped by the lipid bilayer. The procedure was optimized and the resulting lipid-coated polyplex particle was characterized with respect to size, shape, colloidal stability, and encapsulation efficiency.
MATERIALS AND METHODS
Double-Stranded Oligodeoxynucleotides
The sequence of the ODN (5′-CCTTGAAGGGATTTCCCTCC-3′ and 3′-GGAACTTCCCTAAAGGGAGG-5′) contained the NF-κB cis-element (13). Single-stranded 20-mer oligonucleotides and corresponding 5′-fluorescein-conjugated oligonucleotides were purchased from MWG Biotech (Highpoint, NC, USA). Double-stranded ODNs were prepared by annealing the single-stranded oligonucleotides with equal molar amounts of complementary single stranded oligonucleotides at a final ODN concentration of 1 μg/μl.
Lipids
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-91-glycerol] (POPG), PEG-PE, and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL, USA).
Polyethylenimine
Low-molecular weight PEI (MW 2.7 kDa) was synthesized and characterized as described previously (11,14) and generously provided by Dr. T. Kissel (Marburg, Germany). PEI was dissolved in HBG buffer (10 mM HEPES, 5% glucose, pH 7.4) at a final concentration of 0.9 μg/μl.
Preparation of the Lipid-Coated PEI/ODN Particles
For the preparation of PEI/ODN polyplexes, 90 μg of PEI and 100 μg of ODN were separately diluted into 500 μl of 10 mM HEPES buffer (5% glucose, pH 7.4). After 10 min at room temperature, the PEI solution was rapidly transferred to the ODN solution and vortexed briefly, resulting in PEI/ODN polyplexes with an N/P ratio of 6. To anionic POPG (3.0 μmol) diluted in 1.0 ml of chloroform, 2.08 ml of methanol was added followed by 1.0 ml of the preformed PEI/ODN complex (100 μg ODN). After 30 min incubation at room temperature, 1.0 ml of chloroform and 1.0 ml of water were added and the tubes centrifuged for 7 min at 830×g. The aqueous phase was removed, and POPC (6.7 μmol) and PEG-PE (0.3 μmol) were added to the organic phase. After 1.0 ml of 10 mM HEPES buffer (5% glucose, pH 7.4) was added, the tube was vortexed vigorously and sonicated for 1 min. The chloroform was then removed by evaporation under vacuum on a rotary evaporator. The remaining aqueous dispersion was extruded 11 times through two stacks of 100 nm polycarbonate membranes by using a hand held extruder (Avestin, Ottawa, ON, USA). The resulting dispersion was assessed for colloidal stability in PBS, protection of ODN from external environment by fluorescence quenching and enzyme digestion, encapsulation efficiency of ODN into particles, and morphologic structure using transmission electron microscopy (TEM).
Colloidal Stability
The colloidal stability of the lipid-coated particles was determined by monitoring hydrodynamic diameter using a particle size analyzer Nicomp 380ZLS (Particle Sizing Systems, Santa Barbara, CA, USA). The mean diameter of the lipid-coated particles was measured at 5-min intervals, immediately after the particles were diluted with PBS. The scattered light was detected at 23°C at an angle of 90°. A viscosity value of 0.933 mPa s and a refractive index of 1.333 were used for the data analysis. The instrument was routinely calibrated using latex microsphere suspensions (0.09 μm, 0.26 μm; Duke Scientific Corp, Palo Alto, CA, USA).
Protection of ODN from KI Quenching
To the lipid-coated particles prepared with fluorescein-labeled ODN, potassium iodide (KI) as a quencher was sequentially added, and fluorescence emission intensities were measured. The external quencher KI (15) has limited access to the fluorophores attached to the ODN in the lipid-coated particles. Thus, the lipid-coated particles should show less quenching than the free ODN or naked polyplexes. The quenching constants (Ksv) were calculated using the Eq. 1
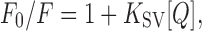 |
1 |
where F0 = fluorescence emission intensity in the absence of KI, F = fluorescence emission intensity in the presence of KI, and [Q] = molar concentration of KI.
Protection of ODN from Enzymatic Degradation
The lipid-coated particles were incubated with DNAse I (100 U/ml) for 30 min at 37°C. The reaction was terminated by adding EDTA to a final concentration 5 mM. The resulting mixtures were treated with Triton X-100 at 1% final concentration and analyzed on a 1% agarose gel in TBE buffer.
Transmission Electron Microscopy Analysis
The lipid-coated particles were applied as a drop on glow-discharged Fomvar silicon monoxide-coated grids (Ted Pella, CA, USA). After 5 min at room temperature, the excess sample was removed by blotting with filter paper and washed five times with H2O. The specimens were then negatively stained by applying a drop of 1% uranyl acetate in H2O. After 2 min at room temperature, the specimens were washed three times with H2O and dried at room temperature for 30 min. The specimens were visualized using a transmission electron microscope (80 kV, Zeiss EM109, Germany).
Determination of ODN Encapsulation Efficiency
To determine ODN entrapping efficiency, the lipid-coated PEI/ODN particles were prepared as described above, and the fractions of Sepharose CL4B column eluent were analyzed for ODN content using OliGreen (Invitrogen) (16). Briefly, 100 μl of each fraction was treated with Triton-X 100 (1.0 wt.%) and heparin (20 U). The release of ODN was measured by the fluorescence intensity emitted by OliGreen after binding ODN, using excitation and emission wavelengths of 485 and 560 nm, respectively. The entrapment efficiency of ODN was calculated as the area under the curve of the first peak relative to the total area under the curve.
RESULTS
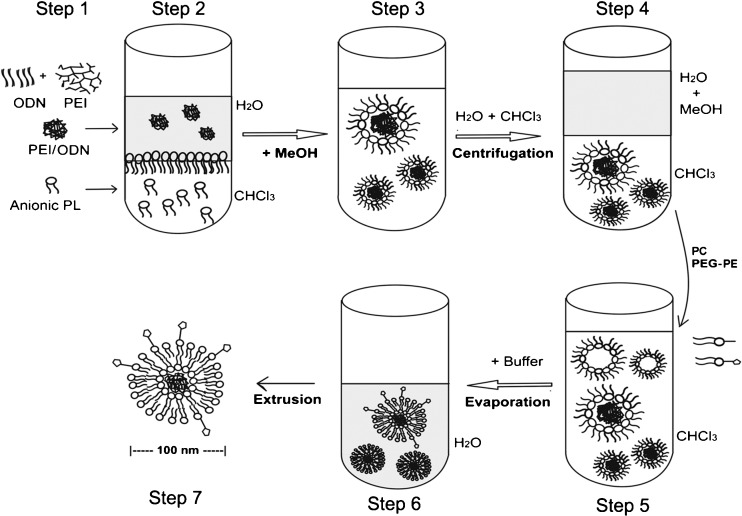
A novel-coated cationic liposomal formulation was previously reported for active entrapping of antisense ODN into a liposomal formulation with small size, high incorporation efficiency, and good stability (17). The procedure was modified and applied to encapsulate the preformed PEI/ODN polyplexes into PEG-stabilized liposomes. While Allen utilized the electrostatic interaction between cationic phospholipid DOTAP and anionic oligodeoxynucleotides in the original study, we used anionic phospholipid POPG to extract the positively charged PEI/ODN polyplexes (Fig. 1).
Fig. 1.
Schematic diagram demonstrating the steps to prepare the lipid-coated PEI/ODN polyplex particles. 1 Formation of the PEI/ODN polyplexes in aqueous buffer, 2 biphasic mixture of the polyplexes in aqueous phase and anionic phospholipids (PL) in chloroform (CHCl3) organic phase, 3 formation of the micelle-like hydrophobic particles with the polyplex inside in overall organic monophase, 4 phase separation and extraction of the hydrophobic particles into organic phase, 5 (a) addition of the hydrophilic polymer-grafted lipid PEG-PE and (b) addition of aqueous buffer and evaporation of organic phase, 6 formation of PEG-stabilized lipid-coated particle with the PEI/ODN polyplex inside, and 7 size reduction by membrane extrusion
The polyplexes between PEI and ODN were first prepared in an aqueous buffer at N/P ratio 6 and then combined with POPG in an organic monophase. An overall organic environment and electrostatic interaction between negatively charged POPG and positively charged PEI/ODN polyplexes resulted in hydrophobic inverted micelle-like particles with the PEI/ODN polyplexes in the core. Upon phase separation, the radio-labeled ODN was completely partitioned to and thus recovered in the organic phase, supporting the formation of the hydrophobic particles (data not shown).
Upon reverse-phase evaporation of the organic solvent in the presence of hydrophilic polymer-grafted lipid PEG-PE, the hydrophobic particles became hydrophilic by the formation of outer lipid layer around the hydrophobic particle through coating process driven by hydrophobic interaction. As a result, stable aqueous dispersion of the lipid-coated particles with the PEI/ODN polyplexes in the core was obtained. The size measurement of the lipid-coated particles (Fig. 1 step 6) by dynamic light scattering showed an average diameter of 300 nm with wide distribution (±100 S.D.), which was reduced to an average diameter of 130 nm with narrow size distribution (±10 S.D.) by membrane extrusion (Fig. 1 step 7). The increased hydrodynamic diameter of the lipid-coated particles, as compared to the naked PEI/ODN polyplexes, indicates the formation of the lipid coat around the polyplexes (data not shown).
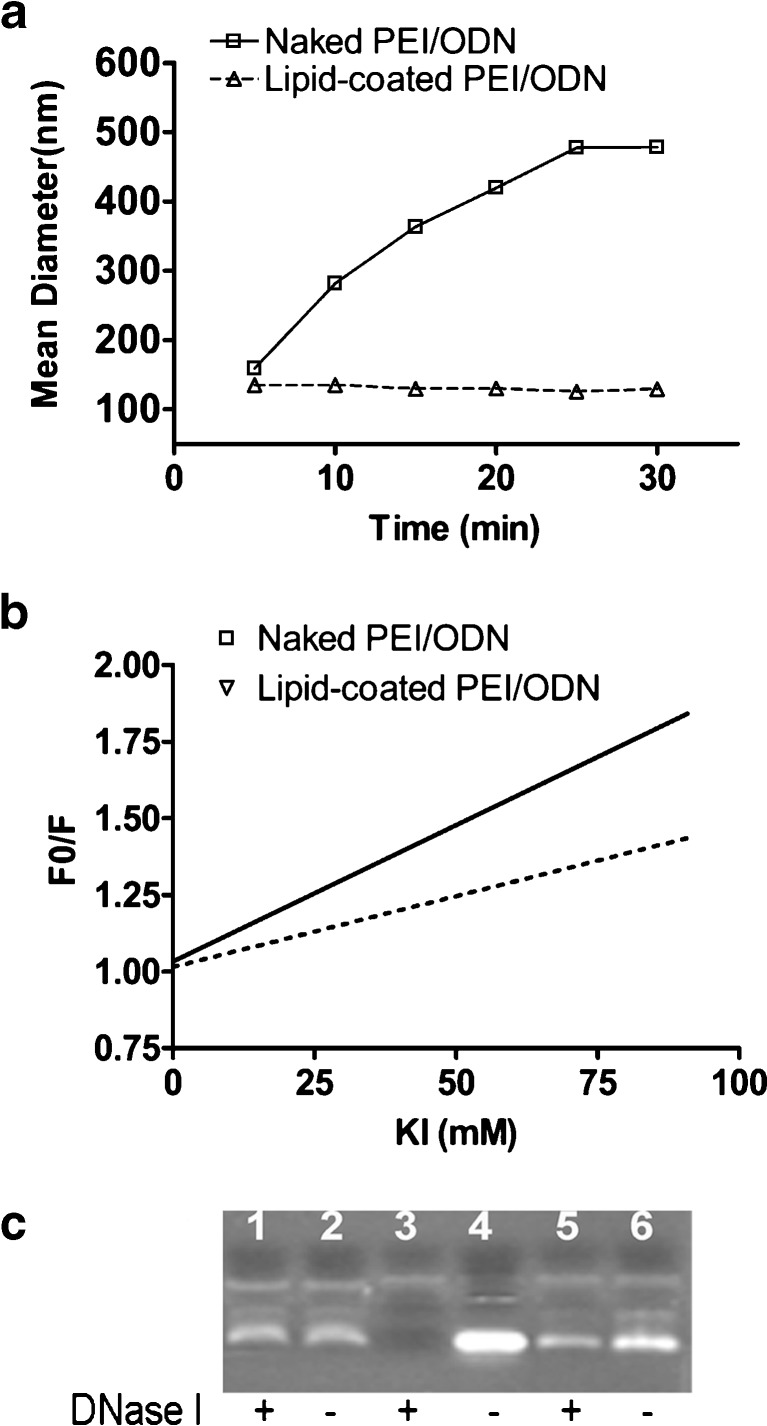
The particles also showed colloidal stability, suggesting steric stabilization and protection of otherwise unstable PEI/ODN polyplexes by the PEG-stabilized lipid coat. The mean diameter of the lipid-coated particles remained constant over 30 min while that of the naked polyplexes continuously increased with time. The time-dependent increase in the mean diameter of the naked polyplexes indicates aggregation, and the constant mean diameter of the lipid-coated particles indicates colloidal stability (Fig. 2a).
Fig. 2.
Formation of the lipid-coated PEI/ODN particles. a Colloidal stability in PBS. b Protection of ODN from potassium iodide (KI) quenching. The decreased quenching constants of the lipid-coated particles indicate that the PEI/ODN polyplexes are shielded by the lipid membrane coats. c Protection of ODN from DNase I digestion. Lanes 1, 2 the lipid-coated PEI/ODN polyplexes, lanes 3, 4 free ODN, lanes 5, 6 naked PEI/ODN polyplexes
Protection of the ODN from the external phase by the stabilized lipid coat was demonstrated by fluorescence quenching or enzyme digestion. The lipid-coated particles showed a decreased quenching constant (Ksv) as compared to the naked polyplexes, supporting that the polyplexes were coated by the lipid membrane and thus protected from the quencher KI in the external phase (Fig. 2b).
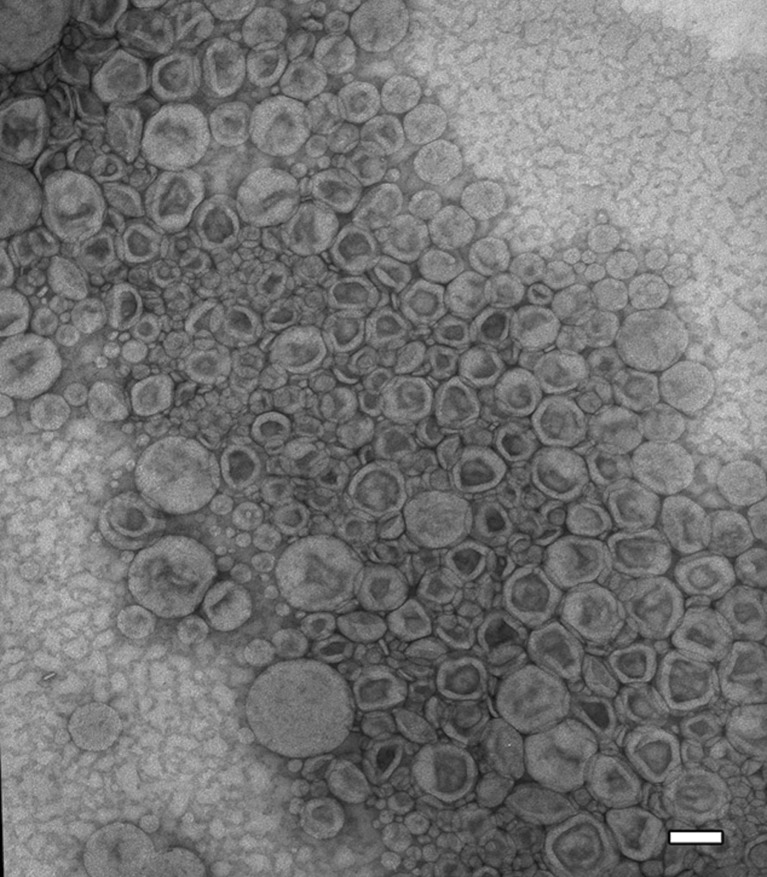
As compared to the moderate protection of the ODN in the naked polyplexes, the lipid-coated particles showed complete protection of the ODN against enzymatic degradation, supporting that the polyplexes in the lipid-coated particles were completely surrounded by the lipid membrane (Fig. 2c). TEM analysis of the lipid-coated particles revealed spherical structures with heavily stained cores surrounded by lightly stained layers, confirming the morphologic structure with the polyplex cores enveloped by the lipid coats (Fig. 3).
Fig. 3.
Transmission electron microscopy image of the lipid-coated particles. The particles showed vesicular structure with lightly stained lipid coats and heavily stained polyplex cores. The bar indicates 100 nm
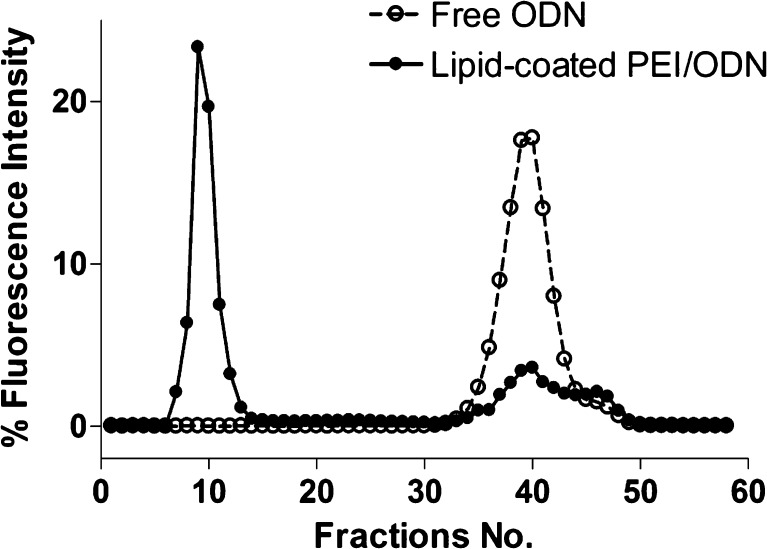
To determine the encapsulation efficiency of ODN, the lipid-coated particles were prepared with 32P-ODN as tracer, and the ODN in the lipid-coated particles was separated from free ODN. Upon SEC separation using Sheparose CL4B column and PBS as eluent, the ODN entrapped in the lipid-coated particles was eluted at void volume. It was determined that approximately 80% of ODN was entrapped in the lipid-coated particles as measured by area under curve of the peak at void volume (Fig. 4).
Fig. 4.
Encapsulation efficiency of ODN. ODN in the lipid-coated particles was eluted at void volume on a Sepharose CL4B column with an encapsulation efficiency of more than 80%
DISCUSSION
Liposomal encapsulation of nucleic acid-based therapeutics such as plasmid DNA, ribozyme, antisense ODN, and siRNA molecules into both conventional and PEG-stabilized liposomes has been studied in several different ways. In most cases, however, the encapsulation efficiency of less than 50% was limiting factor (4,5). In some cases where a higher encapsulation efficiency was achieved, the preparation should employ complicated multi-step procedures such as detergent dialysis (2) and extraction into an organic phase followed by reverse evaporation (3).
As an alternative approach, liposomal encapsulation of polyplexes between polymers and DNA has been proposed. It was reported that polyplexes between DNA and cationic polymer such as poly-l-lysine can be entrapped into folate-targeted anionic liposomes for tumor-specific oligonucleotide delivery (6). It was also shown that polyplexes between oligonucleotides and cationic polypeptides such as protamine sulfate can be entrapped into preformed DOTAP cationic liposomes, resulting ternary oligonucleotide delivery systems, so-called cationic lipid–protamine–DNA particles with enhanced in vivo oligonucleotide transfer activity (18). Polyplexes between PEI and DNA were also shown to be entrapped in preformed anionic liposomes, resulting in nano-sized particles, so-called artificial virus-like particles with high in vitro oligonucleotide transfer activity (7,8).
In this study, liposomal particles entrapping polyplexes between polyethylenimine and oligonucleotides were constructed based on the procedure, previously reported for active entrapping of antisense ODN into cationic liposomes (3,19). The procedure was based on extraction of antisense ODN with DOTAP into organic phase by formation of hydrophobic micelle-like particles followed by reverse-phase evaporation. The procedure employed electrostatic interaction between cationic lipid DOTAP and anionic oligonucleotides to form hydrophobic micelle-like particles, which enabled complete entrapping of 18-mer antisense ODN into hydrophobic micelle-like particles. In this study, the anionic lipid POPG was used to form hydrophobic micelle-like particles entrapping the positively charged polyplexes between polyethylenimines and oligonucleotides. The hydrophobic particles were first recovered into the organic phase, which was then rapidly removed by reverse-phase evaporation in the presence of coating lipids including PEG-PE. As a result, it was a stable aqueous dispersion of hydrophilic particles with the polyplex core and PEG-stabilized lipid membrane coat (Fig. 1).
It was hypothesized that the coating lipids may deposit around the hydrophobic inverted micelle-like particles during the reverse phase evaporation by hydrophobic interaction between lipid side chains, forming the hydrophobic outer lipid membrane coat. This was supported by the TEM analysis of the particles showing vesicular structure with a heavily stained core surrounded by a lightly stained envelope. Although the coating process seems to be incomplete in some cases as shown by the discontinuous lipid membrane around core structure, the membrane extrusion process led to complete lipid coating which could provide colloidal stability as well as protection of ODN from enzymatic degradation. The extrusion procedure also led to size reduction to smaller sizes of 100 nm.
The procedure could provide encapsulation efficiency of 80% comparable to that of the original procedure proposed for antisense ODN entrapping into DOTAP liposomes by Stuart and Allen (19). Although almost all ODN was initially entrapped into the hydrophobic inverted micelle-like particles, the following reverse-phase evaporation and membrane extrusion step diminished the final encapsulation efficiency to around 80%. This may indicate that the hydrophobic micelle-like particles entrapping the polyplexes could be disrupted and thus release the entrapped ODN during the reverse-phase evaporation step. Further optimization of this step to minimize this disruption should lead to higher encapsulation efficiency.
CONCLUSION
In summary, the reverse-phase evaporation technique described in this study presents a novel procedure for the preparation of liposomal formulation encapsulating the preformed polyplexes, which represents a promising delivery system for the various nucleic acid-based therapeutics including siRNA and asODN.
ACKNOWLEDGEMENTS
This research was supported by grant 20110007794 from Korean National Research Foundation (NRF) and grant 5R01-NS045043-02 from National Institutes of Health (NIH).
REFERENCES
- 1.Shi N, Pardridge WM. Noninvasive gene targeting to the brain. Proc Natl Acad Sci USA. 2000;97(13):7567–7572. doi: 10.1073/pnas.130187497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler JJ, Palmer L, Ossanlou M, MacLachlan I, Graham RW, Zhang YP, et al. Stabilized plasmid-lipid particles: construction and characterization. Gene Ther. 1999;6(2):271–281. doi: 10.1038/sj.gt.3300821. [DOI] [PubMed] [Google Scholar]
- 3.Stuart DD, Kao GY, Allen TM. A novel, long-circulating, and functional liposomal formulation of antisense oligodeoxynucleotides targeted against MDR1. Cancer Gene Ther. 2000;7(3):466–475. doi: 10.1038/sj.cgt.7700145. [DOI] [PubMed] [Google Scholar]
- 4.MacLachlan I, Cullis P, Graham RW. Progress towards a synthetic virus for systemic gene theraphy. Curr Opin Mol Ther. 1999;1(2):252–259. [PubMed] [Google Scholar]
- 5.Fenske DB, MacLachlan I, Cullis PR. Long-circulating vectors for the systemic delivery of genes. Curr Opin Mol Ther. 2001;3(2):153–158. [PubMed] [Google Scholar]
- 6.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271(14):8481–8487. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 7.Muller K, Nahde T, Fahr A, Muller R, Brusselbach S. Highly efficient transduction of endothelial cells by targeted artificial virus-like particles. Cancer Gene Therapy. 2001;8(2):107–117. doi: 10.1038/sj.cgt.7700280. [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay P, Ma X, Stieers CL, Kren BT, Steer CJ. Nucleotide exchange in genomic DNA of rat hepatocytes using RNA/DNA oligonucleotides. J Biol Chem. 1999;274(15):10163–10172. doi: 10.1074/jbc.274.15.10163. [DOI] [PubMed] [Google Scholar]
- 9.Heyes J, Palmer L, Chan K, Giesbrecht C, Jeffs L, MacLachlan I. Lipid encapsulation enables the effective systemic delivery of polyplex plasmid DNA. Mol Ther. 2007;15(4):713–720. doi: 10.1038/sj.mt.6300101. [DOI] [PubMed] [Google Scholar]
- 10.Ko YT, Bhattacharya R, Bickel U. Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J Control Release. 2009;133(3):230–237. doi: 10.1016/j.jconrel.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Kunath K, von Harpe A, Petersen H, Fischer D, Voigt K, Kissel T, et al. The structure of PEG-modified poly(ethylene imines) influences biodistribution and pharmacokinetics of their complexes with NF-kappaB decoy in mice. Pharm Res. 2002;19(6):810–817. doi: 10.1023/A:1016152831963. [DOI] [PubMed] [Google Scholar]
- 12.Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16(8):1273–1279. doi: 10.1023/A:1014861900478. [DOI] [PubMed] [Google Scholar]
- 13.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3(8):894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 14.von Harpe A, Petersen H, Li Y, Kissel T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J Control Release. 2000;69(2):309–322. doi: 10.1016/S0168-3659(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 15.Linnertz H, Urbanova P, Amler E. Quenching of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole-modified Na+/K+-ATPase reveals a higher accessibility of the low-affinity ATP-binding site. FEBS Lett. 1997;419(2–3):227–230. doi: 10.1016/S0014-5793(97)01460-9. [DOI] [PubMed] [Google Scholar]
- 16.Uddin MN, Do DP, Pai SB, Gayakwad S, Oettinger CW, D'Souza MJ. A methodology for quantitation and characterization of oligonucleotides in albumin microspheres. Analyst. 2009;134(7):1483–1489. doi: 10.1039/b823554f. [DOI] [PubMed] [Google Scholar]
- 17.Stuart DD, Allen TM. A new liposomal formulation for antisense oligodeoxynucleotides with small size, high incorporation efficiency and good stability. Biochim Biophys Acta. 2000;1463:219–229. doi: 10.1016/S0005-2736(99)00209-6. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4(9):891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 19.Stuart DD, Allen TM. A new liposomal formulation for antisense oligodeoxynucleotides with small size, high incorporation efficiency and good stability. Biochim Biophys Acta. 2000;1463(2):219–229. doi: 10.1016/S0005-2736(99)00209-6. [DOI] [PubMed] [Google Scholar]