Abstract
A process is described using supercritical carbon dioxide to extract organic solvents from drug solutions contained in 30-mL serum vials. We report drying times of less than 1 h with quantitative recovery of sterile drug. A six-log reduction of three spore types used as biological indicators is achieved with direct addition of peracetic acid to a final concentration of approximately 5 mM (~0.04 %) to the drug solution in the vial. Analysis of two drugs, acetaminophen and paclitaxel, indicated no drug degradation as a result of the treatment. Furthermore, analysis of the processed drug substance showed that no residual peracetic acid could be detected in the final product. We have demonstrated an effective means to simultaneously dry and sterilize active pharmaceutical ingredients from organic solvents directly in a dispensing container.
KEY WORDS: drying, paclitaxel, peracetic acid, sterilization, supercritical carbon dioxide
INTRODUCTION
There are approximately 350 injectable drugs commercially available in the USA (1). Injectable drugs must be sterile, or more properly stated, must have a negligible probability (defined as <1:106) that the finished product is contaminated with microbes or spores when starting with an initial bioburden of at least one million colony forming units (CFU) (2). A great many injectable drugs must also be dry in order to have adequate shelf life. Most typically these two objectives of producing sterile, dry drug products are accomplished by preparing an aqueous solution of the drug, filtering the solution through a 0.22-μm filter, filling sterile vials in an appropriately aseptic manner, transporting the filled vials into a pre-sterilized lyophilization chamber, drying the vials under reduced pressure and temperature and finally capping the vials.
Subramaniam et al. (3) described a process for drying drug products directly in dispensing vials using supercritical fluids. They named this process lyophobic precipitation (LP). More recently, (4) that process was improved making it practical for use on multiple vials. In this process drug is dissolved in an appropriate organic solvent and placed in the dispensing vial or primary packaging. The containers are placed into a stainless steel chamber able to withstand pressures in excess of 13.79 MPa (2,000 psi). The chamber is flooded with CO2 at pressure and temperature above the critical point (supercritical carbon dioxide—scCO2) such that the scCO2 and organic solvent are in intimate contact. Use of ultrasonic energy to provide surface agitation results in the rapid migration of the organic layer into the bulk scCO2 and results in precipitation of the drug in the vial. The organic solvent is removed with a flowing stream of scCO2 until the sample is completely dry (i.e., organic solvent level below the limit of detection). Typical conditions for drying with scCO2 are pressures in the range of 8.27 to 9.64 MPa (1,200 to 1,400 psi) and temperatures in the range of 32 to 45 °C.
By about the middle of the twentieth century (5), scCO2 was investigated as a means of sterilization especially for food substances and other products which would be seriously degraded by elevated temperature. Spilimbergo and Bertucco (6) provide a thorough review of attempts to use scCO2 for sterilization of a wide variety of microbes in a wide variety of products. While a certain degree of success is obtained with scCO2 against vegetative forms of several common food contaminants, it was not until recently that methods were described to achieve the sterility assurance level (SAL) of 10−6 against the difficulty to sterilize spore-forming bacteria that is necessary to produce safe injectable drugs. Several laboratories reported that it is possible to achieve a SAL of 10−6 against dry spores using scCO2 with added sterilants (7–10). These laboratories reported successful destruction of spores using scCO2 at pressures ranging from 10.34 to 30.34 MPa (1,500 to 4,400 psi), temperatures ranging from 34 to 80 °C and for exposure times between 30 min and 4 h. Without the added sterilants (most often oxidizing agents such as peracetic acid (PAA) or hydrogen peroxide) little or no killing of spores occurred under the conditions of pressure, temperature, and time mentioned above.
Here we report a process that combines the two techniques for drying and sterilizing using scCO2 with an added sterilant to prepare drug samples directly in the dispensing vial that achieves at least a 6 log reduction of bacterial spores traditionally used to certify sterilization equipment. We used acetaminophen as our model drug substance and three different species of bacterial spores as biological indicators. By combining these two processes it should be possible to produce dry, sterile drug in multiple dispensing vials in a single operation.
MATERIALS AND METHODS
Materials
The following materials were used in this study: acetaminophen (Sigma Aldrich, St. Louis, MO, USA), paclitaxel (Natural Pharmaceuticals, Inc., Beverly, MA, USA), 32 % peracetic acid (Sigma Aldrich, St. Louis, MO, USA), 35 % hydrogen peroxide (Acros, Morris Plains, NJ, USA), 100 % ethanol, anhydrous (Acros, Morris Plains, NJ, USA), methanol (Fisher, Fair Lawn, NJ, USA), and water (HPLC grade, Fisher, Fair Lawn, NJ, USA).
Biological Indicators
Biological indicators (BI) were Geobacillus stearothermophilus ATCC 7953, Bacillus atrophaeus ATCC 9372, and Bacillus pumilus ATCC 27142 from NAMSA supplied as spore suspensions in water for injection at a concentration of approximately 109 spores/mL. Because there is no accepted standard BI for sterilization using scCO2 and added sterilants, we elected to use three indicator strains used by industry for steam (G. stearothermophilus), ethylene oxide or dry heat (B. atrophaeus), and radiation (B. pumilus) sterilization.
Apparatus
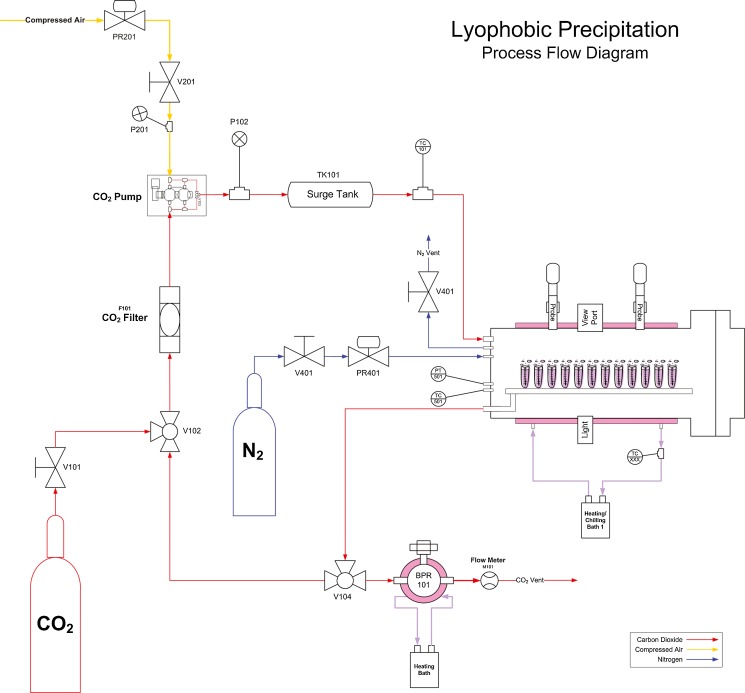
The apparatus used for lyophobic precipitation consists of a high pressure chamber that is roughly a 20 × 50 cm 316 stainless steel cylinder with an inner volume of slightly more than 7 l (Fig. 1). It can accommodate roughly 125 4-mL vials or about two dozen 30-mL vials and up to four sonic probes (Misonix) located in several locations along the length of the chamber (Fig. 2). Carbon dioxide was pumped from a cylinder by a booster pump (Haskel) through a surge tank into the pressure chamber. The apparatus can deliver carbon dioxide at pressures ranging from 2.07 to 13.79 MPa (300 to 2,000 psi) and is normally operated in the range of 7.58 to 8.27 MPa (1,100 to 1,200 psi). Temperature of the chamber can be controlled between ambient and 60 °C and the apparatus is controlled via a computer interface running National Instrument’s LabVIEW software.
Fig. 1.
Schematic of lyophobic precipitation unit. See Apparatus section for detailed description
Fig. 2.
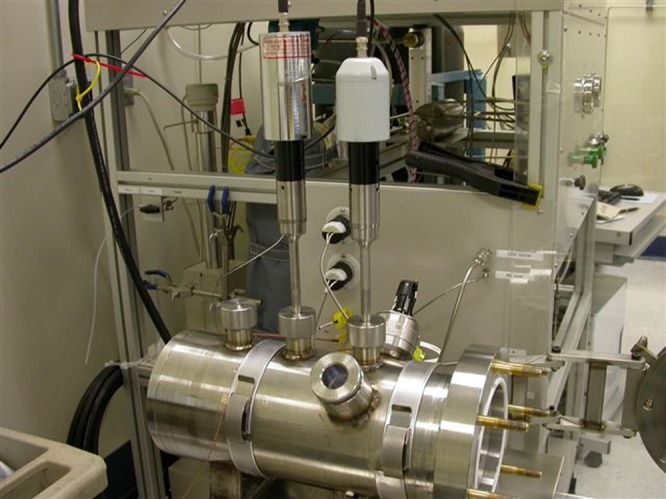
Photograph of LP unit. Complete unit consisting of LP chamber, scCO2 generating equipment, and ultrasonic probes. Apparatus shown with two (of four possible) sonic probes in place
Microbiological Methods
All microbiological assays were performed at WuXi AppTec, Inc. at the Atlanta, GA, facility. Briefly, vials that were dried and sealed using the LP process were shipped to WuXi AppTec where the vials were opened in a sterile hood. The dried samples (drug mimic plus BI) were suspended in 20 mL Fluid D (1 g/L peptic digest containing 1 mL of polysorbate 80; adjusted to pH 7.1). The vials were shaken and the entire contents transferred to a 250-mL jar. The vials were each rinsed with an additional 80 mL of Fluid D which was added to the original 20 mL in the same 250-mL jar. A 5-mL aliquot was removed, heat shocked, and added to tryptic soy agar pour plates for aerobes and anaerobes (Rose Bengal Agar—for mold and yeast). Plates were incubated aerobically for 3–4 days at 30–35 °C (7 days at 20–25 °C for mold and yeast) before counting.
HPLC Methods
HPLC analysis of paclitaxel, acetaminophen, and peracetic acid were performed on a Hitachi L-7000 series HPLC with UV detection. The system is equipped with an auto-sampler and column oven.
Paclitaxel Analysis
Paclitaxel and its degradation products were assayed according to USP Related Compound Test 2 (11). The chromatography medium was a Luna C18 (3 μ) 150 × 4.6 mm column. Mobile phase A was a 3:2 water/acetonitrile mixture and mobile phase B was acetonitrile. The elution conditions were: 0–20 min, 100 % A; 20–60 min, 0–90 % B linear gradient; 60–62 min, 90–0 % B linear gradient; 62–70 min 100 % A. The flow rate was 1.2 mL/min at 35 °C. The column eluent was monitored at a wavelength of 227 nm. Under these conditions paclitaxel had a retention time of 28.58 min.
Acetaminophen Analysis
Acetaminophen and its degradation products were assayed using the HPLC method of Aukunuru et al. (12). The chromatography medium was a Phenomenex Prodigy C8 (5 μ) 150 × 4.6 mm column. Mobile phase A was 0.1 M sodium phosphate pH 2.7, mobile phase B was methanol, and mobile phase C was acetonitrile. The elution conditions were as described in Aukunuru et al. (12). A flow rate of 1 mL/min was used at 30 °C. The column eluent was monitored at a wavelength of 220 nm. Under these conditions acetaminophen had a retention time of 3.88 min.
Peracetic Acid Analysis
Peracetic acid was assayed using the HPLC method of Pinkernell et al. (13). Briefly, methyl p-tolyl sulfide (MTS) is mixed with samples containing PAA under controlled conditions to oxidize the MTS to the corresponding sulfoxide (MTSO). MTSO and MTS concentrations are determined by HPLC and compared to levels produced from a standard curve of known PAA concentrations. The chromatography medium was an Agilant Zorbax SB-C18 (5 μ) 350 × 4.6 mm column. The mobile phase was water/methanol (25:75) and the elution was isocratic with a flow rate of 0.7 mL/min at 30 °C. PAA concentration was calculated from a standard curve of the MTSO concentration determined at a wavelength of 230 nm. For analysis of residual PAA after LP processing 20 mg of acetaminophen dissolved in 1 mL of ethanol was mixed with 5 μL of PAA solution (32 mg/ml) to deliver 160 μg of PAA and dried by LP as described above. The dried samples were dissolved in methanol/water, MTS was added and the amount of MTSO was determined by HPLC/UV analysis. An MTSO standard curve from 16.7 ng to 1.67 μg resulted in a correlation coefficient of 0.9998. Under these conditions the retention time for MTSO was 4.57 min and that for MTS was 12.03 min.
Calculations
Colony forming units are reported as the total per vial of dried drug. Log reduction was determined using the formula: log reduction = log (CFU from untreated sample/CFU from treated sample).
RESULTS
Our initial objective was to determine the optimal conditions to dry acetaminophen in dispensing vials using scCO2 as the drying agent. Approximately 60 mg of acetaminophen were weighed into 30-mL serum vials and 1 mL of ethanol was added to dissolve the drug. Acetaminophen was chosen as the model drug because it is relatively safe to handle, inexpensive, is easily susceptible to oxidative degradation, and provided sufficient bulk to provide potential surface area that might serve to sequester the BI from the effects of various sterilizing attempts.
Standard Drying Conditions
Six vials were placed in the LP unit and carbon dioxide was pumped into the chamber until supercritical conditions were reached. At that time the sonic probes were activated to induce an agitation of the interface between the solvent and the scCO2. Beginning with run parameters as described in Rajewski et al., (4) we determined the minimal time required to remove all liquid (as measured by weight difference) from sets of six 30-mL vials. These initial parameters were: 7.93 MPa (1,150 psi), 38 °C, a power setting of 8 on the sonifier, recirculation of the scCO2, and a sonifier run time of 20 min. Under these conditions several of the vials had visible liquid levels. After trying a series of pressures, temperatures, and times, we determined that increasing pressure or temperature had no effect on overall solvent removal, but that increasing the duration of the application of sonic energy eventually resulted in the apparent complete removal of solvent. We determined that an operating pressure between 8.00 and 8.27 MPa (1,160 and 1,200 psi), a temperature of 37 °C, application of sonic energy (sonifier power setting of 6) for a period of 55 min, and a depressurization cycle of 30 min resulted in a dry product with an average recovery of 100.1 % (±0.5 %) determined by weight (data not shown). These conditions were used as the baseline drying conditions for all subsequent studies.
After determining that drug-containing vials prepared in this manner had no detectable bioburden (data not shown), we spiked vials containing drug in ethanol with 10 μl each of G. stearothermophilus, B. atrophaeus, or B. pumilus (two vials of each BI) to achieve a final inoculation of roughly 2 × 107 spores per vial and dried them using the baseline LP conditions. There was little to no killing of these spores under the drying conditions used (data not shown). Because others had shown the need to have water present during sterilization with scCO2 (14), we included a test tube of water within the LP chamber, but similar to the results of White et al., (9) this had little impact on spore survival.
Enhanced Drying Conditions
Consistent with the findings of White et al. (9) and Zhang et al., (10) elevating the temperature to 50 °C and/or extending the exposure time to 4 h had no effect on survival of spores of all three BI. In each case the log reduction was less than 1 (data not shown).
Addition of Sterilants
The effect of replacing the test tube of water in the LP chamber with a test tube of either PAA (32 %) or hydrogen peroxide (35 %) as a means of exposing the spores to these agents was examined using the baseline LP conditions of 8.10 MPa (1,175 psi) and 37 °C for 55 min with sonication. Table I shows that a small, but measurable, effect was observed and that PAA was more effective at killing each type of spore than was hydrogen peroxide. Addition of both PAA and hydrogen peroxide to the chamber at the same time was similarly ineffective; furthermore, saturating the chamber with PAA by continuous spraying of the PAA solution into the chamber during recirculation of the scCO2 also had little or no effect on killing of spores in the drug vials.
Table I.
Log Reduction of Biological Indicators Exposed to Peroxyacetic Acid or Hydrogen Peroxide in Lyophobic Precipitation Chamber
| Microorganism | Log reduction (n = 2) | |
|---|---|---|
| PAA | H2O2 | |
| G. stearothermophilus | 1.56 | −0.48 |
| B. atrophaeus | 0.73 | 0.17 |
| B. pumilus | 1.62 | 0.38 |
Vials containing 32 % PAA or 35 % H2O2 were placed in the same chamber as vials containing a solution of 60 mg/mL acetaminophen in ethanol and ~106 CFU of each biological indicator and were subjected to lyophobic precipitation at 37 °C, 1,175 psi scCO2 with sonication for 55 min
Direct Addition of Sterilants to Drug Vials
At the relatively low pressure and temperature used for these studies, compared to those used by other investigators, adding PAA or hydrogen peroxide to the chamber did not adequately kill spores in the drug-containing vials. Therefore, we determined if direct addition of PAA or hydrogen peroxide to the spore-laden drug might achieve the desired six-log reduction of the BI. Immediately prior to the start of the LP process, 5 μL of either PAA or hydrogen peroxide was added directly to the vials containing acetaminophen dissolved in ethanol. For PAA, this amount was equal to 1.6 mg (21.1 μmol) and for hydrogen peroxide 1.75 mg (51.5 μmol). All other conditions were identical to the previous experiments. Table II shows clearly that direct addition of sterilant to the dispensing vial was more effective at killing spores under these conditions. Furthermore, PAA appears to be more effective than hydrogen peroxide for inactivating spores under these conditions.
Table II.
Log Reduction of Biological Indicators Exposed to Peroxyacetic Acid or Hydrogen Peroxide Directly in Dispensing Vial
| Microorganism | Log reduction (n = 2) | |
|---|---|---|
| PAA in vial | H2O2 in vial | |
| G. stearothermophilus | 5.84 | 3.90 |
| B. atrophaeus | 7.18 | 4.24 |
| B. pumilus | 5.51 | 4.64 |
Five microliters of either 32 % PAA or 35 % H2O2 were placed directly into 1 mL of a solution of 60 mg/mL acetaminophen in ethanol and ~106 CFU of each biological indicator and subjected to lyophobic precipitation at 37 °C, 1,175 psi scCO2 with sonication for 55 min
To determine if a log reduction of 6 could be achieved, we repeated this experiment using a larger number of spores (~2 × 107) and two different amounts of PAA (5 and 1 μL or 21.1 and 4.2 μmol, respectively). Table III shows that a six-log reduction in CFUs could be achieved for all three spore types with 21.1 μmol of added PAA, but that B. pumilus showed only a 3.6 log reduction when the lower amount of PAA was used. Table III also demonstrates that 5 μL of PAA alone, without use of scCO2 for lyophobic precipitation, could achieve a spore log reduction of 6 for all three microorganisms.
Table III.
Log Reduction of Biological Indicators by Peroxyacetic Acid
| Log reduction (n = 1) | |||
|---|---|---|---|
| 1 μL PAA (0.32 mg/mL) | 5 μL PAA (1.6 mg/mL) | 5 μL PAA (1.6 mg/mL) no LP | |
| G. stearothermophilus | 7.52 | 7.52 | 7.52 |
| B. atrophaeus | 7.74 | 7.74 | 7.74 |
| B. pumilus | 3.61 | 6.30 | 6.54 |
One or five microliters of 32 % peroxyacetic acid were placed directly into 1 mL of a solution of 60 mg/mL acetaminophen in ethanol and ~2 × 107 CFU of biological indicators. Two sets of vials were then subjected to lyophobic precipitation at 37 °C, 1,175 psi scCO2 with sonication for 55 min while the third set was allowed to incubate at room temperature for the duration of the experiment
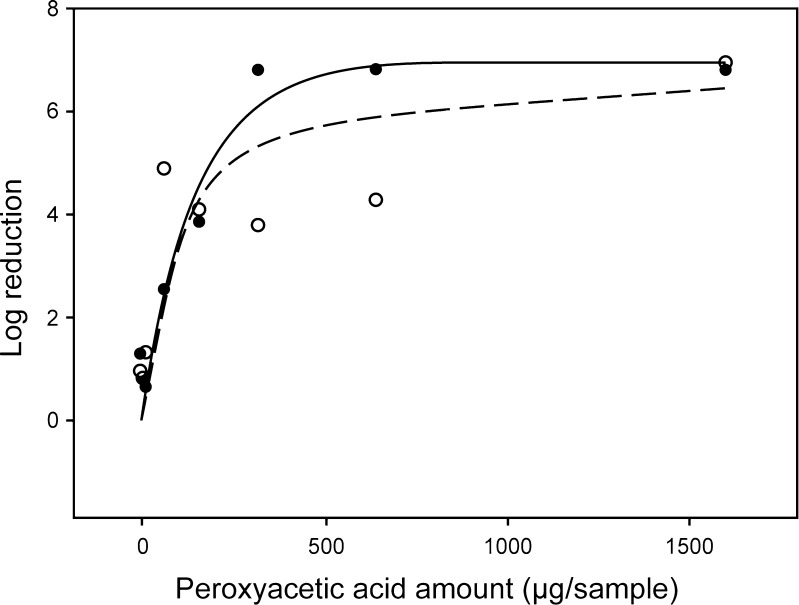
We next determined what amount of PAA had to be added to the samples in order to reach a six-log reduction for B. atrophaeus and B. pumilus. Because G. stearothermophilus was consistently more susceptible than the other two spore types throughout these studies, we ceased working with that microorganism as being insufficiently robust to this process to provide a meaningful challenge to sterilization. We measured spore survival as a function of amount of PAA added directly to sample vials over a range from 1.6 to 1,600 μg (21.1 nmol to 21.1 μmol). Figure 3 shows the results for the B. atrophaeus and B. pumilus spores. A six-log reduction of B. atrophaeus is achieved at a concentration of approximately 270 μg/mL (3.6 μmol/mL) PAA. Results for B. pumilus were similar except that the concentration of PAA required to reach a six-log reduction was approximately 400 μg/mL (5.3 μmol/mL). This result is consistent with the data shown in Table III and indicates that B. pumilus is the most resistant of these three microorganisms to sterilization by direct exposure to PAA and scCO2.
Fig. 3.
Plot of log reduction of B. atrophaeus and B. pumilus as function of amount of PAA in vial. Different amounts of peroxyacetic acid were added to ~2 × 107 CFU of biological indicator in 1 mL of a solution of 60 mg/mL acetaminophen in ethanol then subjected to lyophobic precipitation at 37 °C, 8.1 MPa scCO2 with sonication for 55 min. filled circles, B. atrophaeus; unfilled circles, B. pumilus
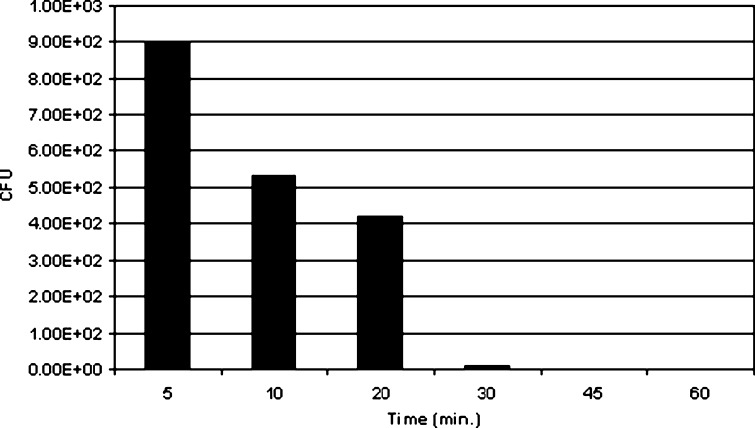
We attempted to determine the minimum exposure time necessary to reach a spore log reduction of 6 using a less than optimal level of PAA. Because PAA at levels above about 250 μg per vial resulted in a six-log reduction within the minimum time required to dry the 1 mL sample, we measured the log reduction over time of exposure using 160 μg of PAA per vial. PAA was added to vials containing acetaminophen in ethanol and ~2 × 107 CFU of either B. atrophaeus or B. pumilus and allowed to incubate at room temperature for 5, 10, 20, 30, 45, and 60 min prior to initiating lyophobic precipitation. As can be seen in Fig. 4, even as little as 5 min of exposure to PAA prior to beginning the drying process results in a significant reduction in the CFU (approximately a 4.8 log reduction) and that by 30 min exposure to 160 μg of PAA all spores are killed. Results for B. pumilus were similar (data not shown). We can conclude that exposure to 160 μg for PAA for 30 min or to greater amounts of PAA for shorter periods of time is sufficient to achieve a six-log reduction of the most resistant spore forms.
Fig. 4.
CFU reduction of B. atrophaeus as function of time of exposure to PAA. Vials containing >107 CFU of biological indicator in 1 mL of a solution of 60 mg/mL acetaminophen in ethanol were spiked with 160 μg of peroxyacetic acid and allowed to incubate at room temperature for the times indicated. The vials were then subjected to lyophobic precipitation at 37 °C, 1,175 psi scCO2 with sonication for 55 min
Effect of PAA Exposure and Lyophobic Precipitation on Drug Stability
If this approach to drying and sterilizing drugs is to have any practical value, two conditions must be met in addition to the ability to achieve SAL 10−6. First, all of the added sterilant must be removed by the process and second, the process must not affect the chemical stability of the drug. We examined both of these factors.
PAA Removal
Residual PAA remaining in vials after drying by lyophobic precipitation was measured as described in Materials and Methods. Analysis of non-dried samples gave large MTSO peaks corresponding to 100 % residual PAA whereas duplicate samples assayed after drying with LP yielded no detectable MTSO peak. The limit of detection for MTSO in our HPLC system was 4.65 ng meaning that the residual PAA in these samples was less than that or something less than 0.003 % of the amount added to the vial.
Paclitaxel and Acetaminophen Stability
To determine the stability of drugs to exposure to the oxidizing agent PAA in concentrations and conditions sufficient to reach a spore log reduction value of 6, we performed the sterilizing LP process with either paclitaxel or acetaminophen as the drug substance. Paclitaxel was prepared as a 60-mg/mL solution in methanol and treated with 160 μg PAA immediately prior to lyophobic precipitation. After drying, the samples were dissolved in acetonitrile and assayed by HPLC/UV as described in Materials and Methods. As is shown in Table IV, duplicate samples of paclitaxel exposed to PAA prior to lyophobic precipitation yielded essentially identical degradation profiles. Furthermore, the degradation profiles were essentially identical to the degradation profile for untreated paclitaxel. A third sample of paclitaxel exposed to PAA for 30 min prior to drying by lyophobic precipitation also gave a degradation profile that was indistinguishable from that of untreated paclitaxel.
Table IV.
Stability Analysis of Paclitaxel Exposed to PAA and Dried by Lyophobic Precipitation
| HPLC analysis of paclitaxel | |||||
|---|---|---|---|---|---|
| Peak identity | R.R.T. | Standard | Sample 1 | Sample 2 | Sample 2a |
| 160 μg PAA | 160 μg PAA | 160 μg PAA30 minutes | |||
| Total % | Total % | Total % | Total % | ||
| Related compound B paclitaxel | 0.21 | 0.03 | 0.03 | 0.03 | 0.03 |
| 0.63 | 0.03 | ||||
| 0.81 | 0.34 | 0.33 | 0.34 | 0.34 | |
| 0.94 | 0.12 | 0.12 | 0.13 | 0.12 | |
| 1 | 100 | 100 | 100 | 100 | |
| 1.22 | 0.01 | ||||
| 1.42 | 0.08 | 0.06 | 0.06 | 0.06 | |
| 1.44 | 0.02 | ||||
| 1.65 | 0.09 | 0.08 | 0.11 | 0.09 | |
| 1.93 | 0.13 | 0.12 | 0.21 | 0.17 | |
| 2.75 | 0.02 | ||||
| Total percent degradation products | 0.81 | 0.78 | 0.89 | 0.83 | |
Peak areas reported as percent of paclitaxel peak. Samples 1 and 2 were two distinct samples dried on two separate days; sample 2a was dried in the same batch as sample 2, but included a 30-min incubation with PAA prior to initiating lyophobic precipitation; RRT relative retention time (relative to paclitaxel peak)
Similarly, acetaminophen (60 mg in 1 mL ethanol) was incubated with 160 μg PAA for 30 min prior to lyophobic precipitation. After drying, the samples were dissolved in methanol and assayed by HPLC/UV as described in Materials and Methods. Additionally, acetaminophen was exposed to higher amounts of PAA (320 and 1,600 μg) without lyophobic precipitation to demonstrate susceptibility to oxidation by PAA and to determine the retention time of any new degradation peaks when chromatographically separated in this HPLC system. Table V shows the results of this study. The samples that were exposed to PAA, but not processed by LP, show two new degradation peaks (RRT 2.9 and RRT 4.4) that do not appear in the untreated drug and large increases in the peaks that appear at RRT 1.9 and RRT 2.4 in the untreated drug. The increases in these four peaks appear to correlate with the amount of PAA added to the sample. By comparison, the two samples that were processed under conditions that would result in a dry, sterile product are indistinguishable from the untreated drug and show no increase in degradation products.
Table V.
Stability Analysis of Acetaminophen Exposed to PAA and Dried by Lyophobic Precipitation
| HPLC analysis of acetaminophen | |||||
|---|---|---|---|---|---|
| Peak identity | R.R.T. | Standard | Sample 1 | Sample 2 | Test (n = 2) |
| n = 2 | Acetaminophen plus 320 μg PAA, no LP | Acetaminophen plus 1.6 mg PAA, no LP | Acetaminophen plus 160 μg PAA, LP | ||
| Total % | Total % | Total % | Total % | ||
| 1.7 | 0.18 | 0.02 | 0.15 | 0.12 | |
| 1.9 | 0.10 | 0.36 | 11.28 | 0.14 | |
| 2.4 | 0.03 | 0.64 | 18.54 | 0.03 | |
| 2.7 | |||||
| 2.9 | 0.01 | 0.69 | |||
| Acetaminophen | 3.9 | 100 | 100 | 100 | 100 |
| 4.4 | 0.11 | 4.23 | |||
| 5.8 | 0.06 | ||||
| 6.5 | 0.05 | 0.01 | 0.10 | 0.02 | |
| 7.3 | 0.07 | 0.04 | 0.07 | ||
| Total percent degradation products | 0.43 | 1.15 | 35.10 | 0.37 | |
Sample 1 is acetaminophen exposed to 320 μg PAA for 30 min prior to HPLC analysis; Sample 2 is acetaminophen exposed to 1.6 mg PAA for 30 min prior to HPLC analysis; Test is average of two samples of acetaminophen exposed to 160 μg PAA then dried by lyophobic precipitation prior to HPLC analysis; RRT relative retention time (relative to acetaminophen peak)
DISCUSSION
We report here the successful combination of two distinct processes using supercritical carbon dioxide to accomplish two tasks that are essential to the production of injectable drug products, namely sterilization and drying. Using a custom-built apparatus for extracting solvent from multiple vials containing drug dissolved in organic solvent, we demonstrated the quantitative recovery of starting material. Using three biological indicators normally used to challenge thermal- or radiation-based sterilization techniques, we demonstrated that a six-log reduction of spores could be obtained with direct exposure of drug to peracetic acid, but not with indirect exposure (PAA added to the chamber, but not into the vials containing drug) nor by the scCO2 drying process alone. Under the conditions used in this study, spore killing was predominantly due to the added PAA with little or no contribution from the organic solvent or the scCO2. Even when added directly into the serum vial, hydrogen peroxide was less effective at killing all three spore types than was PAA.
We deliberately used less rigorous scCO2 conditions (lower pressure, lower temperature, and shorter times) than previous investigators (7–10) who also demonstrated spore killing at a log reduction level of 6 so that we might minimize any potentially deleterious effects on drug substance. In our hands, and under the conditions used, B. pumilus proved to be the most resistant of the three microorganisms tested, whereas B. atrophaeus and G. stearothermophilus tended to show less, but equal susceptibility. Neither of the two drugs tested in this study (paclitaxel and acetaminophen) showed any measurable increase in degradation products relative to unprocessed drug when measured by HPLC/UV analysis.
It is interesting to speculate why it is possible to inactivate these difficult to kill spores while apparently not damaging the drug molecules, especially the easily oxidized acetaminophen. The answer may be related to water content of spores relative to the overall water content of the drug/spore/solvent mixture. It has long been speculated that hydroxyl radicals may be responsible for various sporicidal effects (15,16) observed either from oxidizing agents or radiation. Mohan et al. (17) confirmed by electron spin resonance spectroscopy studies that hydroxyl radicals (and to a lesser extent superoxide radicals) are formed by hydrogen peroxide and peracetic acid in the presence of added spores (107 CFU/mL). They state that “the efficient generation of high concentrations of localized radicals and other oxidizing species appears to be the primary cause for the inactivation of the B. atrophaeus spores…”, quoting Riesenman and Nicholson (18) and Imlay (19). Mohan et al. (17) point out that the high reactivity of hydroxyl radicals allows diffusion of only a few bond lengths before reacting. As Nakashio and Gerhardt (20) showed, the water content of most spores falls in the range of 26 to 55 % which, while low in comparison to vegetative cells, is very much higher than the roughly 0.01 % water content of the reaction mixtures used in these experiments. Furthermore, consistent with the observations made by Mohan et al. (17), the water contained in the spores is in intimate proximity to biomolecules that have been shown to be easily inactivated (15) at concentrations of PAA used in the current studies. Thus our observations of high sporicidal activity with low, or undetectable, chemical damage to the active pharmaceutical ingredient (API) may be explained by the higher likelihood that hydroxyl radicals will be formed in situ by interaction between water and PAA in close proximity to, or within the spores, but not in close proximity to API where the water content is minimal.
In addition to the short half-life of the hydroxyl radical to limiting oxidative damage to spores over API, the fact that PAA is highly soluble in scCO2 (21) helps to explain the rapid and complete removal of the oxidizing agent from the sample in a time frame that limits chemical degradation of API.
Two advantages of lyophobic precipitation as described here for drying drug product are the ability to dry from a wide variety of organic solvents directly in dispensing vials and the relatively short time required to produce a dry product. The current observation of conditions that allow complete sterilization of heavily contaminated vials has the potential to expand the utility of scCO2 for drug processing. It is clear from the data presented here that it is possible to reach conditions that are lethal for very difficult to kill bacterial spores without causing any increase in drug degradation.
CONCLUSION
The process described demonstrates that it is possible to inactivate difficult to kill spores to a log reduction value of 6 and in the same process remove organic solvent directly from dispensing vials containing drug and biological indicator by a combination of added sterilant (PAA) and extraction with supercritical carbon dioxide. Recovery of drug, as measured by weight gain of the vial, is 100 % (±0.5 %) and analysis of two drugs treated by the process shows no increase in degradation products. After processing, the residual level of sterilant in the vials was below the limit of detection by an HPLC assay. The process operates at a temperature of about 37 °C (±2 °C) and pressure of about 8 MPa (1,160 psi) and has a full cycle time of less than 90 min. While much remains to be done before this process could be commercially applicable, the procedure is promising, especially for the preparation of drugs that are easily susceptible to hydrolysis in the presence of water.
Acknowledgments
The authors wish to thank Bala Subramaniam for helpful discussions about free radicals in supercritical fluids and David Johnston for review of this manuscript.
Role of the Funding Source
The authors gratefully acknowledge the National Cancer Institute (1R43CA139643-01) and the Kansas Bioscience Authority for financial support of this work. Neither funding source had any role in study design, data collection, analysis or interpretation, nor writing of the report of the study.
Glossary
ABBREVIATIONS
- CFU
Colony forming unit
- scCO2
Supercritical carbon dioxide
- PAA
Peracetic acid
- LP
Lyophobic precipitation
- BI
Biological indicator
- MTS
Methyl p-tolyl sulfide
- MTSO
Methyl p-tolyl sufoxide
- SAL
Sterility assurance level
- RRT
Relative retention time
References
- 1.Trissel LA. Handbook on injectable drugs. 16. Bethesda: American Society of Health-System Pharmacists; 2011. [Google Scholar]
- 2.United States Pharmacopeia 31. Sterilization and Sterility Assurance of Compendial Articles. Rockville: The United States Pharmacopeial Convention; 2008a. p. 670–5.
- 3.Subramaniam B, Saim S, Rajewski RA, Stella V, inventors; The University of Kansas, assignee. Methods for a particle precipitation and coating using near-critical and supercritical antisolvents. United States patent US 5,833,891. 1998 Nov 10.
- 4.Rajewski RA, Subramaniam B, Niu F, inventors; CritiTech, Inc., assignee. Method for precipitation of small medicament particles into use containers. United States patent US 7,744,923 B2. 2010 Jun 29.
- 5.Fraser D. Bursting bacteria by release of gas pressure. Nature. 1951;167:33–34. doi: 10.1038/167033b0. [DOI] [PubMed] [Google Scholar]
- 6.Spilimbergo S, Bertucco A. Non-thermal Bacteria Inactivation with Dense CO2. Biotechnol Bioeng 2003;84 Dec 20(6):627–38. [DOI] [PubMed]
- 7.Christensen T, Burns D, White A, Ganem B, Eisenhut A, inventors; NovaSterilis Inc., assignee. Sterilization methods and apparatus which employ additive-containing supercritical carbon dioxide sterilants. United States patent US 7,108,832. 2006 Sept 19.
- 8.Hemmer JD, Drews MJ, LaBerge M, Matthews MA. Sterilization of bacterial spores by using supercritical carbon dioxide and hydrogen peroxide. J Biomed Mater Res B. 2007;80:511–518. doi: 10.1002/jbm.b.30625. [DOI] [PubMed] [Google Scholar]
- 9.White A, Burns D, Christensen T. Effective terminal sterilization using supercritical carbon dioxide. J Biotechnol. 2006;123:504–515. doi: 10.1016/j.jbiotec.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Burrows S, Gleason C, Matthews M, Drews M, LaBerge M, et al. Sterilizing Bacillus pumilus spores using supercritical carbon dioxide. J Microbiol Meth. 2006;66:479–485. doi: 10.1016/j.mimet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 11.United States Pharmacopeia 31. Paclitaxel monograph, test 2. Rockville: The United States Pharmacopeial Convention; 2008b. p. 2899–2901.
- 12.Aukunuru JV, Kompella UB, Betageri GV. Simultaneous high performance liquid chromatographic analysis of acetaminophen, salicylamide, phenyltoloxamine, and related products. J Liq Chrom Relat Tech. 2000;23:565–578. doi: 10.1081/JLC-100101473. [DOI] [Google Scholar]
- 13.Pinkernell U, Effkemann S, Nitzsche F, Karst U. Rapid high-performance liquid chromatographic method for the determination of peroxyacetic acid. J Chromatogr A. 1996;730:203–208. doi: 10.1016/0021-9673(95)01346-6. [DOI] [Google Scholar]
- 14.Dillow AK, Dehghani F, Hrkach JS, Foster NR, Langer R. Bacterial inactivation by using near- and supercritical carbon dioxide. Proc Natl Acad Sci. 1999;96:10344–10348. doi: 10.1073/pnas.96.18.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palop A, Rutherford GC, Marquis RE. Inactivation of enzymes within spores of Bacillus megaterium ATCC 19213 by hydroperoxides. Can J Microbiol. 1998;44:465–470. [PubMed] [Google Scholar]
- 16.Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2005;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohan A, Dunn J, Hunt MC, Sizer CE. Inactivation of Bacillus atrophaeus spores with surface-active peracids and characterization of formed free radicals using electron spin resonance spectroscopy. J Food Safety. 2009;74:M411–M417. doi: 10.1111/j.1750-3841.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 18.Riesenman PJ, Nicholson WL. Role of the spore-coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B and solar UV radiation. Appl Environ Microbiol. 2000;66:620–626. doi: 10.1128/AEM.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 20.Nakashio S, Gerhardt P. Protoplast dehydration correlated with heat resistance of bacterial spores. J Bacteriol. 1985;162:571–578. doi: 10.1128/jb.162.2.571-578.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher SA, inventor; American Air Liquide, Inc., assignee. Peracetic acid in an anhydrous sterilant delivery system. United States patent US 7,834,207. 2010 Nov 16.