Abstract
Recently, great attention has been paid to in situ gel-forming chitosan/glycerol-phosphate (chitosan/Gp) solution due to their good biodegradability and thermosensitivity. This in situ gel-forming system is injectable fluid that can be introduced into the body in a minimally invasive manner prior to solidifying within the desired tissue. At the present study, insulin release from chitosan/Gp solution has been investigated. Insulin in different concentrations was loaded in two formulations of chitosan/Gp solution and in vitro drug release was studied over a period of 3 weeks. Results indicated that the release of insulin from chitosan/Gp gel decreases by increasing in Gp salt and initial insulin concentration. Stability of released insulin was investigated by 8-anilino-1-naphthalenesulfonate probe. Results proved that insulin have been released in its native form. Because of simple preparation and administration, prolonged release of insulin and stability of released insulin, this in situ gel-forming system could be used as a controlled release delivery system for insulin.
KEY WORDS: biodegradable, chitosan, controlled release, in situ forming, insulin
INTRODUCTION
Hydrogels are cross-linked, three-dimensional hydrophilic networks that swell but do not dissolve when brought into contact with water. Recently, hydrogel-based drug delivery devices have become a major area of research interest (1–3). In the past few years, great attention has been paid to thermosensitive in situ-forming hydrogels because of their potential application for biomedical systems such as drug delivery, tissue engineering, and enzyme or protein modification (4,5). These systems are injectable fluids that can be introduced into the body in a minimally invasive manner prior to solidifying or gelling within the desired tissue. Recently, the potential applications of biopolymers in these criteria have been investigated (6,7). Among them, chitosan, obtained by alkaline deacetylation of chitin, is one of the most popular biopolymers (8). A thermosensitive chitosan-Pluronic (CP) hydrogel was designed as an injectable cell delivery carrier by grafting Pluronic onto chitosan using EDC/NHS chemistry (9). Although the aqueous CP solution presented a sol–gel transition around 25°C, the transition time is long enough to inject the solution to a target site over 25°C (10). In another work, biodegradable thermosensitive chitosan hydrogel was synthesized by block copolymerization of monomethoxy poly(ethylene glycol) macromere (PEG) onto chitosan backbone, using potassium per sulfate as a free radical initiator (10). Aqueous solutions of PEG-g-chitosan can be prepared at physiological pH values, thereby allowing safe incorporation of bioactive molecules.
A thermosensitive neutral solution based on chitosan/β-glycerophosphate disodium salt (Gp) combinations, first reported by Chenit et al., is an outstanding injectable in situ gel-forming system (11). Neutral solution of chitosan/Gp combination could remain liquid at room temperature, but changed into a gel upon heating at physiological temperature (37°C). Many works had reported the pharmaceutical application of this thermosensitive in situ gel-forming solution (12,13).
Using chitosan/Gp solution, Sharma et al. have developed an in situ gelling system for sustained delivery of ellagic acid (EA) via subcutaneous route (14). Their results indicate the potential of chitosan/Gp solution in improving the bioavailability of ellagic acid as well as sustained release of EA, as demonstrated by the in vitro release profile. They also found that entrapping the nanoparticles of ellagic acid in the chitosan/Gp in situ gel-forming system can be further sustained the EA release (14).
A transparent thermo and pH-sensitive in situ gel-forming system based on quaternized chitosan (HTCC) and α-β-Gp had reported by Wu et al. (15). αβ-glycerophosphate (αβ-Gp) is a mixture of β-glycerophosphate (β-Gp) and α-glycerophosphate (α-Gp), while α-Gp has linear chain structure and shows less steric hindrance than β-Gp. Solutions prepared with HTCC and αβ-Gp had better gelation capacity and was transformed to transparent gel at body temperature (37°C) compared with opaque gels with chitosan and β-Gp salt. HTCC/GP solutions also showed better pH-sensitivity than chitosan/Gp solutions. The entrapped drug in HTCC/Gp hydrogel was released quickly in acidic condition and quite slowly with low initial release in basic condition. (15).
By simply mixing HTCC and poly(ethylene glycol) (PEG) with a small amount of αβ-Gp, a novel nasal drug delivery system was developed (16). Wu et al. showed that when the HTCC/Gp solution cross-linked by PEG, it can be dropped or sprayed easily into nasal cavity, but transformed into viscous gel at body temperature. Therefore, nasal mucociliary clearance rate as well as drug release rate was decreased (16).
Berrada et al. have demonstrated the effectiveness of using the chitosan/Gp in situ gel-forming system to deliver high doses of camptothecin locally to a mouse tumor model (17). After an initial burst of less than 5% in the first day, the formulation showed zero-order release kinetics for the 4 weeks. Besides, tumor growths treated in this manner was hindered for extensively longer periods than those treated with systemically administered camptothecin (17).
Kempe et al. have studied the macro- and microstructure of chitosan/β-GP systems and showed that the microviscosity and pH inside the gels depends on the β-GP concentration (18). They also studied the dynamics of the spin-labeled insulin and its microviscosity inside the gels and during release by EPR spectroscopy. Their results indicate that the insulin was incorporated into the aqueous environment of the gel and was released in its native form.
Although many studies have been showed the high potential of this in situ gelling hydrogel for encapsulating drugs and biological agents, little work has been published in the preparation of sustained insulin delivery formulation using chitosan/Gp solution. Based on biodegradable polymeric matrices, few sustained insulin release products have received FDA approved. Inherent instability of insulin exposing to the conventional emulsion/solvent removal techniques as well as low encapsulation efficiencies and high initial burst release of drug are the some major reasons. Besides, the structural and chemical instability often results in incomplete release of insulin from biodegradable matrices. Achieving an acceptable dose volume of insulin while maintaining satisfactory release profiles from an in situ gel-forming system is the main challenge of this study. Since the chitosan/Gp solutions are essentially designed to be used via parenteral injection, exact observant of their gelation time and temperature in the presence of insulin as well as the stability of incorporated insulin may lead to better design of sustained insulin delivery formulation. So in this work, we evaluate the effect of insulin loading on thermosensitivity of chitosan/Gp solution as well as the chemical stability of incorporated insulin under various drugs loading. In addition, the release profile of insulin under various chitosan and Gp concentrations were investigated.
EXPERIMENTS
Materials
Medium molecular weight chitosans with degree of deacetylation (DDA) of 75% and 85% were purchased from Sigma-Aldrich (St. Louis, MO). The DDA of chitosan samples were determined by H-NMR spectroscopy. H-NMR was performed using a Bruker NMR spectrometer operating at 500 μHz and room temperature. Gp and 8-anilino-1-naphthalenesulfonic acid) (ANS) were purchased from Sigma-Aldrich (St. Louis, MO); glacial acetic acid, potassium hydroxide and Coomassie blue G250 were obtained from Merck (Germany). Insulin was obtained from Novo Nordisk (Iran).
Preparation of Thermosensitive Solutions
Clear solutions of chitosan were prepared by dissolving various amount of chitosan in aqueous solution of acetic acid (0.1 M). The pH of the acetic acid solution has been adjusted to 4.0 by adding droplets of potassium hydroxide solution (1 M). The chitosan solutions were cooled down to 4°C and continuously stirred while adding different amounts of Gp powder. Mixing was carried on for 15 min. The resultant clear chitosan/Gp solutions were stored at 4°C.
Determination of Gelation Time
In this study, gelation was assessed using the inverted tube test, as described by Zhou and coworkers (19). When a test tube containing a solution is titled, it is defined as a sol phase if the solution deforms by flow, or a gel phase if there is no flow. Firstly, 2 ml of chitosan/Gp solutions were maintained for 12 h at 4°C in 5 mL vials with inner diameter of 10 mm to remove air bubbles. The vials were then incubated in a temperature-controlled bath at 37°C. The sol–gel transition time was determined by inverting the vials horizontally every minute. The time at which the gel did not flow was recorded as the gelation time. All measurements were performed in triplicate, data are reported as means ± SD.
Morphological Studies
The structural characteristic of formed gel was observed using SEM XL-series from Philips (The Netherlands) at 15 kV. The gels were rinsed with distilled water, frozen in liquid nitrogen and freeze-dried for 48 h. The sample was fixed on a metal stub with conductive carbon tape, and was coated with platinum under vacuum by an ion sputter.
In Vitro Insulin Release
Insulin was dissolved in 3 ml of phosphate buffer (pH = 7.4) and then was added to chitosan/Gp solutions under stirring at 4°C, up to a final concentration of 0.2 and 0.5 mg/ml. Samples of 0.5 ml were placed in Eppendorf tubes upon nitrogen atmosphere and incubated at 4°C for 24 h. The insulin-loaded chitosan/Gp solution then gelled at 37°C for 1 h. The gels (0.5 ml) were incubated with 1.5 ml of phosphate buffer pH 7.4 at 37°C while stirring at 150 rpm. At several time intervals, 0.5 ml of the buffer was taken as a sample and replaced by new buffer solutions to assure sink conditions. The amount of the released insulin was determined by Bradford protein assay. All measurements were performed in triplicate, data are reported as means ± SD.
The Bradford protein assay is a dye-binding (Coomassie Brilliant Blue G-250) test based on the discrepancy color change of a dye in reply to a variety of concentrations of protein (20). While binding to protein occurs, the absorbance maximum for an acidic solution of Coomassie Brilliant Blue G-250 shifts from 465 nm to 595 nm. 0.1 g Coomassie Brilliant Blue G-250 was added to 50 mL 96% ethanol, then 10 mL of 85% orthophosphoric acid was added and diluted to 1000 mL. The solution was filtered and stored in a brown glass bottle in a dark environment at room temperature. The above solution was added to the insulin solutions released from chitosan/Gp hydrogel and their absorbance were measured vs the reagent blank at 600 nm in ELISA reader (Statfax 2100, UK).
Insulin Structural Stability
Overall structural stability of released insulin from chitosan/Gp in situ gel-forming system was compared to lyophilized pure insulin using fluorescence spectroscopy. Hydrophobic dyes like 1-anilinonaphthalene-8-sulfonate (ANS) are widely used to monitor changes in surface hydrophobicity of macromolecules. 3D structural changes of protein may alter intensity and/or λem of ANS. It has a high capacity to bind to hydrophobic regions of moderately unfolded proteins, leading to an enhancement in fluorescence (21). A fresh ANS stock solution was prepared in double distilled water. An aliquot of 1 μL of the ANS solution was added to 1 mL of buffer to a final concentration of 10 μM. A blank spectrum without protein was recorded before insulin solution was added to the ANS solution. Fluorescence measurements (Spectrofluorophoto Meter, Shimadzu, Japan) were performed immediately after the addition of insulin to the ANS solution. Emission spectra were recorded from 400 to 550 nm with excitation at 370 nm.
RESULTS AND DISCUSSION
The aim of this study was to investigate the capacity of thermosensitive chitosan solutions to be used as injectable insulin sustained delivery system. To achieve this goal, insulin-loaded chitosan/Gp in situ gel-forming systems were prepared and their gelation time and released profile were analyzed as described below.
Characteristics of Chitosan/Gp Hydrogels
The characteristics of the Chitosan/Gp thermosensitive solutions prepared with different formulations (Table I) show that the pH values of solution were increased with increases of Gp salt concentration. While only a little pH increase were recorded with increases of DDA or chitosan concentration. Gp is a polyol counter ionic mono-head salt which could increase the pH of the solution due to the neutralizing effect of the phosphate groups (base). The pH of all solutions was in physiological range; therefore, the prepared gels could be potentially used in controlled drug delivery systems.
Table I.
Characteristics of Chitosan/Gp Hydrogels
| Sample | Starting Solution | Final hydrogel | ||||
|---|---|---|---|---|---|---|
| Chitosana (% w/v) | Gp (mol/L) | pHb | Gelation Timeb (min) | Reversibility (sol–gel–sol) | Syringeability | |
| Ch1 | 1.0 | 0.45 | – | No gel | No gel | Easily |
| Ch2 | 1.0 | 0.55 | – | No gel | No gel | Easily |
| Ch3 | 1.5 | 0.45 | 7.29 | 50 | + | Drop-wise |
| Ch4 | 1.5 | 0.55 | 7.34 | 37 | + | Drop-wise |
| Ch5 | 2.0 | 0.45 | 7.30 | 8 | + | Hardly |
| Ch6 | 2.0 | 0.55 | 7.35 | 4 | – | Hardly |
| Ch7 | 1.0 | 0.45 | 7.36 | >100 | + | Easily |
| Ch8 | 1.0 | 0.55 | 7.44 | >100 | + | Easily |
| Ch9 | 1.5 | 0.45 | 7.37 | 55 | + | Drop-wise |
| Ch10 | 1.5 | 0.55 | 7.44 | 40 | + | Drop-wise |
| Ch11 | 2.0 | 0.45 | 7.38 | 3 | – | Hardly |
| Ch12 | 2.0 | 0.55 | 7.45 | 2 | – | Hardly |
aDDA of chitosan in samples Ch1 to Ch6 is 75%, while in samples Ch7 to Ch12 is 85%
bAll measurements were performed in triplicate; data are reported as means ± SD
The thermosensitivity of chitosan/Gp solution at 37°C was investigated by inverted test tube method (Fig. 1). The gelation mechanism of chitosan/Gp solution was already reported (22–24). Briefly, electrostatic attraction between oppositely charged ammonium groups of chitosan and the phosphate moiety of Gp salt allows for attractive hydrophobic and hydrogen bonding between chitosan chains, which are the main molecular forces responsible for the sol/gel transition in chitosan/Gp solutions.
Fig. 1.
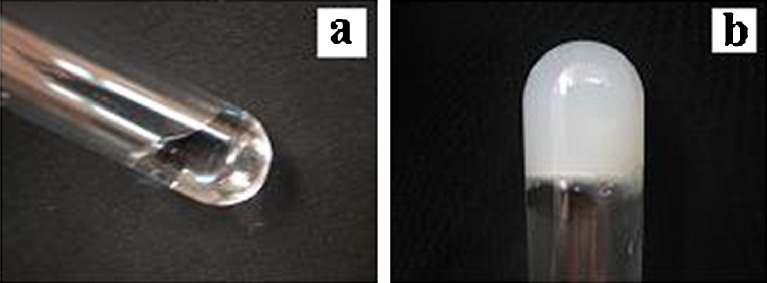
The chitosan/Gp solution at room temperature a and the formed hydrogel at 37°C b
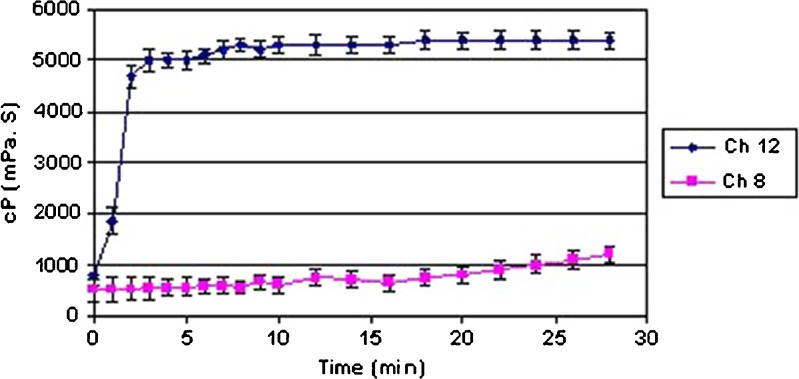
Our results are in good agreement with above theory (Table I). No temperature-induced gelation of chitosan/Gp solutions was observed for samples Ch1 and Ch2; formation of gel for samples Ch7 and Ch8 was completed after heating the mixture at 37°C for more than 100 min. However, higher DDA or chitosan concentration leads to complete sol-to-gel behavior. It seems that the concentration and DDA of chitosan as well as the Gp salt concentration will affected the thermosensitive characteristics of these in situ gel-forming systems. Comparing the gelation behavior of chitosan/Gp solutions of various polymer concentrations (Table I), a reverse relation between time of gelation and chitosan concentration could be found (Ch3 vs Ch5 or Ch9 vs Ch11). The same results obtained in rheological analysis (Brookfield DV-E viscometer, Brookfield Engineering Laboratories, Inc., USA). Figure 2 shows the change of viscosities of hydrogels prepared with different concentrations of chitosan (Ch8 and Ch12). The hydrogel was maintained at 37°C and the viscosity of the hydrogel was measured every 1–2 min. As it can be seen, the viscosity of Ch8 (with 1.0% w/v chitosan concentration) was nearly unchanged during 30 min, whereas the viscosity of hydrogel prepared with a chitosan concentration of 2.0% w/v (sample Ch12) increased quickly after 2 min. In other words, solutions with higher polymer concentrations which correspond to more –OH and –NH groups for intermolecular hydrogen binding; form gel faster than those with lower concentrations. The same results were recorded for DDA effect on gelation time. For example, gelation time of Ch6 with DDA 75% and Ch12 with DDA 85% was 4 and 2 min, respectively. Obviously, by increasing the DDA, which is corresponds to the substituted amine groups in a chitosan chain, the gelation time of polymeric solution decreases.
Fig. 2.
Viscosities of chitosan/Gp hydrogels as a function of time at 37°C
The influence of Gp salt concentration on the thermosensitivity of chitosan solution was also investigated (Table I). Apparently, by increasing the concentration of Gp salt, the required time for gelation decreases. Ch4 solution with 0.55 M Gp salt took 37 min to form gel whereas for Ch3 with 0.45 M Gp salt, the gelation time was 50 min. The same situation was observed for other pairs. With the increase of Gp salt concentration, more chitosan amino groups were neutralized. Therefore, electrostatic repulsive force between chitosan chains was damaged, and polymer chains were aggregated more easily. This led to obvious reduction in gelation time. This result is in agreement with Kempe’s group finding (18). They showed that higher β-Gp concentrations lead to faster gelation at constant temperature. They also showed that increasing β-GP concentrations lead to a lowering of the gelation temperature from about 50°C at 6% β-Gp to 37°C (16% β-Gp) (18).
Therefore, reaching an optimum concentration and DDA of chitosan as well as Gp salt concentration is the critical point required to start the gelling process of chitosan solutions. Ch1 and Ch2 do not match this criterion, so no temperature-induced gelation was observed for them.
The thermal-reversibility of chitosan/Gp gels was also studied by inverted test tube method (Table I). This result is in good agreement with other reports (22). It seems that the thermo-reversibility or thermo-irreversibility of chitosan/Gp gels is mainly dependent on the competition between the different molecular forces involved in gel formation. For example, at low concentration of Gp salt, gelation may mainly occur by hydrophobic interactions. Since the hydrophobic forces are known to be temperature dependent, cooling the solution causes this weak gel to be changed to the liquid solution. At high concentration of Gp salt, the hydrogen bonds between chitosan chains and chitosan–water molecules is predominate due to the neutralization effects of Gp salt. Since hydrogen bonds are not temperature dependent, cooling the gel did not affect the gel structure (22).
The syringeability of the final solutions greatly decreased with the increase of chitosan concentration. The lowest syringeability was observed with chitosan concentration of 2% (w/v). The DDA of chitosan and Gp salt concentration had no effect on syringeability of final solutions (Table I).
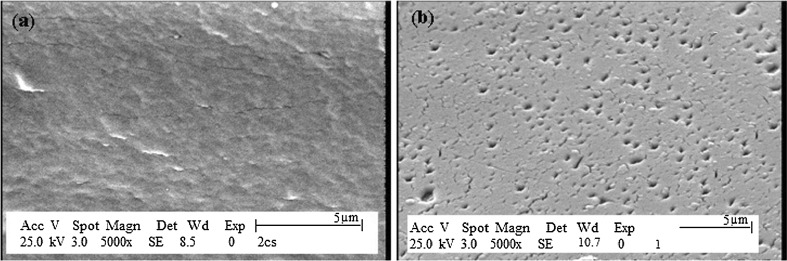
The SEM photograph of the Ch5 sample prepared with 2% (w/v) of chitosan (DDA = 75%) and 0.45 mol/L Gp salt is shown in Fig. 3a. It shows that the formed gel had a rough and non-porous surface, while the swelled gel in phosphate buffer (pH = 7.4) had a porous structure as shown in Fig. 3b. Protein and peptide molecules could easily penetrate in this porous matrix of chitosan/Gp gel.
Fig. 3.
SEM photographs of chitosan/Gp hydrogel a dry hydrogel, b swelled hydrogel in phosphate buffer for 1 h
In Vitro Release of Insulin
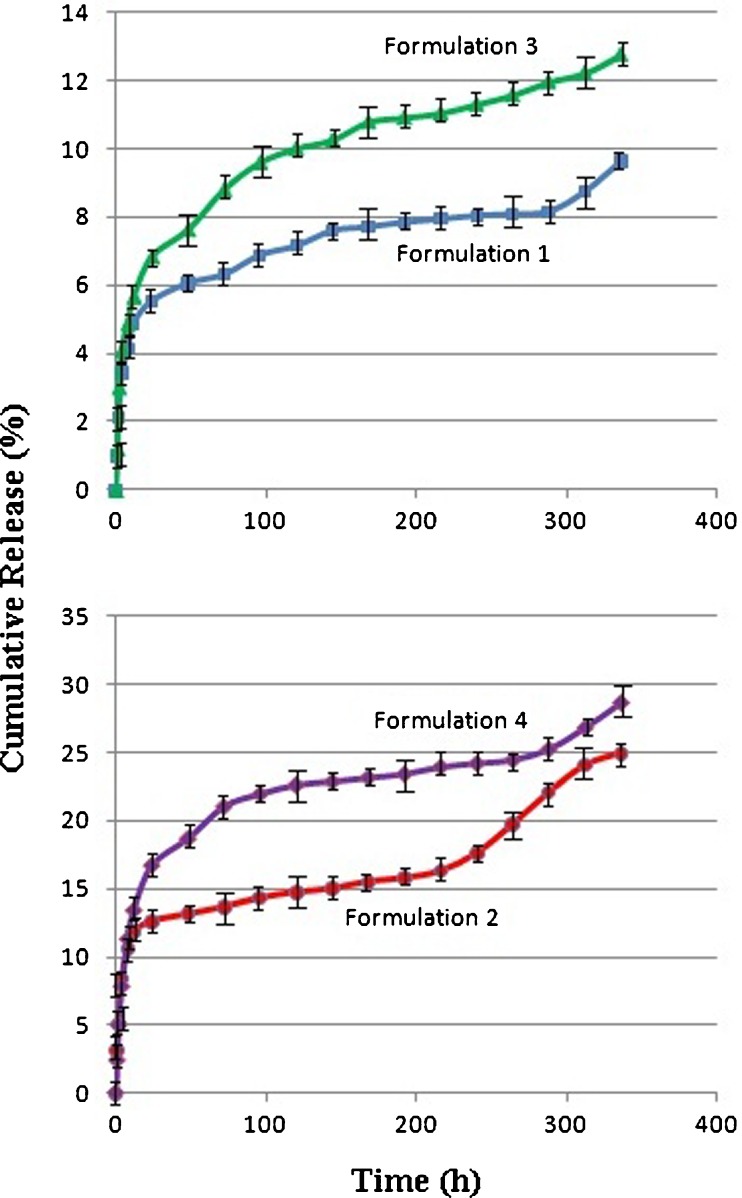
Insulin was trapped in the chitosan/Gp gel (Table II), and its cumulative release behavior was investigated in vitro as shown in Fig. 4. As it can be seen, the release pattern for all formulations was biphasic, comprising an initial burst effect followed by an almost sustained continuous phase. The initial burst release was relatively low; 19%, 13%, 8% and 6% of drug were released within 48 h from formulations 4, 2, 3, and 1, respectively. A similar release profile was observed by Wu et al., where HTCC-PEG-Gp gel was used as a nasal insulin delivery carrier (19). This behavior could be assigned to the immediate dissolution and release of the accumulated drug on the surface or in the tunnels of gels during the gelation process. This part of the drug could diffuse rapidly when the gel came into contact with the release medium.
Table II.
Insulin-loaded Chitosan/Gp Samples (DDA = 75%)
| Formulation | Gp (mol/L) | Insulin (mg/mL) | Chitosan (% w/v) |
|---|---|---|---|
| 1 | 0.55 | 0.5 | 2 |
| 2 | 0.55 | 0.2 | 2 |
| 3 | 0.45 | 0.5 | 2 |
| 4 | 0.45 | 0.2 | 2 |
Fig. 4.
Insulin release profile from chitosan/Gp hydrogels, (n = 3)
After an initial burst release, the insulin entrapped into the chitosan/Gp gel was released slowly. The release rates of insulin show that the gel characteristics affected the release efficiency (Tables II and III and Fig. 4). Both rate and total drug release were decreased by increasing the Gp salt ratio in formulations 2 and 4. As discussed before, addition of Gp salt to aqueous solution of chitosan can directly modulate electrostatic and hydrophobic interactions, and especially hydrogen bonding between chitosan chains, which is the main molecular forces involved in gel formation. Increasing the Gp salt concentration will result in extensive hydrogen bonding via OH–NH and O–HN of chitosan chains, which formed the tightest network and effectively hindered the drug release from the gel. The same conclusion could be made by comparing the release profile of insulin from gels made with 0.45 mol/l Gp (Formulations 1 and 3). These results are supported by experiments from Kempe et al., who explored the impact of the chitosan/β-Gp gel on insulin status and release rate (18). Based on their results, the β-Gp concentration impacts the molecular mobility of insulin in a sense that higher concentrations showed a slower mobility. They find that the chitosan/Gp gels with the higher β-Gp content, higher gel strength, and a higher macroviscosity, have a lower microviscosity inside the gel networks (18).
Table III.
Kinetic Profile of Insulin Release
| Formulations | Release rate (mg/h) | Zero-order | Higuchi |
|---|---|---|---|
| R 2 | R 2 | ||
| F1 | 0.009 | 0.7490 | 0.9341 |
| F2 | 0.020 | 0.7950 | 0.8787 |
| F3 | 0.016 | 0.7283 | 0.9372 |
| F4 | 0.026 | 0.7163 | 0.9143 |
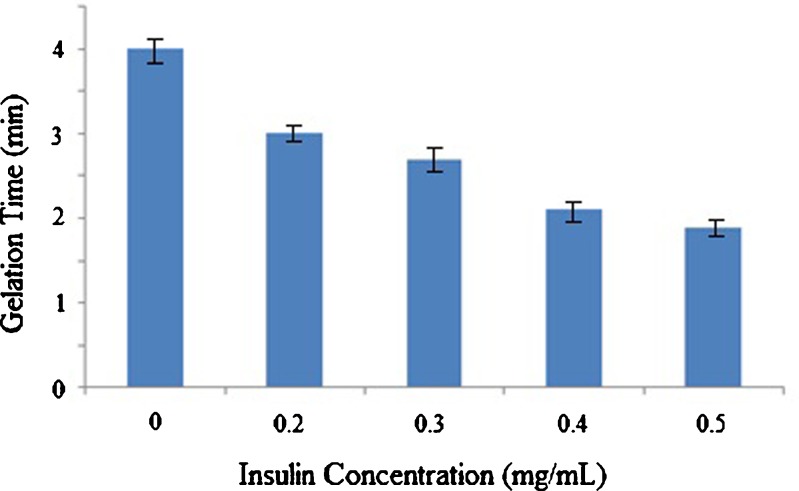
In the present study, the effect of initial drug loading on insulin release profile was also investigated (Tables II and III and Fig. 4). Both the rate and total drug release were increased by decreasing the insulin concentration in chitosan/Gp gel (Formulations 3 and 4). It is also obvious that in a higher insulin concentration, the initial release became lower. During the first 48 h, about 8% of the drug was released from the gel with 0.5 mg/mL insulin (Gp 0.55 mol/l). When the insulin concentration decreased to 0.2 mg/mL, the initial release during the first 48 h increased to 19%. Wu et al. (19) have reported a similar release profile, where increasing the initial drug loading led to decrease in the insulin release rate. It seems that the amino and hydroxyl groups of insulin have a high potential to increase the number of intermolecular hydrogen bonding in chitosan/Gp gel, which strengthened the cross-linked network. Obviously, diffusing of insulin from a rigid network is slower than a floppy one. To check this theory, the effect of insulin concentration on gelation time of chitosan/Gp solutions was evaluated (Fig. 5). As it is obviously shown in Fig. 5, increasing the insulin concentration has shortened the gelation time. This finding can also support the idea of contribution of insulin in gel formation and strength.
Fig. 5.
Effect of insulin concentration on gelation time of chitosan/Gp solution (chitosan: 2% (w/v), Gp salt: 0.55 M) at 37°C, (n = 3)
The insulin release patterns from the prepared gels were also determined in this study. In Table III, the insulin release data were fit according to zero-order and Higuchi kinetic models of drug release and it was found that for all formulations the data were better fitted to Higuchi kinetic models.
Insulin Structural Stability
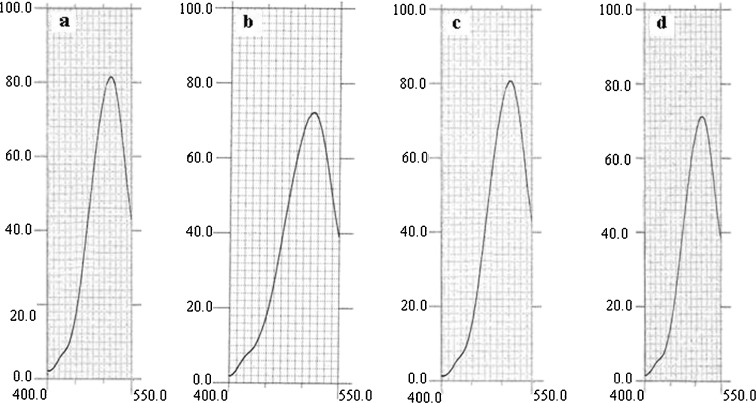
Stability of released insulin from chitosan/Gp gel was studied in vitro using extrinsic fluorescence spectroscopy analysis. Insulin released from different gel was incubated with ANS. No changes in Emax and fluorescence intensity were observed (Fig. 6). Results showed that insulin retains its native structure. Upon addition of insulin to ANS solution, no change in ANS fluorescence intensity or emission maximum was seen (Fig. 6c and d). This specified that our formulation does not have any significant effects on insulin folded structure. Furthermore, since the insulin show little interaction with ANS, no significant structural changes are induced through solubilization in acidic chitosan/Gp solution.
Fig. 6.
Extrinsic fluorescence of a ANS, b pure insulin, c insulin in formulation 1 and d insulin in formulation 2 at λ ex = 370 nm and λ em = 400–550 nm
CONCLUSION
It was concluded that the release of insulin from chitosan/Gp thermosensitive gel decreases by increasing in Gp salt and initial insulin concentration. Furthermore, it was proved that the insulin incorporation into the chitosan/Gp gel has no impact on insulin stability. Because of simple preparation and administration, prolonged release of insulin and stability of released insulin, this in situ gel-forming system could be used as insulin controlled release delivery system.
ACKNOWLEDGEMENTS
This project was supported by a grant from Vice Chancellor of Research, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran. The results described in this paper were part of a Pharm D student thesis proposal related to Ms Hanieh Naghizadeh.
REFERENCES
- 1.Khodaverdi E, Rajabi O, Farhadi F, Jalali A, Mirzazadeh Tekie F. Preparation and investigation of poly (N-isopropylacrylamide–acrylamide) membranes in temperature responsive drug delivery. IJBMS. 2010;13(3):102–110. [Google Scholar]
- 2.El-Leithy ES, Shaker DS, Ghorab MK, Abdel-Rashid RS. Evaluation of mucoadhesive hydrogels loaded with diclofenac sodium–chitosan microspheres for rectal administration. AAPS PharmSciTech. 2010;11(4):1695–1702. doi: 10.1208/s12249-010-9544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia J, Dong C, Zhang W, Cui Y, Liu J. Evaluation of pharmacokinetic and pharmacodynamic relationship for oral sustained-release atenolol pellets in rats. J Pharm Biomed Anal. 2011;55(2):342–348. doi: 10.1016/j.jpba.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Ganji F, Vasheghani-Farahani E. Hydrogels in controlled drug delivery systems. IPJ. 2009;18(1):63–88. [Google Scholar]
- 5.Fu J, Feng X, Yuan H, Yan L, Kuang X, Xia Z, et al. Study of ocular pharmacokinetics of in situ gel system for S(−)-satropane evaluated by microdialysis. J Pharma Biomed Anal. 2008;48(3):840–843. doi: 10.1016/j.jpba.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Horkay F, Magda J, Alcoutlabi M, Atzet S, Zarembinski T. Structural, mechanical and osmotic properties of injectable hyaluronan-based composite hydrogels. Polymer. 2010;51(19):4424–4430. doi: 10.1016/j.polymer.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes SR, Walker DR, Goldberg EP. Injectable cell-biopolymer gels for neural tissue repair. IFMBE Proc. 2009;24:367–368. doi: 10.1007/978-3-642-01697-4_125. [DOI] [Google Scholar]
- 8.Buranachai T, Praphairaksit N, Muangsin N. Chitosan/polyethylene glycol beads crosslinked with tripolyphosphate and glutaraldehyde for gastrointestinal drug delivery. AAPS PharmSciTech. 2010;11(3):1128–1137. doi: 10.1208/s12249-010-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD. Thermosensitive chitosan–Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009;5(6):1956–1965. doi: 10.1016/j.actbio.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Ganji F, Abdekhodaie MJ. The effects of reaction conditions on block copolymerization of chitosan and poly(ethylene glycol) Carbohyd Polym. 2010;81:799–804. doi: 10.1016/j.carbpol.2010.03.049. [DOI] [Google Scholar]
- 11.Chenite A, Buschmann M, Wang D, Chaput C, Kandani N. Rheological characterization of thermogelling chitosan/glycerol-phosphate solutions. Carbohyd Polym. 2001;4691:39–47. doi: 10.1016/S0144-8617(00)00281-2. [DOI] [Google Scholar]
- 12.Ruel-Gariépy E, Shive M, Bichara A, Berrada M, Garrec DL, Chenite A, et al. Thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur J Pharm Biopharm. 2004;57(1):53–63. doi: 10.1016/S0939-6411(03)00095-X. [DOI] [PubMed] [Google Scholar]
- 13.Ruel-Gariépy E, Leclair G, Hildgen P, Gupta A, Leroux J-CA. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J Contr Release. 2002;82(2–3):373–383. doi: 10.1016/S0168-3659(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 14.Sharma G, Italia JL, Sonaje K, Tikoo K, Ravi Kumar MNV. Biodegradable in situ gelling system for subcutaneous administration of ellagic acid and ellagic acid loaded nanoparticles: evaluation of their antioxidant potential against cyclosporine induced nephrotoxicity in rats. J Contr Release. 2007;118:27–37. doi: 10.1016/j.jconrel.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Su Z-G, Ma G-H. A thermo- and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int J Pharm. 2006;315:1–11. doi: 10.1016/j.ijpharm.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Wei W, Wang L-Y, Su Z-G, Ma G-H. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials. 2007;28:2220–2232. doi: 10.1016/j.biomaterials.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Berrada M, Serreqi A, Dabbarh F, Owusu A, Gupta A, Lehnert S. A novel non-toxic camptothecin formulation for cancer chemotherapy. Biomaterials. 2005;26:2115–2120. doi: 10.1016/j.biomaterials.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Kempe S, Metz H, Bastrop M, Hvilsom A, Contri RV, Mäder K. Characterization of thermosensitive chitosan-based hydrogels by rheology and electron paramagnetic resonance spectroscopy. Eur J Pharm Biopharm. 2008;68:26–33. doi: 10.1016/j.ejpb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Zhou HY, Zhang YP, Zhang WF, Chen XG. Biocompatibility and characteristics of injectable chitosan-based thermosensitive hydrogel for drug delivery. Carbohyd Polym. 2011;83(4):1643–1651. doi: 10.1016/j.carbpol.2010.10.022. [DOI] [Google Scholar]
- 20.Changrani NR, Chonkar A, Adeghate E, Singh J. Effects of streptozotocin-induced type 1 diabetes mellitus on total protein concentrations and cation contents in the isolated pancreas, parotid, submandibular, and lacrimal glands of rats. Ann N Y Acad Sci. 2006;1084:503–519. doi: 10.1196/annals.1372.019. [DOI] [PubMed] [Google Scholar]
- 21.Fu X, Zhang X, Chang Z. 4,40-Dianilino-1,10-binaphthyl-5,50-sulfonate, a novel molecule having chaperone-like activity. Biochem Bioph Res Co. 2005;329:1087–1093. doi: 10.1016/j.bbrc.2005.01.164. [DOI] [PubMed] [Google Scholar]
- 22.Ganji F, Abdekhodaie MJ, Ramazany A. Gelation time and degradation rate of chitosan as a thermosensitive injectable hydrogel. J Sol-Gel Sci Techn. 2007;42:47–53. doi: 10.1007/s10971-006-9007-1. [DOI] [Google Scholar]
- 23.Cho J, Heuzey MC, Begin A, Carreau PJ. Physical gelation of chitosan in the presence of beta-glycerophosphate: the effect of temperature. Biomacromolecules. 2005;6:3267–3275. doi: 10.1021/bm050313s. [DOI] [PubMed] [Google Scholar]
- 24.Li XY, Kong XY, Wang XH, Shi S, Guo G, Luo F, et al. Gel–sol–gel thermo-gelation behavior study of chitosan–inorganic phosphate solutions. Eur J Pharm Biopharm. 2010;75:388–392. doi: 10.1016/j.ejpb.2010.04.015. [DOI] [PubMed] [Google Scholar]