Abstract
Egg phosphatidylcholine is commonly used as an emulsifier in formulations administered parenterally. However, synthetic phosphatidylcholine (PC) emulsifiers are now widely available and may be desirable substitutes for egg-derived phospholipids due to stability, purity, and material source considerations. In earlier work, we demonstrated that a squalene–1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) emulsion provided equivalent physical stability compared to a squalene–egg PC emulsion. In the present manuscript, we evaluate the physical stability of vaccine adjuvant emulsions containing a range of other synthetic phosphatidylcholine emulsifiers. Besides the POPC emulsion, the 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) emulsion showed good particle size and visual stability compared to emulsions made with other synthetic phospholipids. Moreover, comparable immune responses were elicited by squalene emulsions employing various synthetic PC or egg PC emulsifiers in combination with an inactivated influenza vaccine or a recombinant malaria antigen, and these responses were generally enhanced compared to antigen without adjuvant. Therefore, we show that (1) some synthetic PCs (DMPC, POPC, and to a lesser extent 1,2-dioleoyl-sn-glycero-3-phosphocholine) are effective stabilizers of squalene emulsion over a range of storage temperatures while others are not (1,2-distearoyl-sn-glycero-3-phosphocholine, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, and 1,2-dilauroyl-sn-glycero-3-phosphocholine) and (2) the immunogenicity of stable squalene emulsions is similar regardless of PC source.
Electronic supplementary material
The online version of this article (doi:10.1208/s12249-012-9771-x) contains supplementary material, which is available to authorized users.
Key words: oil-in-water emulsion, phosphatidylcholine, squalene, vaccine adjuvant
INTRODUCTION
Metabolizable oil-in-water emulsions have been demonstrated to be safe and effective vaccine adjuvants, nutritional supplements, and drug delivery vehicles. Various emulsifier compositions exist, with squalene being the preferred metabolizable oil for vaccine adjuvant applications (1–3). Commonly used emulsifiers include Pluronics®, Tweens®, Spans®, and phospholipids. Emulsifier selection is based on emulsion stabilizing capacity and/or biological activity, since emulsifiers are membrane active by definition and have been shown to have various biological effects related to immune stimulation (4).
Lecithin and its main component, phosphatidylcholine (PC), have been successfully utilized as emulsifiers in safe and effective parenterally delivered emulsions. Perhaps the widest use of phospholipid emulsifiers is in the application of intravenous nutritional supplementation (5), such as in Intralipid®, a soybean oil/egg lecithin emulsion. However, there may be some disadvantages associated with egg lecithin (or egg PC) as an emulsifier, and advantages to substituting egg-derived phospholipids with synthetic phospholipids. First, there is considerable heterogeneity of structure: egg PC may contain at least 17 different PC species (6), whereas various synthetic PCs are available commercially at 99 % purity. Second, egg lecithin consists of multiple monounsaturated and polyunsaturated acyl chains, which are prone to oxidative degradation, whereas various synthetic PCs are composed of saturated acyl chains and are therefore more chemically stable (7). Third, the egg phospholipids are derived from an animal source instead of being synthetically produced.
We have previously published the physical stability profile of squalene emulsions stabilized by egg PC (8,9). Moreover, we showed that synthetic 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC; a main component of egg PC) provided equivalent stability to a squalene emulsion compared to egg PC. However, no other synthetic PCs were evaluated. Synthetic, homogeneous phospholipids have become widely available and are now relatively inexpensive. By appropriate selection of synthetic phospholipid, the chemical stability of the emulsifier and the physical stability of the emulsion could be optimized. Biological equivalence must also be addressed; changes in source or purity of emulsifiers in vaccine adjuvant emulsions have previously been shown to dramatically affect vaccine potency (10,11). In the present manuscript, we seek to (1) build on our previous work by evaluating the physical stability of squalene emulsions containing other synthetic PCs (besides POPC), and (2) compare the biological activity of the synthetic PC emulsions as well as an egg PC emulsion in the context of malaria and influenza vaccine formulations.
MATERIALS AND METHODS
Adjuvant Formulations
Shark liver squalene (≥98 % purity) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Egg phosphatidylcholine (egg PC), POPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), and 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) were obtained from Avanti Polar Lipids, Inc. at 99 % purity (Alabaster, AL, USA). All emulsion formulations were prepared by high-speed mixing an oil phase (containing squalene and phospholipid) and an aqueous phase followed by high-pressure homogenization, as described previously (8). The final concentration of phospholipid was 25 mM. Formulations were monitored for stability over 6 months at 5°C, room temperature (RT), 37°C, and 60°C. Particle size, zeta potential, viscosity, hemolysis, and light scattering optical profiling measurements were performed as described previously (8). Briefly, particle size was determined at the indicated timepoints by diluting an aliquot of each emulsion 1:100-fold in water and measuring the scattering intensity-biased size average (Z-avg) by dynamic light scattering (Malvern Zetasizer APS). Zeta potential was measured shortly after emulsion manufacture by diluting an aliquot of each emulsion 1:20-fold in water and measuring zeta potential by microelectrophoresis (Malvern Zetasizer Nano-ZS). A 20-mL sample of each emulsion was removed from storage at 5°C and allowed to equilibrate to room temperature before measuring viscosity by a rotational viscometer (Brookfield DV-E). Emulsion hemolytic activity was assayed following a modified version of a previously published technique (12); a suspension of red blood cells from a human volunteer was mixed in saline with each emulsion for ∼20 min, following which the sample was centrifuged, the supernatant was diluted into an ethanol/HCl (39:1, v/v) mixture, and the absorbance was measured at 398 nm. Laser scattering optical profiling measurements were collected every 10 min for 4 h on undiluted emulsion samples at 60°C using a 870 nm laser and a charge-coupled detector (LUMiReader).
Immunization, Serum Collection, and Immunological Assays
Female, 6–7 weeks old, BALB/c mice (Charles River Laboratories, Wilmington, MA, USA) were immunized by intramuscular injection in each hind quadricep. Two distinct antigens were tested: (1) 2007–2008 Fluzone inactivated split-virus vaccine, incorporating the representative influenza strains A/Solomon Islands/2/2006 (H1N1), A/Wisconsin/67/2005 (H3N2) and B/Malaysia/2506/2004; and (2) recombinant Plasmodium berghei circumsporozoite protein (PbCSP) produced in-house using the codon-harmonized construct developed by Walter Reed Army Institute of Research. Influenza and malaria vaccine formulations, immunization regimens, and serum collection were as described earlier (8), except that the influenza antigen dose was 0.02 μg total HA. All procedures were performed under specific pathogen-free conditions and in accordance with the regulations and guidelines of the Infectious Disease Research Institute animal care and use committee.
Antibody Responses
Mice were immunized twice, 3 weeks apart. Sera were analyzed for antigen-specific IgG, IgG1, IgG2a antibodies, and hemagglutination inhibition (HI) antibody activity, as described previously, with arbitrary anti-PbCSP units assigned by comparison with a standard curve whereas endpoint titers were determined for influenza antibodies (8). A bone marrow ELISPOT assay was used to determine the induction of vaccine-specific long-lived antibody-secreting plasma cells in samples collected 4 weeks following the Fluzone boost immunization with and without adjuvant as previously described with minor modifications (13).
Antigen-Specific Cytokine Responses
MultiScreen 96-well filtration plates (Millipore, Bedford, MA, USA) were coated with rat anti-mouse IL-5 capture Ab (eBioscience, San Diego, CA, USA) and incubated overnight at 4°C. Plates were washed with PBS, blocked with RPMI 1,640 and 10 % FBS for at least 1 h at RT, and then washed again. Spleens were harvested 4 weeks after the second Fluzone injection. Single cell suspensions were prepared and seeded at 2 × 105 cells per well in duplicate with either media alone, concanavilin A (0.75 μg/ml), 5 hemagglutinating units (HAU) inactivated A/Solomon Islands/2/2006(H1N1) or 2 HAU inactivated A/Wisconsin/67/2005(H3N2) for 48 h at 37°C. The plates were then washed with 0.1 % PBS-Tween 20 and incubated overnight with a biotin-conjugated rat anti-mouse IL-5 secondary Ab (eBioscience). The filters were developed using the VectaStain ABC avidin peroxidase conjugate and Vectastain AEC substrate kits according to the manufacturer’s protocol (Vector Laboratories). The reaction was stopped by washing the plates with deionized water, plates dried in the dark, and spots counted using an automated ELISPOT reader (C.T.L. Serie3A Analyzer, Cellular Technology Ltd.). Data were analyzed using ImmunoSpot® software (CTL Analyzer LLC).
Statistical Analysis
All mouse experiments analyzed five individual animals per group per timepoint. ELISPOT counts and log10-transformed antibody titers were compared using ANOVA with Tukey’s multiple comparison test. HI titers were compared by ANOVA with Tukey’s multiple comparison test using the log2-transformed HI titers.
RESULTS
Physical Stability of Emulsions
Table I describes the composition of the emulsifiers employed in this study. Egg PC is a heterogeneous phosphatidylcholine mixture with various acyl chain lengths and degrees of saturation, although a major component has been identified as POPC (6,15). In contrast, the synthetic phospholipids shown in Table I are highly pure (≥99 %) and have well-defined main phase transition temperatures (Tm) as lipid assemblies. DOPC and POPC, which both contain monounsaturated acyl chains, have low phase transition temperatures (<0°C) due to the packing disorder imposed by the unsaturated chains. Egg PC, which contains monounsaturated and polyunsaturated acyl chains, also has a low Tm. DLPC consists of saturated acyl chains but they are only 12 carbons in length, which results in a low Tm. DMPC, DPPC, and DSPC have longer saturated acyl chains (14, 16, and 18 carbons, respectively) and their phase transition temperatures increase according to chain length. For example, DPPC configuration at room temperature is the highly ordered gel phase, whereas above 41°C, the lipid forms a liquid crystalline phase which is characterized by more packing disorder due to temperature-induced changes in acyl chain conformation (16).
Table I.
Composition of Emulsifiers
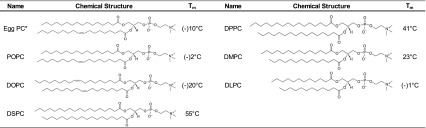
Structures and transition temperatures are taken from the manufacturer, except in the case of egg PC where the phase transition temperature was obtained from (14)
Egg PC structure is representative of only one species; multiple structures comprise egg PC
Table II displays the physical and hemocompatibility properties of the emulsions shortly after their manufacture. The viscosity values are close to that of water (∼1 cP), indicative of the low oil content in these emulsions (10 % v/v when viscosity is measured, but diluted to 2 % v/v for immunization). Zeta potential values are negative for emulsions employing phospholipids with low Tm values (e.g., DOPC, DLPC), whereas emulsions with higher Tm phospholipids are positive (DSPC, DPPC). None of the emulsions displays notable hemolytic activity when incubated with a suspension of RBCs, although the DLPC emulsion appears slightly more hemolytic than the others.
Table II.
Emulsion Physical and Hemocompatibility Characterization
| Phospholipid | Dynamic viscosity (cP) | Zeta potential (mV) | Hemolysis (%) |
|---|---|---|---|
| DOPC | 1.64 | (−)16.1 ± 0.7 | 0.2 ± 0.0 |
| DSPC | 1.72 | (+)5.0 ± 2.1 | 0.2 ± 0.0 |
| DPPC | 1.48 | (+)0.3 ± 0.2 | 0.2 ± 0.0 |
| DMPC | 1.49 | (−)3.2 ± 0.4 | 0.2 ± 0.0 |
| DLPC | 1.44 | (−)13.8 ± 0.7 | 1.0 ± 0.2 |
Compared to (9) for corresponding data on egg PC and POPC emulsions. Inherent instrumental error of the viscometer is ± 0.12 cP. The positive control for 100 % hemolysis was deionized water
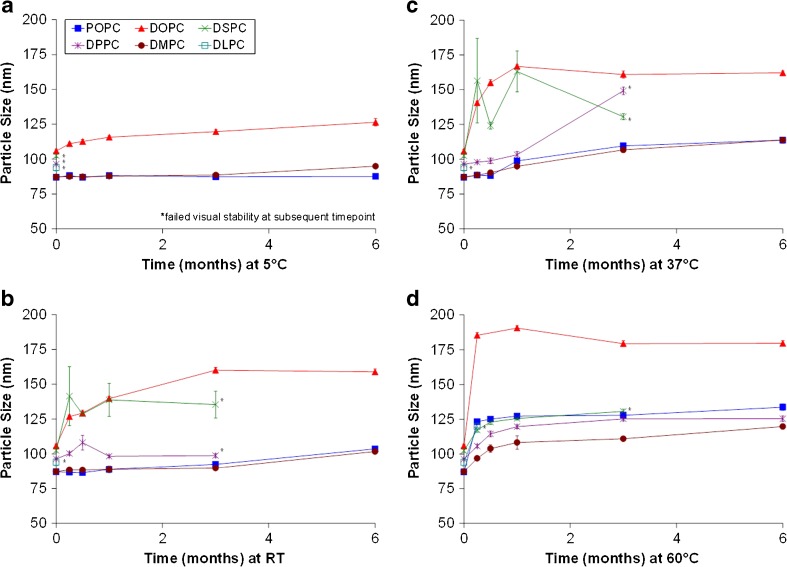
We recently reported that particle size stability of a synthetic POPC–squalene emulsion stored at 5°C or room temperature was equivalent or improved compared to an egg PC–squalene emulsion (9). We sought to build on this work by manufacturing squalene emulsions with various other synthetic phospholipids besides POPC; Fig. 1 shows the particle size stability of the emulsions stored at different temperatures. The initial size of the POPC emulsion was reported earlier (9); here, we have monitored the long-term stability of this same lot for comparison to the other synthetic PC emulsions. Obvious anomalies in physical appearance such as phase separation qualified emulsions as visually unstable. Taking into account data from all temperatures, the DMPC and POPC emulsions demonstrated greater particle size stability than the other emulsions studied. Minimal particle size change was evident at 5°C, and gradual particle size change was apparent with increasing temperature (Fig. 1). Comparatively, the DOPC emulsion droplet size change was minimal at 5°C, but noticeable at the higher storage temperatures. DPPC emulsion stability was highly dependent on temperature. When stored above the DPPC Tm (41°C), the DPPC emulsion showed good stability compared to the other emulsions. However, at the three storage temperatures below the Tm, the DPPC emulsion was either visually unstable or showed more particle size change than more stable emulsions. Interestingly, the DLPC emulsion, containing the shortest saturated chain emulsifier, was visually unstable at early timepoints at all four storage temperatures. The DSPC emulsion also became visually unstable before the 6-month timepoint at all storage temperatures. Size polydispersity values were similar among the more stable emulsions (Electronic Supplementary Material (ESM) Fig. 1).
Fig. 1.
Emulsion particle size stability at various storage temperatures. Shown are results following storage at a 5°C, b RT, c 37°C, and d 60°C. Particle size was measured using Malvern Instruments APS, with error bars representing standard size deviation of three separate aliquots from one batch of each emulsion
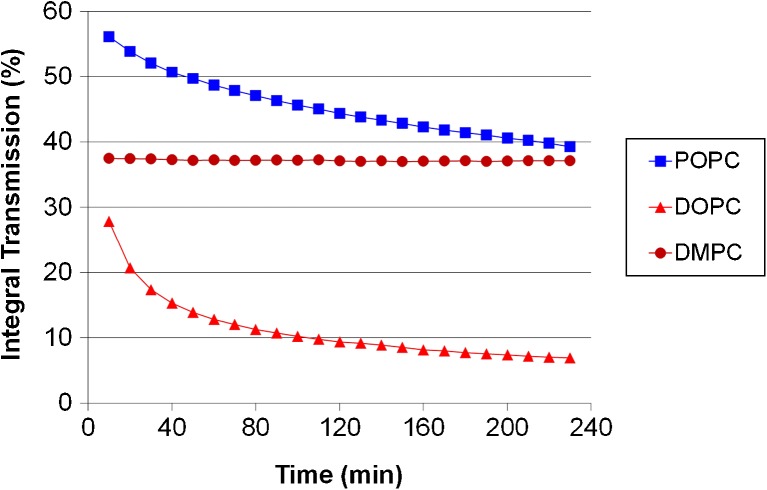
Laser scattering optical profiling provides complementary information regarding emulsion stability. Changes in the emulsion due to creaming, coalescence, etc. are detected as changes in light transmission through the vertical profile of the emulsion (8). The laser scattering optical profiles of the emulsions were measured every 10 min over a period of 4 h at 60°C (ESM Fig. 2). In order to compare data from different emulsions, the integral transmission between a specific region (25–30 mm) in the vertical profile of the sample are plotted in Fig. 2. A decrease in integral transmission is indicative of coalescence or particle size growth since larger particles will scatter more light, allowing less to be transmitted to the detector. An increase in integral transmission is generally representative of creaming or phase separation. The DMPC emulsion shows little change compared to the POPC or DOPC emulsions, indicating superior stability at this temperature. These optical profile data confirm the particle size and visual stability observations described above. DLPC, DPPC, and DSPC emulsions are not shown since these emulsions were already classified as visually unstable at 5°C before the optical profiling measurements had been conducted.
Fig. 2.
Laser scattering optical profiling analysis of emulsion stability. Integral transmission profiles of the 25–30 mm region of cuvettes containing emulsion, measured over 4 h at 60°C
Taken together, the physical stability data indicate that among the synthetic lipids DMPC, POPC, and to a lesser extent DOPC are the most effective emulsifiers of squalene oil. Particle size and sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis following mixing the emulsions with an inactivated influenza vaccine indicated good compatibility (data not shown). Therefore, the above stable synthetic PC emulsions, along with an egg PC emulsion, were subsequently evaluated for biological activity in a mouse model.
Emulsions Selectively Enhance Antibody Responses to a Recombinant Malaria Protein
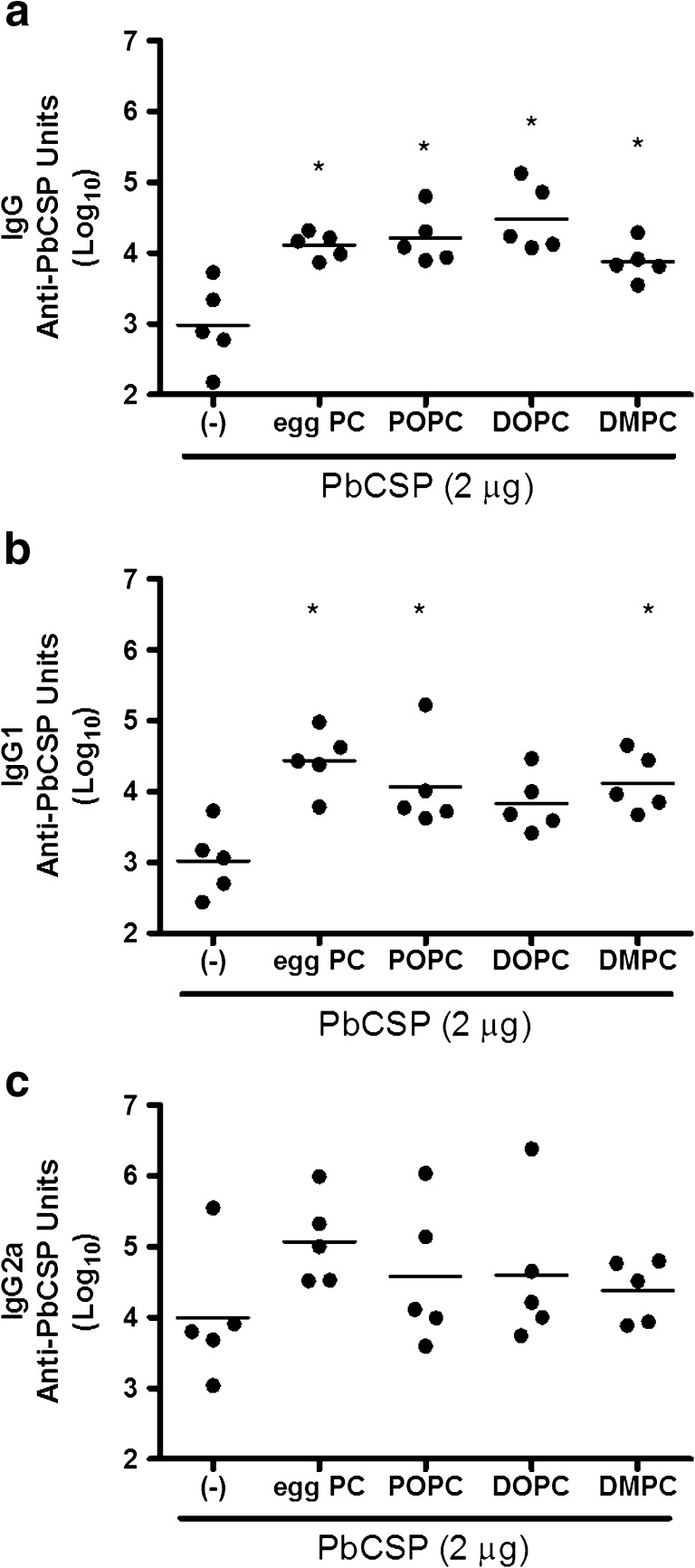
Antibody responses against a recombinant malaria antigen, PbCSP, were measured in individual mouse sera 2 weeks after a second immunization with protein alone or with protein combined with emulsion formulations. The anti-PbCSP IgG antibody levels were significantly higher in mice immunized with PbCSP injected in the presence of emulsions than mice receiving protein alone (Fig. 3a, p values < 0.05). IgG1 antibodies were also higher in mice immunized with PbCSP with egg PC, POPC, and DMPC emulsions compared to mice immunized with protein alone (Fig. 3b, p values < 0.05). In contrast, differences between the various groups were not detected in the anti-PbCSP IgG2a antibody levels (Fig. 3c, p values > 0.05). These data indicate that antigen-specific IgG1 antibodies are selectively enhanced by including emulsions during exposure to antigen.
Fig. 3.
Effect of emulsions on antibody responses induced by immunization with recombinant malaria antigen. BALB/c mice were immunized twice with PbCSP antigen formulated with emulsions containing egg PC or synthetic PC, including POPC, DOPC, and DMPC. Antigen-specific IgG a, IgG1 b, and IgG2a c were determined by ELISA. n = 5 per group, and data are shown as values from individual mice (Log10) with the bar representing the mean. *p value < 0.05 versus immunization with protein alone
Emulsions Enhance Antibody Responses to Influenza Proteins
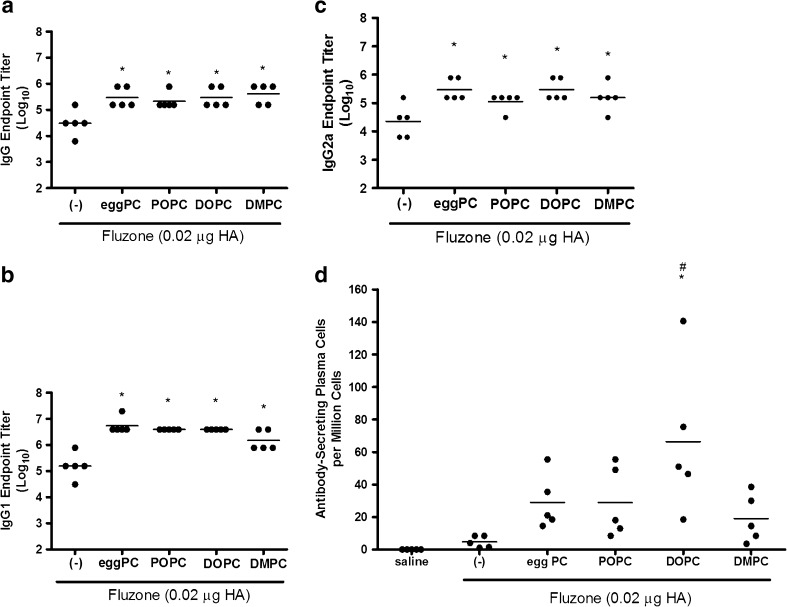
We have previously observed elevated antibody responses in animals injected with Fluzone formulated with emulsions incorporating egg-derived PC (8,13), but have not investigated if these effects are observed when synthetic lipids are incorporated. To determine if emulsions elevated the antibody response to native influenza proteins, we immunized mice with a low dose of Fluzone vaccine in the presence or absence of emulsion formulation incorporating natural or synthetic phospholipids. Compared to vaccination with the Fluzone vaccine alone, total IgG, IgG1, and IgG2a antibody titers were higher for all groups that received Fluzone with emulsion formulations (Fig. 4).
Fig. 4.
Effect of adjuvant formulation in humoral immune responses induced by immunization with inactivated split-virus influenza vaccine (Fluzone). BALB/c mice were immunized with Fluzone formulated with emulsions containing egg PC or synthetic PC, including POPC, DOPC, and DMPC. Antigen-specific IgG a, IgG1 b, and IgG2a c were determined by ELISA. IgG-secreting bone marrow plasma cells against Fluzone were determined by ELISPOT d. Results for a–c are shown as the endpoint titer (Log10), with each dot representing individual mice and the bar representing the mean. Results for d also represent individual mice in each group, with the bar representing the mean ASPC/group. *p value < 0.05 versus immunization with vaccine alone and # p value < 0.05 versus immunization with the vaccine containing the DMPC emulsion
We also assessed the effect of emulsion formulations on the generation of antigen-specific antibody secreting plasma cells (ASPC) within the bone marrow, as these long-lived cells secrete antibody for extended periods of time after antigenic exposure and can provide a basis for long-term protection. Overall, our results indicate that similar numbers of ASPC were generated among the various emulsion formulation groups. Only the vaccine containing DOPC emulsion, however, induced a significantly higher number of long-lived plasma cells than Fluzone vaccine alone (Fig. 4d).
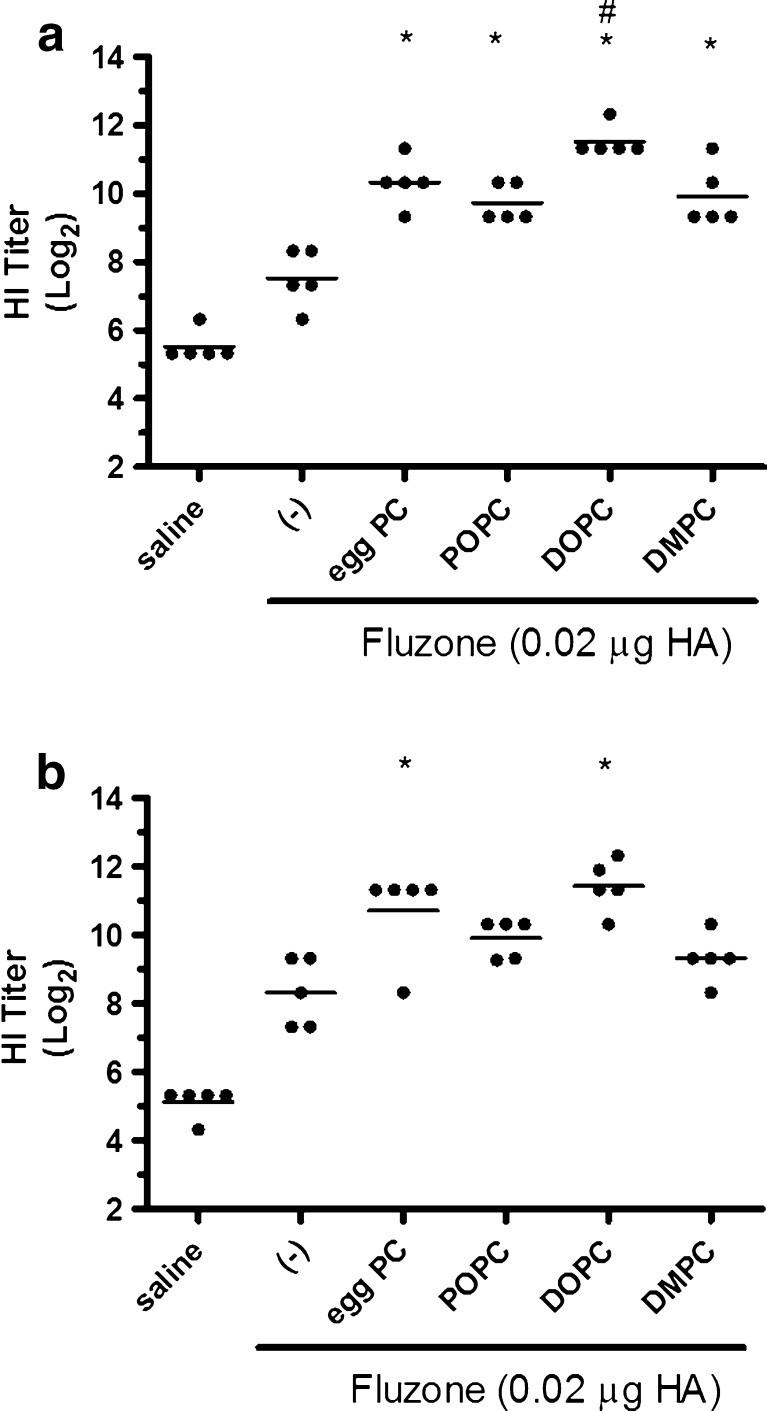
The HI assay is a meaningful predictive indicator of influenza vaccine efficacy, with a titer of ≥40 generally considered enough to provide protection (17). To determine if emulsion formulations could generate higher quality antibodies capable of enhancing protection afforded by the Fluzone vaccine, we compared HI titers of mice vaccinated with Fluzone alone or Fluzone with various emulsion formulations. When measured 4 weeks after the final immunization, the addition of emulsion formulation induces higher HI titers than the Fluzone vaccine alone against both the A/Solomon Islands/3/2006 (H1N1) and the A/Wisconsin/67/2005 (H3N2) vaccine components (Fig. 5). Against the A/Solomon Islands/3/2006 (H1N1) component, all vaccines containing emulsion induced higher HI titers than Fluzone alone. Against the A/Wisconsin/67/2005 (H3N2) component, egg PC and DOPC emulsions induced higher HI titers than Fluzone alone.
Fig. 5.
HI titers were enhanced by immunization with Fluzone formulated with egg PC or synthetic PCs. BALB/c mice were immunized with Fluzone and various emulsions, then serum HI titers determined 4 weeks after boosting. a HI titers against the A/Solomon Islands/3/2006 (H1N1) component of Fluzone. b HI titers against the A/Wisconsin/67/2005 (H3N2) component of Fluzone. Data are shown as the (Log2) titer for each individual animal, with the bar representing the mean. *p value < 0.05 versus immunization with vaccine alone and # p value < 0.05 versus immunization with the vaccine containing POPC emulsion or DMPC emulsion
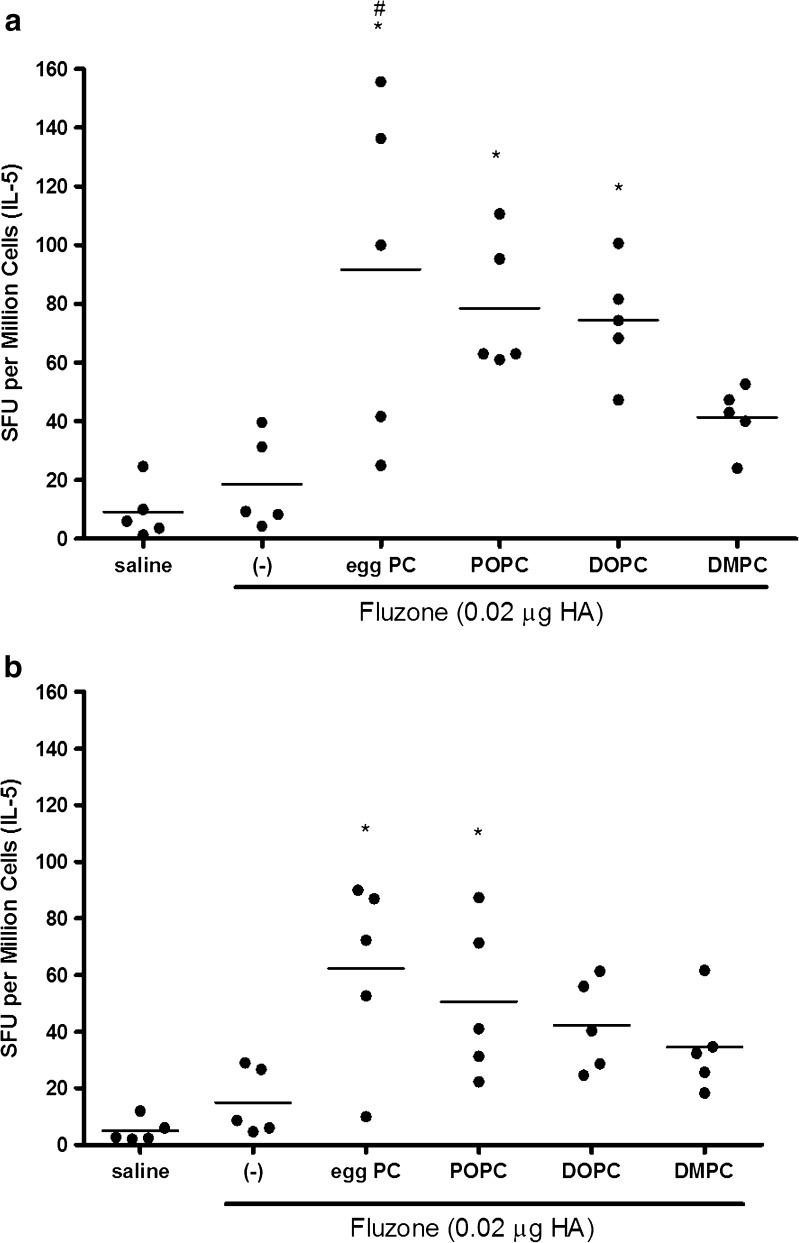
Additional experiments indicated that, with the exception of the DMPC emulsion, the number of IL-5-producing antigen-specific splenocytes from mice immunized with Fluzone plus emulsions was enhanced compared to cells from mice immunized with Fluzone alone (Fig. 6, p values < 0.05). Together, the antibody and cytokine secretion data indicate an enhanced Th2-type immune response is elicited by the vaccines containing these emulsions.
Fig. 6.
IL-5 producing cells are increased after immunization with Fluzone formulated with emulsions. Splenocytes from BALB/c mice immunized with Fluzone and various emulsions were stimulated with either a 5 HAU of inactivated A/Solomon Islands/3/2006(H1N1) or b 2 HAU-inactivated A/Wisconsin/67/2005(H3N2) and IL-5 producing cells determined by ELISPOT. Data is represented as the number of spot forming units (SFU) per million splenocytes for individual mice/group, with the bar representing the mean, n = 5 per group. *p value < 0.05 versus immunization with vaccine alone and # p value < 0.05 versus immunization with the vaccine containing the DMPC emulsion
DISCUSSION
Antibody responses elicited with both the malaria and influenza vaccines were enhanced in an equivalent manner regardless of synthetic or egg-derived PC as emulsifier components. This finding is meaningful for continued development of PC-emulsified emulsions based on synthetic instead of natural components. The literature has shown why the biological equivalence of this type of component substitution should not be taken for granted; there are several instances when substitution of one phospholipid emulsifier with another has resulted in differences in biological activity. For instance, Yasuda et al. found that liposomes composed of synthetic phosphatidylcholines showed a direct correlation between immunogenicity (as indicated by the number of antibody-secreting cells in the spleen) and lipid phase transition temperature when used to immunize mice intraperitoneally with a synthetic lipid-based antigen; higher phase transition was found to directly correlate with increased immunogenicity (18). This correlation between phase transition temperature and immunogenicity was not apparent in the current work, although the phospholipids with the highest phase transition temperatures (DPPC and DSPC) were not evaluated immunologically due to poor emulsion stability. In another example of the importance of emulsifier source, recent clinical trial results with a water-in-oil vaccine adjuvant emulsion, Montanide® ISA 51, revealed that the replacement of animal-source emulsifier with plant-source material may have been the cause for significantly reduced in vivo efficacy (11). The immunological data in the present work demonstrate that influenza and malaria vaccines adjuvanted with emulsions made with different natural or synthetic phosphatidylcholines elicit similar antibody responses. Moreover, the qualitative immunogenicity of the emulsions employed in this work are in line with literature reports; for instance, emulsions (without TLR agonists) appear to induce only modest increases in IgG2a responses compared to antigen alone (19). Subtle differences were evident between emulsions in experiments with the influenza vaccine, illustrating that the lipid emulsifiers may differ slightly in biological effects. However, in general, it appears that egg PC can be replaced by synthetic phospholipids without a detrimental effect on biological activity in the context of a simple recombinant antigen malaria vaccine or a more complex inactivated split-virus influenza vaccine.
Besides biological activity, it is important to consider physical emulsion stability as it relates to emulsifier acyl chain structure. It has occasionally been reported that purified PC molecules do not effectively stabilize emulsions (20). For example, a DOPC/DPPC/1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) synthetic mix did not produce emulsions as stable as that observed with egg lecithin when used to emulsify perfluorocarbons (21). However, the claim that pure synthetic PCs do not emulsify effectively is easily refuted by other reports (22), which make clear that stability is dependent on the structure and properties of both oil and emulsifier (23). The spontaneous curvature or packing structure of the emulsifiers determines their effectiveness in stabilizing emulsions (22). Emulsifier spontaneous curvature or packing is affected by (a) the phospholipid phase behavior, (b) the degree of unsaturation of the lipid acyl chains, and (c) the phospholipid miscibility in the oil (22). In the present work, the importance of lipid phase transition is apparent since emulsions containing the phospholipids with the highest phase transition temperatures (DPPC and DSPC) were not stable at most storage temperatures based on size and visual appearance. Interestingly, DPPC produced a stable emulsion only when stored above its main phase transition temperature. Nevertheless, phase transition temperature was not the only determining factor for physical stability; note that the DMPC emulsion showed good stability (at all storage temperatures, including temperatures above and below the phase transition) compared to the DLPC emulsion even though DMPC has a higher phase transition temperature. Thus, the complexity of the emulsifier–oil interactions is evidenced by the fact that the stability of the emulsions described in the present work could not be predicted solely based on phospholipid phase transition temperature, acyl chain length, or saturation. Likewise, the effect of lipid phase transition temperature and acyl chain structure on emulsion stability was difficult to predict in the context of glyceryl trioctanoate emulsions: DLPC, DMPC, and DPPC emulsions were more stable than DSPC, 1,2-dilinoleoyl-sn-glycero-3-phosphocholine, and DOPC emulsions (24).
Emulsion droplet size growth can occur by two mechanisms: Ostwald ripening (Laplace pressure differences cause oil molecules to diffuse from smaller droplets to larger droplets), or coalescence (separate oil droplets merge and form a single droplet; 25). Since squalene is essentially insoluble in water, it is unlikely that Ostwald ripening is responsible for the size growth in the emulsions of the present work (26–29). Thus, coalescence is the likely mechanism for the size growth reported here. The rate of coalescence is expected to increase with higher temperatures due to increased rates of diffusion and droplet interaction events, and the size data presented here are consistent with this expectation. Emulsifiers that do not provide an effective steric or electrostatic stabilization to the oil droplet via a well-packed interfacial layer may be responsible for increased rates of coalescence. Our findings indicate that, overall, the unsaturated lipid emulsifiers were more effective than the saturated lipids in stabilizing squalene emulsions, with the exception of DMPC which produced a highly stable emulsion even though it contains only saturated acyl chains. This emphasizes again that it is difficult to predict emulsion stability based solely on the phase transition and acyl chain structure of the phospholipid emulsifiers.
Finally, we note that various physical characterization data shown in this manuscript correlate well with previous literature reports. For example, the relationship of more negative zeta potential values for liquid crystalline phase lipids versus gel phase lipids has been reported previously; it is attributed to increased disorder and packing defects in liquid phase membranes which causes exposure of the polar head groups and allows for more available binding sites for solution anions (30,31). Furthermore, the slightly increased hemolytic tendency of the DLPC emulsion corresponds with another report from the literature that DLPC and other short-chain phospholipids induce hemolysis via creation of nonspecific pores in lipid bilayers through lipid phase separation, allowing permeation of ions (32).
CONCLUSION
In the present work, it was shown that vaccine-adjuvant emulsions containing squalene oil and synthetic phospholipid emulsifiers, namely DMPC or POPC, demonstrated long-term particle size stability at various temperatures. In general, egg PC and synthetic PC emulsions induced similar immune responses in combination with a simple recombinant malaria antigen or an inactivated split-virus influenza vaccine. Thus, substitution of egg phosphatidylcholine with synthetic phosphatidylcholine did not result in loss of vaccine adjuvant biological activity. Ongoing work in our laboratory will compare stability and immunogenicity of squalene–phospholipid emulsions with other classes of nonphospholipid surfactant structures, namely polysorbate 80 and poloxamer 188.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
DOC 384 kb
Acknowledgments
The authors wish to thank Susan Lin, Sandra Sivananthan, Tim Dutill, Kristen Forseth, Tony Phan, Farah Mompoint, Tara Evers, Alison Bernard, and Marah Hay for skilled technical assistance and Dr. Martin Friede for helpful discussions. This work was supported in part by National Institutes of Health contract HHSN272200800045C and grant 42387 from the Bill and Melinda Gates Foundation. The authors gratefully acknowledge Dr. Evelina Angov for kindly providing the codon-harmonized PbCSP construct developed by Walter Reed Army Institute of Research.
References
- 1.Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14:3286–3312. doi: 10.3390/molecules14093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy LH, Couvreur P. Squalene: a natural triterpene for use in disease management and therapy. Adv Drug Del Rev. 2009;61:1412–1426. doi: 10.1016/j.addr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Brito LA, Chan M, Baudner B, Gallorini S, Santos G, O’Hagan DT, et al. An alternative renewable source of squalene for use in emulsion adjuvants. Vaccine. 2011;29:6262–6268. doi: 10.1016/j.vaccine.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 4.Yang YW, Wu CA, Morrow WJW. Cell death induced by vaccine adjuvants containing surfactants. Vaccine. 2004;22:1524–1536. doi: 10.1016/j.vaccine.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 5.Forchielli ML, Bersani G, Tala S, Grossi G, Puggioli C, Masi M. The spectrum of plant and animal sterols in different oil-derived intravenous emulsions. Lipids. 2010;45:63–71. doi: 10.1007/s11745-009-3371-x. [DOI] [PubMed] [Google Scholar]
- 6.Vernooij EAAM, Kettens-van den Bosch JJ, Crommelin DJA. Rapid determination of acyl chain position in egg phosphatidylcholine by high-performance liquid chromatography/electrospray mass spectrometry. Rapid Comm Mass Spec. 1998;12:83–86. doi: 10.1002/(SICI)1097-0231(19980131)12:2<83::AID-RCM120>3.0.CO;2-M. [DOI] [Google Scholar]
- 7.de Man JM. Chemical and physical properties of fatty acids. In: Chow CK, editor. Fatty acids in foods and their health implications. Boca Raton: CRC Press; 2008. pp. 17–46. [Google Scholar]
- 8.Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine. 2011;29:9563–9572. doi: 10.1016/j.vaccine.2011.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox CB, Lin S, Sivananthan SJ, Dutill TS, Forseth KT, Reed SG, et al. Effects of emulsifier concentration, composition, and order of addition in squalene-phosphatidylcholine oil-in-water emulsions. Pharm Dev Technol. 2011;16:511–519. doi: 10.3109/10837450.2010.495397. [DOI] [PubMed] [Google Scholar]
- 10.Hilleman MR. Personal historical chronicle of six decades of basic and applied research in virology, immunology, and vaccinology. Immunol Rev. 1999;170:7–27. doi: 10.1111/j.1600-065X.1999.tb01325.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Kammula US, Hughes MS, Restifo NP, Schwarz SL, et al. Different adjuvanticity of incomplete Freund’s adjuvant derived from beef or vegetable components in melanoma patients immunized with a peptide vaccine. J Immunother. 2010;33:626–629. doi: 10.1097/CJI.0b013e3181dac9de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bock TK, Muller BW. A novel assay to determine the hemolytic activity of drugs incorporated in colloidal carrier systems. Pharm Res. 1994;11:589–591. doi: 10.1023/A:1018987120738. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–5963. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 14.Amselem S, Zawoznik E, Yogev A, Friedman D. Emulsomes, a new type of lipid assembly. In: Barenholz Y, Lasic DD, editors. Handbook of Nonmedical Applications of Liposomes: From design to microreactors. New York: CRC Press. 1995;209–23.
- 15.Vernooij EAAM, Brouwers JFHM, Kettenes-van den Bosch JJ, Crommelin DJA. RP-HPLC/ESI MS determination of acyl chain positions in phospholipids. J Sep Sci. 2002;25:285–289. doi: 10.1002/1615-9314(20020401)25:5/6<285::AID-JSSC285>3.0.CO;2-U. [DOI] [Google Scholar]
- 16.Fox CB, Uibel RH, Harris JM. Detecting phase transitions in phosphatidylcholine vesicles by Raman microscopy and self-modeling curve resolution. J Phys Chem B. 2007;111(39):11428–11436. doi: 10.1021/jp0735886. [DOI] [PubMed] [Google Scholar]
- 17.Falsey AR. Half-dose influenza vaccine. Arch Intern Med. 2008;168:2402–2403. doi: 10.1001/archinte.168.22.2402. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda T, Dancey GF, Kinsky SC. Immunogenicity of liposomal model membranes in mice: dependence on phospholipid composition. Proc Natl Acad Sci. 1977;74:1234–1236. doi: 10.1073/pnas.74.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baudner BC, Ronconi V, Casini D, Tortoli M, Kazzaz J, Singh M, et al. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020) Pharm Res. 2009;26:1477–1485. doi: 10.1007/s11095-009-9859-5. [DOI] [PubMed] [Google Scholar]
- 20.Mikrut B. Case study: formulation of an intravenous fat emulsion. In: Burgess DJ, editor. Injectable dispersed systems: formulation, processing, and performance. Boca Raton: Taylor and Francis; 2005. pp. 415–425. [Google Scholar]
- 21.Yoon JK, Burgess DJ. Interfacial properties as stability predictors of lecithin-stabilized perfluorocarbon emulsions. Pharm Dev Tech. 1996;1:333–341. doi: 10.3109/10837459609031428. [DOI] [PubMed] [Google Scholar]
- 22.Kabalnov A, Tarara T, Arlauskas R, Weers J. Phospholipids as emulsion stabilizers: phase behavior versus emulsion stability. J Coll Inter Sci. 1996;184:227–235. doi: 10.1006/jcis.1996.0615. [DOI] [PubMed] [Google Scholar]
- 23.Mollet H, Grubenmann A. Formulation technology: emulsions, suspensions, solid forms. New York: Wiley; 2001. [Google Scholar]
- 24.Nii T, Ishii F. Properties of various phosphatidylcholines as emulsifiers or dispersing agents in microparticle preparations for drug carriers. Colloids Surf B: Biointerfaces. 2004;39(1–2):57–63. doi: 10.1016/j.colsurfb.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG, Vedvick T. Monitoring the effects of component structure and source and formulation stability and adjuvant activity of oil-in-water emulsions. Colloids Surf B: Biointerfaces. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Bibette J, Morse DC, Witten TA, Weitz DA. Stability criteria for emulsions. Phys Rev Lett. 1992;69(16):2439. doi: 10.1103/PhysRevLett.69.2439. [DOI] [PubMed] [Google Scholar]
- 27.Capek I. Degradation of kinetically-stable o/w emulsions. Adv Coll Inter Sci. 2004;107(2–3):125–155. doi: 10.1016/S0001-8686(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 28.Dalgleish DG. Adsorption of protein and the stability of emulsions. Trends Food Sci Technol. 1997;8(1):1–6. doi: 10.1016/S0924-2244(97)01001-7. [DOI] [Google Scholar]
- 29.McClements DJ. Critical review of techniques and methodologies for characterization of emulsion stability. Crit Rev Food Sci Nutr. 2007;47:611–649. doi: 10.1080/10408390701289292. [DOI] [PubMed] [Google Scholar]
- 30.Tatulian SA. Binding of alkaline-earth metal cations and some anions to phosphatidylcholine liposomes. Eur J Biochem. 1987;170:413–420. doi: 10.1111/j.1432-1033.1987.tb13715.x. [DOI] [PubMed] [Google Scholar]
- 31.Tatulian SA. Effect of lipid phase transition on the binding of anions to dimyristoylphosphatidylcholine liposomes. Biochim Biophys Acta. 1983;736:189–195. doi: 10.1016/0005-2736(83)90283-3. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y, Mashino K, Inoue K, Nojima S. Mechanism of human erythrocyte hemolysis induced by short-chain phosphatidylcholines and lysophosphatidylcholine. J Biochem. 1983;94:833–840. doi: 10.1093/oxfordjournals.jbchem.a134425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DOC 384 kb