Abstract
Hydroxypropyl methylcellulose (HPMC) tablets containing nicotine–magnesium aluminum silicate (NCT-MAS) complex particles and pH modifiers, namely, sodium chloride, citric acid, and magnesium hydroxide, were prepared using the direct compression method. The effects of HPMC viscosity grades and pH modifiers on NCT release and permeation of the matrix tablets were examined. The results showed that the higher the viscosity grade of HPMC that was used in the tablets, the lower was the unidirectional NCT release rate found. The unidirectional NCT permeation was not affected by the viscosity grade of HPMC because the NCT diffusion through the mucosal membrane was the rate-limiting step of the permeation. Incorporation of magnesium hydroxide could retard NCT release, whereas the enhancement of unidirectional NCT release was found in the tablets containing citric acid. Citric acid could inhibit NCT permeation due to the formation of protonated NCT in the swollen tablets at an acidic pH. Conversely, the NCT permeation rate increased with the use of magnesium hydroxide as a result of the neutral NCT that formed at a basic microenvironmental pH. The swollen HPMC tablets, with or without pH modifiers, gave sufficient adhesion to the mucosal membrane. Furthermore, the addition of magnesium hydroxide to the matrix tablets was the major factor in controlling buccal delivery of NCT. This study suggests that the NCT-MAS complex-loaded HPMC tablets, which contained magnesium hydroxide, are potential buccal delivery systems of NCT.
Key words: buccal tablet, hydroxypropyl methylcellulose, magnesium aluminum silicate, nicotine, pH modifier
INTRODUCTION
Buccal drug administration provides several advantages when compared with oral drug administration. Buccal delivery allows drugs to avoid first-pass hepatic metabolism, resulting in greater bioavailability and facilitating drug withdrawal (1). Traditionally, the drug delivery system of this route is in the form of tablets and films. The films have been widely employed, but a large proportion of the drug dose could be swallowed before drug absorption occurs across the buccal mucosa because of an initial burst release of the drug from the films (2). To solve this problem, a sustained-release tablet was designed to help reduce this unwanted effect and extend the duration of drug action. Moreover, the mucoadhesive properties of the tablets were also important because adhesion of the drug delivery system to the buccal mucosa was essential during administration (3). For this reason, tablet formulations of bioadhesive polymer were necessary to enhance the mucoadhesive property and sustain drug release. Hydroxypropyl methylcellulose (HPMC) has been widely used for this purpose (4–6).
Nicotine (NCT), obtained from tobacco plants, is a volatile and strongly alkaline liquid. NCT is highly soluble in both water and hydrophobic solvents (7). It has well-separated pKa values; the pKa1 and pKa2 are 3.04 and 7.84, respectively (8), which leads to the formation of diprotonated, monoprotonated, and neutral NCT at acidic, neutral, and basic pH levels, respectively. NCT has been widely used in smoking cessation therapy for relieving withdrawal symptoms. It is a candidate for buccal delivery because of its low bioavailability after oral administration (7) and its ability to permeate buccal mucosa (8–10). Due to the volatility and susceptibility to the oxidative degradation of free-base NCT, many researchers have sought an adsorbent material for NCT to prevent evaporation and improve stability. The adsorbents, such as cellulose powder (11), cation exchange resin (12), and magnesium aluminum silicate (13), were employed to carry NCT in powdered form.
Magnesium aluminum silicate (MAS) is a mixture of montmorillonite and saponite clays (14), both of which have silicate layer structures. Each layer comprises tetrahedrally coordinated silica atoms fused into an edge-shared octahedral plane, with either aluminum hydroxide or magnesium hydroxide (14,15). The silicate layers of MAS have weakly positively charged edges and negatively charged surfaces. The negatively charged surfaces of the silicate layers strongly interact with NCT at different pH levels (16), leading to the formation of NCT-MAS complexes. This interaction allows NCT to intercalate into the silicate layers of MAS (13). The NCT-MAS complex particles could improve the thermal stability of NCT and give a sustained release of NCT after the initial burst release in pH 6 phosphate buffer (13). For these reasons, the NCT-MAS complex-loaded matrix tablets have been developed and evaluated (17). The tablets containing the complexes prepared at basic pH gave remarkably higher NCT permeation rates than those containing the complexes prepared at acidic and neutral pH levels. This result indicated that the pH level during the preparation method of the complexes gave an NCT species that affected the mucosal delivery of NCT. Thus, modulating the microenvironmental pH of the tablets by adding pH modifiers may alter not only NCT release from the matrix tablets but also NCT permeation through the mucosal membrane. The use of an additive in the NCT buccal tablets to increase pH, such as magnesium hydroxide, has been developed (18). Unfortunately, the effect the amount of pH-increasing agent has on NCT release and permeation characteristics of the matrix tablets is not available in the literature.
Therefore, the aim of this study was to investigate the effect of pH modifiers, namely, sodium chloride (neutral compound), citric acid (acidic compound), and magnesium hydroxide (basic compound), on NCT release and permeation of the matrix tablets containing NCT-MAS complexes prepared at basic pH. HPMC was used for this investigation because it is a nonionic bioadhesive polymer that cannot electrostatically interact with positively or negatively charged compounds in the tablets. Moreover, the effect of the viscosity grade of HPMC on the characteristics of the NCT-MAS complex-loaded matrix tablets was also examined. Additionally, unidirectional release and permeation of NCT using a cellulose acetate membrane and a porcine esophageal mucosa as model membranes, respectively, were investigated for simulating a condition of tablet administration on the mucosa surface. Based on the results of NCT release and permeation, the rate control of the NCT delivery was investigated for a better understanding of when to incorporate pH modifiers into HPMC matrix tablets.
MATERIALS AND METHODS
Materials
MAS (Veegum®HV) and NCT were obtained from R.T. Vanderbilt Company, Inc. (Norwalk, CT, USA) and Fluka (Buchs, Switzerland), respectively. Hydroxypropyl methylcellulose, of viscosity grades of 10–20 cP (low viscosity, LV-HPMC) and 40–60 cP (medium viscosity, MV-HPMC), was purchased from Onimax Co., Ltd. (Bangkok, Thailand). High-viscosity grade HPMC (HV-HPMC), 80–120 cP, was obtained from S.M. Chemical Supplies Co., Ltd. (Bangkok, Thailand). Sodium chloride, citric acid monohydrate, and magnesium hydroxide were purchased from Merck Ltd. (Bangkok, Thailand). Magnesium stearate (Mallinckrodt Inc., St Louis, MO) was used as a lubricant for tableting. All other reagents that were used were of analytical grade and were used as received.
Preparation of NCT-MAS Complexes
A 4% (w/v) MAS suspension was prepared using hot water and was cooled to room temperature before use. An NCT solution (2%, w/v) was prepared using deionized water as the solvent. Fifty milliliters of the 4% (w/v) MAS suspension was then mixed with 50 ml of the 2% (w/v) NCT solution in an Erlenmeyer flask. The pH of the NCT-MAS dispersion was adjusted by adding small amounts of 1 M HCl into the flask while swirling until the final pH of the dispersions was at 9, as measured with a pH meter (WalkLAB TI9000, Singapore). To achieve NCT adsorption equilibrium on MAS, the dispersions were then incubated with shaking at 37°C for 24 h (16). Following incubation, the NCT-MAS complexes were collected by filtration, washed twice using 20 ml of deionized water, and dried at 50°C for 24 h. The dry NCT-MAS complexes were ground using a mortar and pestle, sieved through a 180-μm sieve, and stored in a desiccator.
Characterization of the NCT-MAS Complexes
Determination of NCT Content
Twenty-five milligrams of the NCT-MAS complexes were weighed and dispersed in 100 ml of 2 M HCl for 12 h. The supernatant was then collected and filtered and the NCT content analyzed using a UV–Visible spectrophotometer (Shimadzu UV1201, Kyoto, Japan) at a wavelength of 259 nm.
Particle Size Determination
The particle sizes of the NCT-MAS complexes were measured using a laser diffraction particle size analyzer (Mastersizer2000 model Hydro2000SM, Malvern Instrument Ltd., UK). The samples were dispersed in 70 ml of pH 6 phosphate buffer in a small volume sample dispersion unit and stirred at a rate of 50 Hz for 30 s before the measurement. The particle sizes (volume-weighted mean diameter) were reported.
Characterization of HPMC
Particle Size Determination
The particle sizes of HPMC powder with different viscosity grades were determined using a laser diffraction particle size analyzer (Mastersizer2000 model Scirocco2000SM, Malvern Instrument Ltd.). The particle sizes (volume-weighted mean diameter) were presented.
Measurement of Viscosity
HPMC dispersions at a concentration of 2% (w/v) in distilled water were prepared. The viscosity of the HPMC dispersion was investigated using a small sample adapter for the Brookfield digital rheometer (model DV-III, Brookfield Engineering Labs. Inc., USA). The sample temperature was controlled at 32 ± 1°C. A rheogram of the samples was obtained by plotting between shear stress (y-axis) and shear rate (x-axis) at various revolution rates when a spindle (no. 34) was used. The slope of the rheogram was calculated as the viscosity of the dispersion.
Preparation of NCT-MAS Complex-Loaded HPMC Tablets
All tablets were produced using the direct compression method. Each tablet consisted of 120 mg NCT-MAS complexes (equivalent to 15.8 mg of NCT) and 80 mg HPMC. In the case of pH modifier addition, the constant quantity of HPMC was performed because it was a matrix-forming agent in the tablets. Sodium chloride, citric acid, or magnesium hydroxide was added in the content of 5, 10, or 20% (w/w) of the tablet weight (200 mg), the tablet weights being 210, 220, or 240 mg, respectively. Magnesium stearate (1%, w/w, of the tablet weight) was used as a lubricant. The NCT-MAS complexes were mixed with HPMC and a pH modifier in a rotomixer for 3 min; magnesium stearate was then blended with the mixture for 1 min before tableting. The mixtures were filled into 10-mm flat-faced punches and dies, and then a 23-MPa compression pressure was applied with a hydrostatic press (model 3126, Shimadzu) without holding time. The resulting tablets were stored in a desiccator prior to use.
Characterization of NCT-MAS Complex-Loaded HPMC Tablets
Thickness and Hardness
The thicknesses of the tablets were measured using a Vernier caliper (Mitutoyo, Japan). The hardness of the tablets was measured with a Stokes tablet hardness tester.
In Vitro Release Studies
NCT release of the NCT-MAS complex-loaded HPMC tablets was studied using two apparatuses. NCT released from the whole tablets was tested using a USP dissolution apparatus 1 (basket method, VanKel 7000, USA). The tablets were placed into the basket with a rotation speed of 50 rpm. The release medium was 500 ml of pH 6 phosphate buffer, and the temperature was controlled at 37.0 ± 0.5°C. Seven-milliliter samples were collected and replaced with fresh medium at various time intervals. The amount of NCT released was analyzed using a UV–Visible spectrophotometer (Shimadzu UV1201) at a wavelength of 259 nm.
Unidirectional NCT release of the tablets was characterized using a modified USP dissolution apparatus 2 that was reported previously (17). Briefly, the distance between the paddle and vessel bottom was set to 1 cm, and the dissolution medium used was 300 ml of pH 6 phosphate buffer at 37.0 ± 0.5°C. A cellulose acetate membrane (pore size = 0.45 μm), which had been hydrated in pH 6 phosphate buffer overnight, was tightly attached at the lowest end of a polypropylene tube (inner diameter = 1.8 cm) using a nylon cable tie. This tube was vertically placed in a dissolution vessel, and the distance between the tube and the vessel wall was approximately 1.8 cm. The end of the tube was adjusted so that the membrane was wetted and in contact with the medium. The tablets were placed into the tube and wetted using 2 ml of pH 6 phosphate buffer. The rotation speed of the paddle was set to 50 rpm. Samples (7 ml) were collected and replaced with fresh medium at various time intervals. The amount of NCT released was analyzed using HPLC.
In Vitro Permeation Studies
Unidirectional NCT permeation of the tablets was also performed using a modified USP dissolution apparatus 2. Porcine esophageal mucosa was employed in this study because it had a lipid composition similar to porcine buccal mucosa, but a simpler preparation method (19). Esophageal mucosa of a crossbred pig (hybrid types of Duroc Jersey–Landrace–Large White) weighing 80–100 kg was obtained from a local slaughterhouse (Non Muang Village, Khon Kaen, Thailand). The porcine esophageal tube was opened longitudinally and immersed in 0.9% sodium chloride at 60°C for 1 min (19,20). The epithelium was then peeled away from the connective tissue and stored at −20°C. The frozen mucosal membranes were brought to room temperature by immersion in pH 7.4 isotonic phosphate buffer for 15 min. The mucosal membrane was then mounted and tightly attached to the end of a polypropylene tube. The dissolution vessel contained 300 ml of pH 7.4 isotonic phosphate buffer at 37.0 ± 0.5°C; the methods and experimental conditions were the same as the previous release testing.
Analysis of NCT Release and Permeation
The release mechanisms of NCT released from the whole tablet and the unidirectional tablet were analyzed using a power law (21,22), as shown in Eqs. 1 and 2, respectively, as follows:
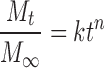 |
1 |
and
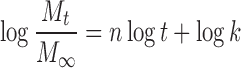 |
2 |
where Mt/M∞ is the fractional NCT release at time t, k is the kinetic constant, and n is the release exponent indicative of the drug release mechanism. A release exponent of n = 0.5 indicates a diffusion-controlled drug release (Fickian diffusion), whereas a release exponent of n = 1 corresponds to a polymer swelling/erosion-controlled release mechanism. Thus, the release exponents between these two extreme values indicate a so-called anomalous transport, which is a complex transport mechanism that is a mixture of both drug diffusion and swelling/erosion of polymer.
The NCT release and permeation rates of the tablets were analyzed using both zero-order and Higuchi models (23), which can be expressed as Eqs. 3 and 4, respectively, as follows:
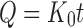 |
3 |
and
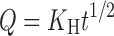 |
4 |
where Q is the amount of NCT released, t is time, and K0 and KH are the zero-order and Higuchi release rates, respectively.
Measurement of Mucoadhesive Properties
The mucoadhesive properties of the tablets were measured using a texture analyzer (TA.XT plus, Stable Micro Systems, UK) with a 50-N load cell equipped with a bioadhesive test rig.
The tablet was attached to a 10-mm diameter cylindrical probe using a two-sided adhesive tape. Esophageal mucosa of pig was also obtained from a local slaughterhouse (Non Muang Village, Khon Kaen, Thailand). The mucosal membrane from the porcine esophagus (approximately 2 × 2 cm), without heat treatment and elimination of the connective tissue that had been hydrated with pH 7.4 isotonic phosphate buffer for 20 min, was placed on the stage of the bioadhesive holder and gently blotted with tissue paper to remove excess water on the surface of the mucosal membrane. Next, 200 μl of pH 6 phosphate buffer was pipetted onto the membrane surface before testing. The probe and attached tablet were moved down at a constant speed of 1 mm s−1 with 0.5 N contact force and 2 min contact time. Immediately afterwards, the probe was moved upwards with a constant speed of 0.5 mm s−1. The relationship between the force and tablet displacement was plotted. The maximum detachment force (Fmax) and work of adhesion (Wad, the area under the force versus distance curve) were calculated using the Texture Exponent 32 program, version 4.0.9.0 (Stable Micro Systems).
HPLC for NCT Analysis
The NCT concentration of the samples from release and permeation testing was determined using HPLC (Perkin Elmer Series, USA). Reversed-phase HPLC using a C-18 column (Waters Spherisorb® S5 ODS2, 5-μm particle size, 4.6 × 250 mm, Ireland) connected to a guard column was employed. The mixture of 0.05 M sodium acetate/methanol/triethylamine in the ratio of 88:12:0.5 by volume was used as a mobile phase, and the final pH of the mobile phase was adjusted to 4.2 with glacial acetic acid. The flow rate of the mobile phase was 1 ml min–1, and the detector was a UV–Visible detector at a wavelength of 259 nm. The retention time of NCT was approximately 7.0 min. Under these conditions, good linearity and reproducibility were shown over the range 1.0–100.0 μg ml–1 NCT.
Statistical Analysis
One-way ANOVA with the least significant difference test for multiple comparisons was performed using SPSS program for MS Windows, release 11.5 (SPSS Thailand Co., Ltd., Bangkok, Thailand), to assess the statistical significance of physical properties as well as NCT release and permeation rate of the tablets. The significance of the difference was determined at a 95% confident limit (α = 0.5) and considered to be significant at a level of P <0.05.
RESULTS AND DISCUSSION
Characteristics of NCT-MAS Complexes and HPMC
The size of NCT-MAS complex particles was 94.03 ± 1.28 μm (n = 3) and the NCT content was 13.20 ± 0.04%, w/w (n = 3). The particle size of HPMC with different viscosity grades is listed in Table I. The MV-HPMC showed the smallest particle size of 75.7 μm, whereas the particle sizes of the LV-HPMC and HV-HPMC were over the range of 96.2–106.8 μm. In the viscosity determination, the relationship between shear stress and shear rate provided good linearity, indicating Newtonian flow. Therefore, the slope of such a relationship was the viscosity of HPMC dispersion. The viscosity of HPMC dispersions, as shown in Table I, was less than that claimed by the manufacturer due to the higher temperature used in this study.
Table I.
Characteristics of HPMC Powder and Dispersion and NCT-MAS Complex-Loaded Matrix Tablets Prepared Using Different Viscosity Grades of HPMC
| HPMC characteristics | LV-HPMC | MV-HPMC | HV-HPMC |
|---|---|---|---|
| Powders | |||
| Particles sizea (μm) | 106.8 ± 3.1 | 75.7 ± 0.2 | 96.2 ± 1.3 |
| Dispersions | |||
| Viscositya (cP) | 7.80 ± 0.02 | 24.00 ± 0.01 | 52.24 ± 0.01 |
| Tablets | |||
| Thicknessa (mm) | 1.81 ± 0.04 | 1.82 ± 0.03 | 1.83 ± 0.03 |
| Hardnessa (N) | 66.6 ± 8.2 | 100.7 ± 3.5 | 118.4 ± 1.8 |
| Release of whole tabletsa | |||
| n | 0.77 ± 0.05 (R 2 = 0.996) | 1.21 ± 0.06 (R 2 = 0.995) | 0.61 ± 0.05 (R 2 = 0.991) |
| K 0 (% min−1) | 0.21 ± 0.01 (R 2 = 0.939) | 0.55 ± 0.05 (R 2 = 0.992) | 0.11 ± 0.01 (R 2 = 0.939) |
| K H (% min−0.5) | 3.84 ± 0.25 (R 2 = 0.995) | 7.14 ± 0.72 (R 2 = 0.929) | 2.63 ± 0.05 (R 2 = 0.996) |
| Unidirectional releasea | |||
| n | 0.59 ± 0.02 (R 2 = 0.999) | 0.60 ± 0.01 (R 2 = 0.997) | 0.54 ± 0.01 (R 2 = 0.998) |
| K 0 (μg min−1) | 10.57 ± 0.36 (R 2 = 0.973) | 9.54 ± 0.46 (R2 = 0.979) | 6.61 ± 0.15 (R 2 = 0.973) |
| K H (μg min−0.5) | 259.6 ± 9.6 (R 2 = 0.994) | 242.1 ± 11.5 (R 2 = 0.994) | 162.2 ± 4.2 (R 2 = 0.993) |
| Unidirectional permeationa | |||
| K 0 (μg min−1) | 4.66 ± 0.92 (0.969) | 4.33 ± 0.75 (R 2 = 0.990) | 3.96 ± 0.49 (R 2 = 0.997) |
| K H (μg min−0.5) | 122.1 ± 24.2 (0.993) | 117.3 ± 19.4 (R 2 = 0.985) | 109.3 ± 12.3 (R 2 = 0.978) |
| Mucoadhesive propertyb | |||
| F max (mN) | 541.5 ± 173.6 | 486.7 ± 122.3 | 600.3 ± 140.2 |
| W ad (mN mm) | 525.7 ± 87.7 | 436.6 ± 93.3 | 577.5 ± 127.4 |
Particle size of HPMC powder was measured using a laser diffraction particle size analyzer. Of the HPMC dispersion, 2% (w/v) was used for viscosity determination at 32 ± 1°C
aData are the mean ± SD, n = 3
bData are the mean ± SD, n = 5
Effect of HPMC Viscosity Grade on Characteristics of NCT-MAS Complex-Loaded Tablets
The thickness and hardness of the tablets that were prepared using different viscosity grades of HPMC are listed in Table I. The HPMC viscosity grade did not affect the thickness of the tablets that were prepared. On the other hand, the hardness of the tablets statistically increased (P < 0.05) with increasing viscosity grade of HPMC, and the use of HV-HPMC presented the highest tablet hardness. This result was in agreement with a previous study (24). The tablets gave acceptable physical properties because HPMC had a good compressibility and showed plastic deformation under compression with small elastic recovery when using low compression speed (24), which was similar to the use of hydrostatic press for tableting that had a slow speed for tablet compression.
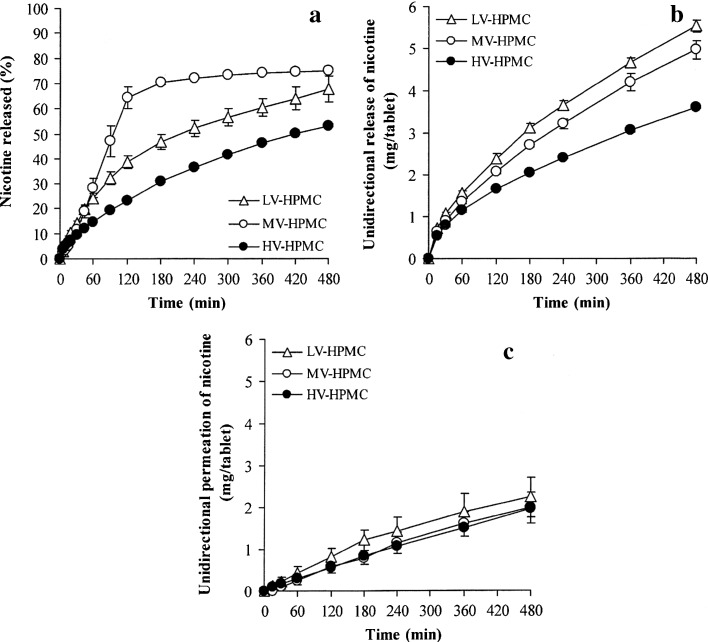
The NCT release of the whole tablets is presented in Fig. 1a, and the NCT release parameters are listed in Table I. It can be observed that NCT release was not related to the viscosity grade of the HPMC used. The release exponent, n value, of the MV-HPMC tablets was more than unity, whereas those of the LV- and HV-HPMC tablets were in the range of 0.61–0.77. This result suggested that the NCT release of the MV-HPMC tablets was controlled by a matrix erosion mechanism, whereas both NCT diffusion and matrix erosion controlled the release of the LV-HPMC and HV-HPMC tablets. The NCT release data of the MV-HPMC tablets showed good correlation when using the zero-order equation with an R2 higher than 0.99 (Table I). In contrast, the Higuchi model presented a better determination coefficient (R2) than the zero-order model for the LV- and HV-HPMC tablets. Furthermore, the MV-HPMC tablets provided the highest K0 and KH values. The LV-HPMC tablets gave lower K0 and KH values than the HV-HPMC tablets. Generally, increasing the viscosity grade of HPMC caused slower drug release from the HPMC tablets (25) due to a higher viscosity gel barrier that was created around the tablets when exposed to the dissolution medium. This phenomenon could be explained for only the NCT release of the LV- and HV-HPMC tablets, but not for the MV-HPMC tablets. The NCT release of the LV- and HV-HPMC tablets mainly followed a matrix diffusion-controlled mechanism, indicating that a continuous gel barrier could be formed around the tablets. In the case of the MV-HPMC tablets, an incomplete swelling of MV-HPMC particles may have occurred because the fracture of some NCT-MAS complex particles, which have a larger particle size than the MV-HPMC powder, could cover the surface of the MV-HPMC particles that underwent plastic deformation under compression. Covering the surface of these particles resulted in a slow water uptake and swelling of the MV-HPMC particles to form a continuous gel barrier around the tablets. An incomplete gel formation may lead to rapid erosion and NCT release of the tablets.
Fig. 1.
NCT release profiles of whole tablets (a) and unidirectional NCT release (b) and permeation (c) of NCT-MAS complex-loaded HPMC tablets prepared using different viscosity grades of HPMC. Each value represents the mean ± SD of triplicate experiments
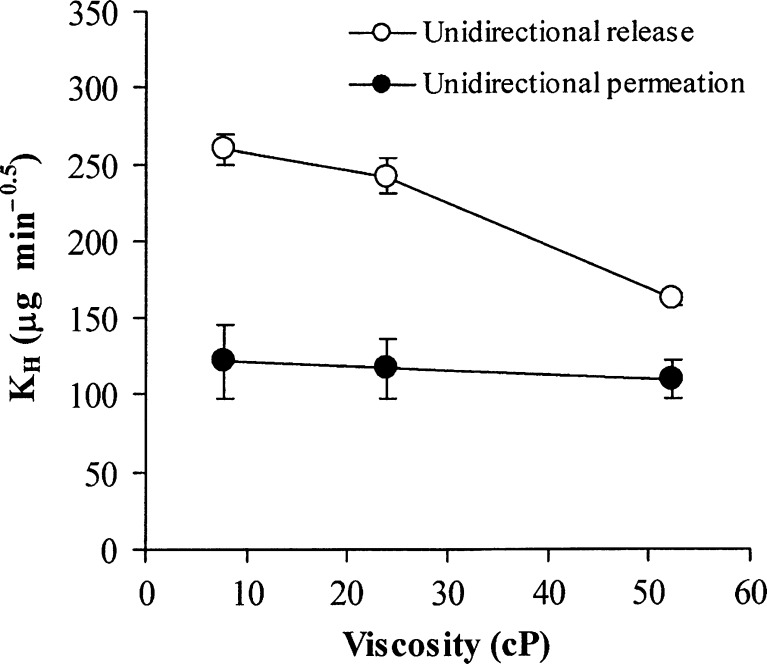
Unidirectional release and permeation of NCT from NCT-MAS complex-loaded HPMC tablets using different viscosity grades of HPMC are shown in Fig. 1b, c, respectively. The release and permeation parameters of NCT are listed in Table I. The release exponents of all tablets were over the range of 0.54–0.60. The NCT release rate computed using the Higuchi model gave a better determination coefficient than that using the zero-order model. For these results, it was indicated that the NCT release was controlled by a matrix diffusion-controlled mechanism with only a small impact on polymer swelling, which can be observed from the value of the release exponent that was slightly higher than 0.5. The NCT release rate of the tablets using different viscosity grades of HPMC was related to the viscosity value of 2% (w/v) HPMC that could be represented by the viscosity of the gel barrier that formed surrounding the swollen tablets (Fig. 2). The NCT release rate tended to decrease with increasing viscosity of HPMC. This result was in contrast with the NCT release of the whole tablets because erosion of the swollen tablets did not involve the unidirectional release of NCT. Therefore, the gel barrier could be completely created on the surface of the swollen tablets that were located on the cellulose acetate membrane. Apart from the unidirectional release, the NCT permeation rate could be computed with both the Higuchi and the zero-order models. It can be observed that the NCT permeation rate did not change significantly when increasing the viscosity value of HPMC (Fig. 2). This result suggested that the NCT diffusion across the mucosal membrane was the rate-limiting step of the NCT permeation.
Fig. 2.
Relationship between viscosity of 2% (w/v) HPMC dispersion and unidirectional release and permeation rate of tablets, calculated using Higuchi’s equation. Each point represents the mean ± SD of triplicate experiments
The mucoadhesive properties, Fmax and Wad, of the NCT-MAS complex-loaded matrix tablets when using different viscosity grades of HPMC are presented in Table I. It was found that the viscosity grade of HPMC did not influence the Fmax and Wad values on porcine esophageal mucosa. HPMC is a nonionic polymer that possesses a mucoadhesive property (1,26) because it contains numerous hydroxyl groups that can form hydrogen bonds. It has been proposed that the interaction between the mucus on the mucosal membrane and hydrophilic polymers occurs by physical entanglement and chemical interactions, such as hydrogen bonding (26). Due to the mucoadhesive properties of HPMC and NCT-MAS complexes (17), the NCT-MAS complex-loaded HPMC tablets adhered sufficiently onto the mucosal membrane.
Effect of pH Modifier on the Characteristics of NCT-MAS Complex-Loaded HPMC Tablets
The NCT-MAS complex-loaded MV-HPMV tablet was selected as a control tablet for investigating the effect of pH modifiers, including sodium chloride, citric acid, and magnesium hydroxide, on the characteristics of the tablets. Incorporation of pH modifiers caused a change in the thickness and hardness of the tablets (Table II). The tablet thickness seemed to increase with increasing amounts of pH modifier. Incorporation of 5–20% sodium chloride did not affect the hardness of the tablets, whereas 20% citric acid caused a statistical decrease in tablet hardness (P < 0.05), but 5–10% citric acid caused no change. On the other hand, a small amount of magnesium hydroxide brought about a significant decrease in the tablet hardness (P < 0.05), and a similar tablet hardness was found by further increasing the magnesium hydroxide content (10 and 20%) in the tablets. It is possible to explain that sodium chloride possessed an intermediate plastic deformation with a low degree of fragmentation under the compression pressure (27,28), which was similar to HPMC. Incorporation of sodium chloride into HPMC did not affect deformation and interparticle bonding, leading to no change in the tablet hardness when sodium chloride was added. Conversely, the highest quantity of citric acid significantly decreased the tablet hardness because large amounts of citric acid may reduce interparticle bonding of HPMC. Magnesium hydroxide, an inorganic material, possessed hard and brittle particles (28) that deformation by fragmentation occurred under compression pressure. The fragmentation of magnesium hydroxide to small particles could obviously reduce interparticle bonding and interlocking of HPMC particles, resulting in a decrease in the tablet hardness.
Table II.
Physical Properties and NCT Release Rates of Whole NCT-MAS Complex-Loaded MV-HPMC Tablets Containing Different pH Modifiers
| pH modifier | Thickness (mm) | Hardness (N) | K 0 (% min−1) | K H (% min−0.5) |
|---|---|---|---|---|
| Control tablet | 1.82 ± 0.03 | 100.7 ± 3.5 | 0.55 ± 0.05 (R 2 = 0.992) | 7.14 ± 0.72 (R 2 = 0.929) |
| 5% NaCl | 1.83 ± 0.05 | 98.8 ± 2.2 | 1.30 ± 0.09 (R 2 = 0.993) | 12.8 ± 0.84 (R 2 = 0.968) |
| 10% NaCl | 1.96 ± 0.04 | 99.6 ± 2.7 | 1.25 ± 0.08 (R 2 = 0.980) | 13.6 ± 0.96 (R 2 = 0.964) |
| 20% NaCl | 2.01 ± 0.07 | 106.4 ± 4.5 | 1.18 ± 0.25 (R 2 = 0.993) | 10.5 ± 2.16 (R 2 = 0.945) |
| 5% Citric acid | 1.92 ± 0.05 | 98.8 ± 9.5 | 1.28 ± 0.12 (R 2 = 0.972) | 11.5 ± 1.18 (R 2 = 0.949) |
| 10% Citric acid | 1.94 ± 0.04 | 104.3 ± 9.5 | 1.52 ± 0.10 (R 2 = 0.915) | 13.9 ± 0.91 (R 2 = 0.933) |
| 20% Citric acid | 2.18 ± 0.03 | 70.6 ± 2.7 | 1.52 ± 0.19 (R 2 = 0.945) | 14.9 ± 2.04 (R 2 = 0.922) |
| 5% Mg(OH)2 | 1.83 ± 0.03 | 62.5 ± 3.6 | 0.10 ± 0.01 (R 2 = 0.951) | 2.47 ± 0.13 (R 2 = 0.997) |
| 10% Mg(OH)2 | 1.97 ± 0.03 | 59.4 ± 2.6 | 0.11 ± 0.01 (R 2 = 0.952) | 2.71 ± 0.13 (R 2 = 0.996) |
| 20% Mg(OH)2 | 2.01 ± 0.02 | 61.9 ± 4.9 | 0.11 ± 0.01 (R 2 = 0.977) | 2.64 ± 0.06 (R 2 = 0.995) |
Data are the mean ± SD, n = 3
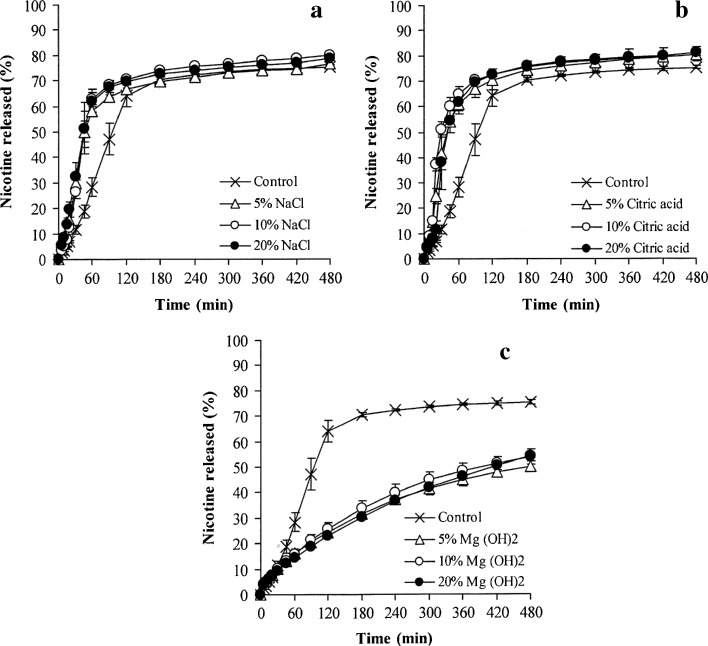
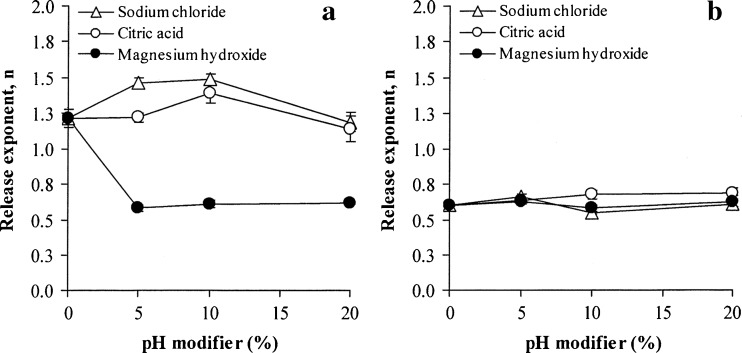
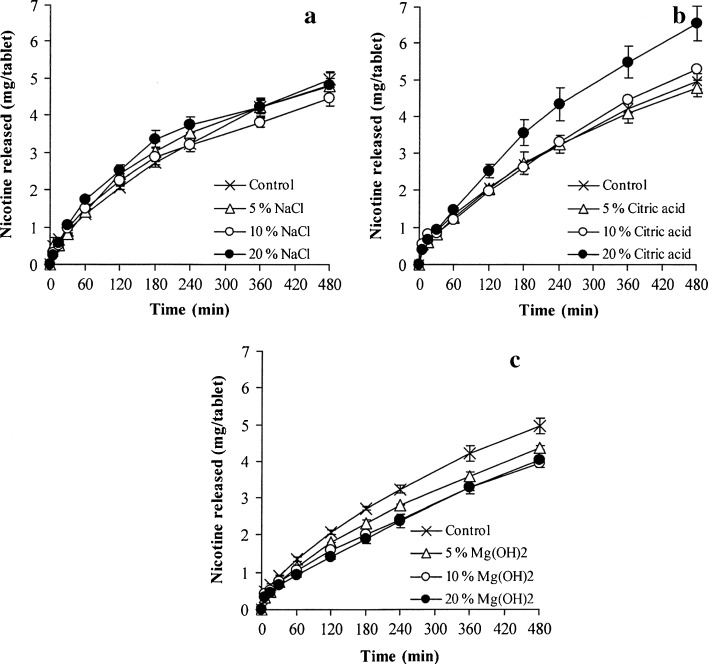
The effect of pH modifiers on NCT release of whole tablets is presented in Fig. 3, and the release exponent of NCT is shown in Fig. 4a. As observed from the NCT release profiles, sodium chloride and citric acid could accelerate NCT release (Fig. 3a, b, respectively), but a retardation of NCT release was found in the tablets containing magnesium hydroxide (Fig. 3c). The release exponents of the tablets containing varying quantities of sodium chloride and citric acid were higher than unity and similar to the control tablets (Fig. 4a). This finding indicated that a matrix erosion mechanism was the predominant factor in controlling NCT release. The NCT release rates of the tablets containing various contents of sodium chloride and citric acid are presented in Table II. The NCT release of the tablets containing sodium chloride showed a good fit with the zero-order model (R2 higher than 0.98), whereas neither the Higuchi model nor the zero-order model could fit with that of the tablets containing citric acid, which could be observed from a determination coefficient that was in the range of 0.91–0.97. However, it was clear that sodium chloride and citric acid could promote the release of NCT from the tablets, but the extent of release did not correlate with the amount of sodium chloride and citric acid that was added. It is possible that the high water solubility of sodium chloride and citric acid could accelerate the swelling and erosion of the HPMC tablets, which could cause matrix erosion of the tablets and a higher NCT release rate compared to the control tablets. Moreover, the high water solubility of citric acid, 1 g in 1.69 ml at 20°C (29), gave a greater NCT release rate than sodium chloride, which has a water solubility of 1 g in 2.78 ml (29). In contrast to the tablets containing magnesium hydroxide, the release exponent was found to be in the range of 0.58–0.62, even though only a low content of magnesium hydroxide was added to the tablets. This range led to a good fit for NCT release with the Higuchi model (Table II). The NCT release rate of the tablets with magnesium hydroxide was statistically lower (P < 0.05) than that of the control tablets. This finding suggested that incorporating magnesium hydroxide could reinforce and maintain the matrix of the tablets, which could result from an inorganic gel formation of magnesium hydroxide in the tablet matrix after being exposed to the dissolution medium, leading to matrix erosion retardation and a matrix diffusion-controlled mechanism of NCT release.
Fig. 3.
Effect of pH modifiers on the NCT release of whole tablets of NCT-MAS complex-loaded HPMC tablets containing various amounts of sodium chloride (a), citric acid (b), and magnesium hydroxide (c). Each value represents the mean ± SD of triplicate experiments
Fig. 4.
Release exponent (n value) of whole tablets (a) and unidirectional tablets (b) of NCT-MAS complex-loaded HPMC tablets prepared using different pH modifiers. Each value represents the mean ± SD of triplicate experiments
The effect of sodium chloride, citric acid, and magnesium hydroxide on unidirectional NCT release from the tablets is shown in Fig. 5a–c, respectively, and the release exponent is summarized in Fig. 4b. It can be observed that the release exponent of all tablets was over the range of 0.55–0.69, which is indicative of a drug diffusion- and polymer swelling-controlled mechanism. The addition of pH modifiers did not influence the NCT release kinetics. However, the release exponent of the unidirectional NCT release was obviously lower than that of the NCT release of the whole tablets, particularly the control tablets and the tablets containing sodium chloride and citric acid (Fig. 4a), because of the limitation on the erosion process of the swollen matrix tablets when using a cellulose acetate membrane. The NCT release rates of the tablets are listed in Table III. The Higuchi model gave a better fit with the release of NCT from the unidirectional test than the zero-order model. The addition of sodium chloride did not remarkably affect the release of NCT when compared with the control tablets. This result suggested that sodium ions dissolved in the swollen tablets could not accelerate an ion exchange process with NCT that was intercalated in the silicate layers of MAS. On the other hand, 10 and 20% citric acid could significantly increase the NCT release rate (P < 0.05) when compared with the control tablets. This result was similar to the previous report (30). This similarity was due to the higher water solubility of citric acid that could allow it to be rapidly dissolved and leach out from the swollen tablets. This leaching led to a decrease in the tortuosity of the swollen matrix and resulted in faster NCT release. In the case of magnesium hydroxide, a significant retardation of NCT release (P < 0.05) was found in comparison with the control tablets, but was not proportional to the content of magnesium aluminum silicate added. This result suggested that the formation of an inorganic gel of magnesium hydroxide could reinforce and increase the tortuosity of the swollen matrix, resulting in slower diffusion and release of NCT from the tablets.
Fig. 5.
Effect of pH modifiers on the unidirectional NCT release of NCT-MAS complex-loaded HPMC tablets containing various amounts of sodium chloride (a), citric acid (b), and magnesium hydroxide (c). Each value represents the mean ± SD of triplicate experiments
Table III.
Unidirectional Release and Permeation of NCT and Mucoadhesive Properties of NCT-MAS Complex-Loaded MV-HPMC Tablets Containing Different pH Modifiers
| pH modifier | Unidirectional NCT releasea | Unidirectional NCT permeationa | Mucoadhesive propertyb | |||
|---|---|---|---|---|---|---|
| K 0 (μg min−1) | K H (μg min−0.5) | K 0 (μg min−1) | K H (μg min−0.5) | F max (mN) | W ad (mN mm) | |
| Control tablet | 9.54 ± 0.46 (R 2 = 0.979) | 242.1 ± 11.5 (R 2 = 0.994) | 4.33 ± 0.75 (R 2 = 0.990) | 117.3 ± 19.4 (R 2 = 0.985) | 486.7 ± 122.3 | 436.6 ± 93.3 |
| 5% NaCl | 9.43 ± 0.58 (R 2 = 0.932) | 235.8 ± 13.6 (R 2 = 0.988) | 4.58 ± 0.16 (R 2 = 0.992) | 125.6 ± 4.3 (R 2 = 0.990) | 435.7 ± 101.6 | 433.5 ± 169.6 |
| 10% NaCl | 9.98 ± 0.42 (R 2 = 0.926) | 215.8 ± 9.8 (R 2 = 0.994) | 3.59 ± 0.52 (R 2 = 0.992) | 108.6 ± 13.7 (R 2 = 0.989) | 437.5 ± 122.1 | 368.3 ± 67.2 |
| 20% NaCl | 9.45 ± 0.31 (R 2 = 0.895) | 240.8 ± 9.4 (R 2 = 0.986) | 4.16 ± 0.22 (R 2 = 0.989) | 116.4 ± 6.4 (R 2 = 0.981) | 446.7 ± 88.1 | 449.2 ± 160.5 |
| 5% Citric acid | 9.23 ± 0.50 (R 2 = 0.969) | 237.6 ± 13.8 (R 2 = 0.995) | 0.78 ± 0.08 (R 2 = 0.965) | 19.24 ± 2.02 (R 2 = 0.942) | 468.2 ± 96.3 | 461.7 ± 92.4 |
| 10% Citric acid | 10.19 ± 0.57 (R 2 = 0.987) | 268.1 ± 5.2 (R 2 = 0.986) | 0.91 ± 0.22 (R 2 = 0.989) | 24.92 ± 5.99 (R 2 = 0.963) | 485.0 ± 102.6 | 392.6 ± 108.2 |
| 20% Citric acid | 13.20 ± 1.25 (R 2 = 0.975) | 336.8 ± 33.4 (R 2 = 0.993) | 0.32 ± 0.03 (R 2 = 0.959) | 8.90 ± 0.67 (R 2 = 0.945) | 471.6 ± 89.6 | 464.2 ± 151.1 |
| 5% Mg(OH)2 | 8.44 ± 0.17 (R 2 = 0.977) | 213.5 ± 4.4 (R 2 = 0.994) | 8.39 ± 0.53 (R 2 = 0.993) | 227.4 ± 13.4 (R 2 = 0.988) | 548.6 ± 53.8 | 531.9 ± 42.0 |
| 10% Mg(OH)2 | 7.34 ± 0.22 (R 2 = 0.990) | 188.1 ± 5.9 (R 2 = 0.989) | 10.19 ± 0.91 (R 2 = 0.991) | 277.0 ± 25.7 (R 2 = 0.990) | 448.8 ± 76.3 | 420.4 ± 72.6 |
| 20% Mg(OH)2 | 7.76 ± 0.35 (R 2 = 0.993) | 196.0 ± 8.2 (R 2 = 0.977) | 8.63 ± 0.21 (R 2 = 0.991) | 235.4 ± 5.9 (R 2 = 0.990) | 452.9 ± 52.7 | 484.7 ± 42.3 |
aData are the mean ± SD, n = 3
bData are the mean ± SD, n = 5
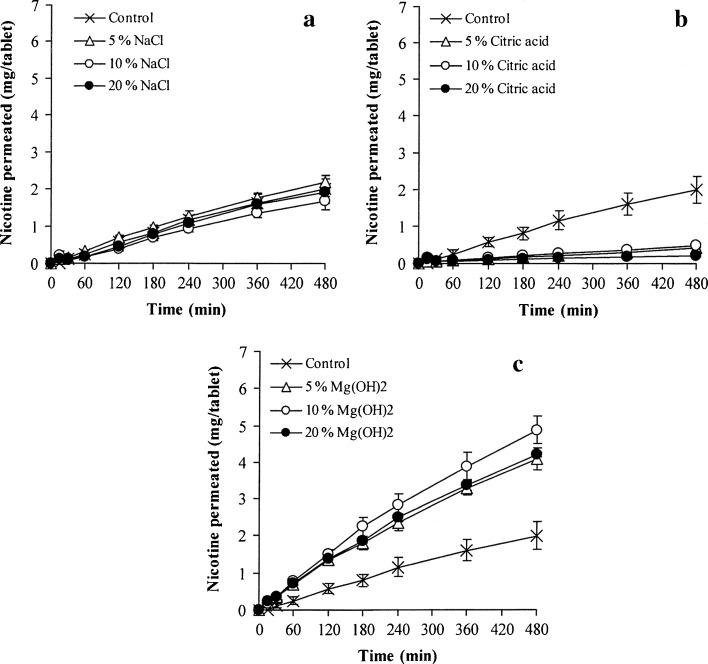
The unidirectional permeation of the tablets containing various amounts of sodium chloride, citric acid, and magnesium hydroxide was also investigated, as shown in Fig. 6a–c, respectively. The NCT permeation rates were calculated using the Higuchi and zero-order models and are reported in Table III. The NCT permeation could be described using the zero-order model rather than the Higuchi model because of a greater determination coefficient value, suggesting that the NCT diffusion across the mucosal membrane was the rate-limiting step of the permeation. The tablets containing sodium chloride gave a similar NCT permeation rate when compared with the control tablets. The incorporation of citric acid gave a significantly lower NCT permeation rate (P < 0.05) than the control tablets, and 20% citric acid gave the highest retardation effect (P < 0.05). Citric acid could enhance the NCT release from the swollen matrix, but NCT molecules that were released did not readily permeate across the mucosal membrane because NCT in the protonated form under acidic conditions has a low permeability for the mucosal membrane (8,9). On the other hand, magnesium hydroxide provided a statistical enhancement in NCT permeation (P < 0.05) when compared with the control tablets, even through a small quantity (5%) of magnesium hydroxide was used. The basic microenvironmental pH of the swollen tablets containing magnesium hydroxide could prevent the protonation of NCT, instead giving a neutral species that showed increased permeability across the mucosal membrane, leading to an enhanced permeation of NCT. Additionally, the use of 10% magnesium hydroxide showed the highest NCT permeation rate, suggesting that microenvironmental basic pH in the swollen tablets could be sufficient for inducing the formation of neutral NCT. Curiously, the addition of more than 10% magnesium hydroxide did not enhance NCT permeation.
Fig. 6.
Effect of pH modifiers on the unidirectional NCT permeation of NCT-MAS complex-loaded HPMC tablets containing various amounts of sodium chloride (a), citric acid (b), and magnesium hydroxide (c). Each value represents the mean ± SD of triplicate experiments
The mucoadhesive properties of the tablets containing pH modifiers are listed in Table III. The pH modifiers that were added did not affect the Fmax and Wad values of the tablets, which suggested that the MV-HPMC particles still had swelling properties on the tablet surface that incorporated with the particles of pH modifiers. The rapid swelling and disentanglement of MV-HPMC molecules could interact with mucin on the surface of the porcine esophageal mucosa, which resulted in similar mucoadhesive properties when adding a pH modifier to the tablets.
Rate Control Studies of the Matrix Tablets
In the development of the drug delivery system, the delivery of drug to circulating blood must be controlled by the drug delivery system that is administered, which is not controlled by the mucosal membrane. Thus, the rate control studies of the NCT-MAS complex-loaded matrix tablets were modified from the method that was reported previously (31,32). The fractional rate control provided by the device (buccal tablet) and the mucosal membrane could be computed using the NCT release and permeation data from the unidirectional testing and the following equations:
 |
where AP is the amount of NCT that permeates across the mucosal membrane at 8 h and AR is the amount of NCT released through the cellulose acetate membrane at 8 h. These equations are based on the same testing method of the unidirectional NCT release and permeation.
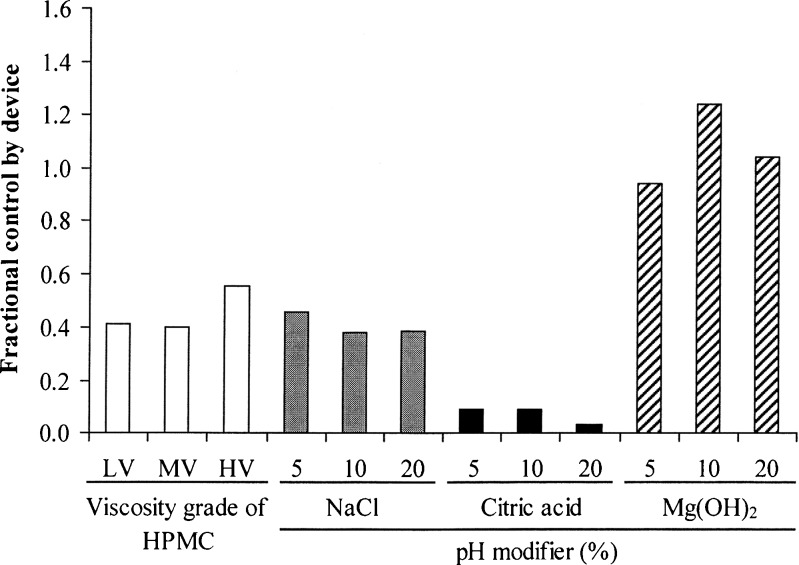
The fractional control by the buccal tablets is presented in Fig. 7. In the evaluation of different HPMC viscosity grades, the tablets using HV-HPMC gave a remarkably greater Fd value than those using LV- and MV-HPMC. This result suggested that an increase in swollen gel viscosity could retard and control NCT release onto the mucosal membrane before NCT permeation, leading to a decrease in NCT permeation. Moreover, incorporation of sodium chloride did not influence the Fd value when compared with the tablets using MV-HPMC (the control tablet). The reduction in the Fd value was found when adding citric acid because of the lower permeability of the protonated NCT that formed on the mucosal membrane. On the other hand, the Fd values of the tablets containing magnesium hydroxide were close to unity. This result indicated that the NCT permeation across the mucosal membrane was identical to the release of NCT from the tablets because of the formation of neutral NCT under basic pH.
Fig. 7.
Fractional control by device of NCT-MAS complex-loaded HPMC tablets prepared using different viscosity grades of HPMC and pH modifiers
CONCLUSION
The NCT-MAS complex-loaded HPMC tablets were successfully prepared by the direct compression method. The higher the viscosity grade of HPMC used in the tablets, the lower was the unidirectional NCT release rate. The unidirectional NCT permeation was not affected by the viscosity grade of HPMC. The incorporation of magnesium hydroxide could retard NCT release, whereas the enhancement of unidirectional NCT release was found in the tablets containing citric acid. Citric acid could inhibit the NCT permeation rate due to the formation of protonated NCT under acidic pH. Conversely, the NCT permeation rate increased with the use of magnesium hydroxide, resulting from a neutral NCT species that formed under basic pH. The swollen HPMC tablets, with or without pH modifiers, showed mucoadhesive properties toward the mucosal membrane. Furthermore, the buccal delivery of NCT could be controlled mainly by the matrix tablets with the addition of magnesium hydroxide. This study suggests that the NCT-MAS complex-loaded HPMC tablets containing magnesium hydroxide presented a promising buccal delivery system of NCT.
Acknowledgments
The authors would like to thank the Thailand Research Fund (Bangkok, Thailand) for research funding (grant no. RSA5280013) and the Faculty of Pharmaceutical Sciences, Khon Kaen University (Khon Kaen, Thailand) for technical support. We are very pleased to acknowledge the Graduate School, Khon Kaen University (Khon Kaen, Thailand) for a scholarship for S.K.
References
- 1.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Pongjanyakul T, Suksri H. Alginate–magnesium aluminum silicate films for buccal delivery of nicotine. Colloids Surf B. 2009;74:103–113. doi: 10.1016/j.colsurfb.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Park CR, Munday DL. Development and evaluation of a biphasic buccal adhesive tablet for nicotine replacement therapy. Int J Pharm. 2002;237:215–226. doi: 10.1016/S0378-5173(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 4.Desai KG, Kumar TM. Preparation and evaluation of a novel buccal adhesive system. AAPS PharmSciTech. 2004;5(3):Article 35. [DOI] [PMC free article] [PubMed]
- 5.Hassan N, Khar RK, Ali M, Ali J. Development and evaluation of buccal bioadhesive tablet of an anti-emetic agent ondansetron. AAPS PharmSciTech. 2009;10:1085–1092. doi: 10.1208/s12249-009-9304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Movassaghian S, Barzegar-Jalali M, Alaeddini M, Hamedyazdan S, Afzalifar R, Zakeri-Milani P, Mohammadi G, Adibkia K. Development of amitriptyline buccoadhesive tablets for management of pain in dental procedures. Drug Dev Ind Pharm. 2011;37:849–854. doi: 10.3109/03639045.2010.546403. [DOI] [PubMed] [Google Scholar]
- 7.Dollery SC. Therapeutic drugs. Edinburgh: Churchill Livingstone; 1991. pp. N65–N72. [Google Scholar]
- 8.Nair MA, Chetty DJ, Ho H, Chien YW. Biomembrane permeation of nicotine: mechanistic studies with porcine mucosae and skin. J Pharm Sci. 1997;86:257–262. doi: 10.1021/js960095w. [DOI] [PubMed] [Google Scholar]
- 9.Chen LH, Chetty DJ, Chien YW. A mechanistic analysis to characterize oramucosal permeation properties. Int J Pharm. 1999;184:63–72. doi: 10.1016/S0378-5173(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 10.Adrian CL, Olin HBD, Dalhoff K, Jacobsen J. In vivo human buccal permeability of nicotine. Int J Pharm. 2006;311:196–202. doi: 10.1016/j.ijpharm.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Mihranyan A, Andersson SB, Ek R. Sorption of nicotine to cellulose powders. Eur J Pharm Sci. 2004;22:279–286. doi: 10.1016/j.ejps.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Cheng YH, Watts P, Hinchcliffe M, Hotchkiss R, Nankervis R, Faraj NF, Smith A, Davis SS, Illum DL. Development of a novel nasal nicotine formulation comprising an optimal pulsatile and sustained plasma nicotine profile for smoking cessation. J Control Release. 2000;79:243–254. doi: 10.1016/S0168-3659(01)00553-3. [DOI] [PubMed] [Google Scholar]
- 13.Pongjanyakul T, Khunawattanakul W, Puttipipatkhachorn S. Physicochemical characterizations and release studies of nicotine–magnesium aluminum silicate complexes. Appl Clay Sci. 2009;44:242–250. doi: 10.1016/j.clay.2009.03.004. [DOI] [Google Scholar]
- 14.Alexandre M, Dubois P. Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater Sci Eng. 2000;28:1–63. doi: 10.1016/S0927-796X(00)00012-7. [DOI] [Google Scholar]
- 15.Kibbe HA. Handbook of pharmaceutical excipients. 3. Washington: American Pharmaceutical Association; 2000. pp. 295–298. [Google Scholar]
- 16.Suksri H, Pongjanyakul T. Interaction of nicotine with magnesium aluminum silicate at different pHs: characterization of flocculate size, zeta potential and nicotine adsorption behavior. Colloids Surf B. 2008;65:54–60. doi: 10.1016/j.colsurfb.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Kanjanabat S, Pongjanyakul T. Preparation and characterization of nicotine–magnesium aluminum silicate complex-loaded sodium alginate matrix tablets for buccal delivery. AAPS PharmSciTech. 2011;12:683–692. doi: 10.1208/s12249-011-9633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ìkinci G, Senel S, Wilson CG, Şumnu M. Development of buccal bioadhesive nicotine tablet formulation for smoking cessation. Int J Pharm. 2004;277:173–178. doi: 10.1016/j.ijpharm.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-del Consuelo I, Jacques Y, Pizzolato G, Guy RH, Falson F. Comparison of the lipid composition of porcine buccal and esophageal permeability barriers. Arch Oral Biol. 2005;50:981–987. doi: 10.1016/j.archoralbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-del Consuelo I, Falson F, Guy RH, Jacques Y. Ex vivo evaluation of bioadhesive films for buccal delivery of fentanyl. J Control Release. 2007;122:135–140. doi: 10.1016/j.jconrel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–111. [PubMed] [Google Scholar]
- 22.Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm. 2009;364:328–343. doi: 10.1016/j.ijpharm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 24.Nokhodchi A, Ford JL, Rowe PH, Rubinstein MH. The effects of compression rate and force on the compaction properties of different viscosity grades of hydroxypropylmethylcellulose 2208. Int J Pharm. 1996;129:21–31. doi: 10.1016/0378-5173(95)04236-9. [DOI] [Google Scholar]
- 25.Campos-Aldrete ME, Villafuerte-Robles L. Influence of the viscosity grade and the particle size of HPMC on metronidazole release from matrix tablets. Eur J Pharm Biopharm. 1997;43:173–178. doi: 10.1016/S0939-6411(96)00004-5. [DOI] [Google Scholar]
- 26.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Alderborn G, Pasanen K, Nyström C. Studies on direct compression of tablets. XI. Characterization of particle fragmentation during compaction by permeametry measurements of tablets. Int J Pharm. 1985;23:79–86. doi: 10.1016/0378-5173(85)90224-8. [DOI] [Google Scholar]
- 28.Roopwani R, Buckner IS. Understanding deformation mechanisms during powder compaction using principal component analysis of compression data. Int J Pharm. 2011;418:227–234. doi: 10.1016/j.ijpharm.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Schilling SU, Bruce CD, Shah NH, Malick AW, McGinity JW. Citric acid monohydrate as a release-modifying agent in melt extruded matrix tablets. Int J Pharm. 2008;361:158–168. doi: 10.1016/j.ijpharm.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Espinoza R, Hong E, Villafuerte L. Influence of admixed citric acid on the release profile of pelanserin hydrochloride from HPMC matrix tablets. Int J Pharm. 2000;201:165–173. doi: 10.1016/S0378-5173(00)00406-3. [DOI] [PubMed] [Google Scholar]
- 31.Guy RH, Hadgraft J. Rate control in transdermal drug delivery? Int J Pharm. 1992;82:R1–R6. doi: 10.1016/0378-5173(92)90183-3. [DOI] [Google Scholar]
- 32.Pongjanyakul T, Prakongpan S, Priprem A. Acrylic matrix type nicotine transdermal patches: in vitro evaluations and batch-to-batch uniformity. Drug Dev Ind Pharm. 2003;29:843–853. doi: 10.1081/DDC-120024180. [DOI] [PubMed] [Google Scholar]