Abstract
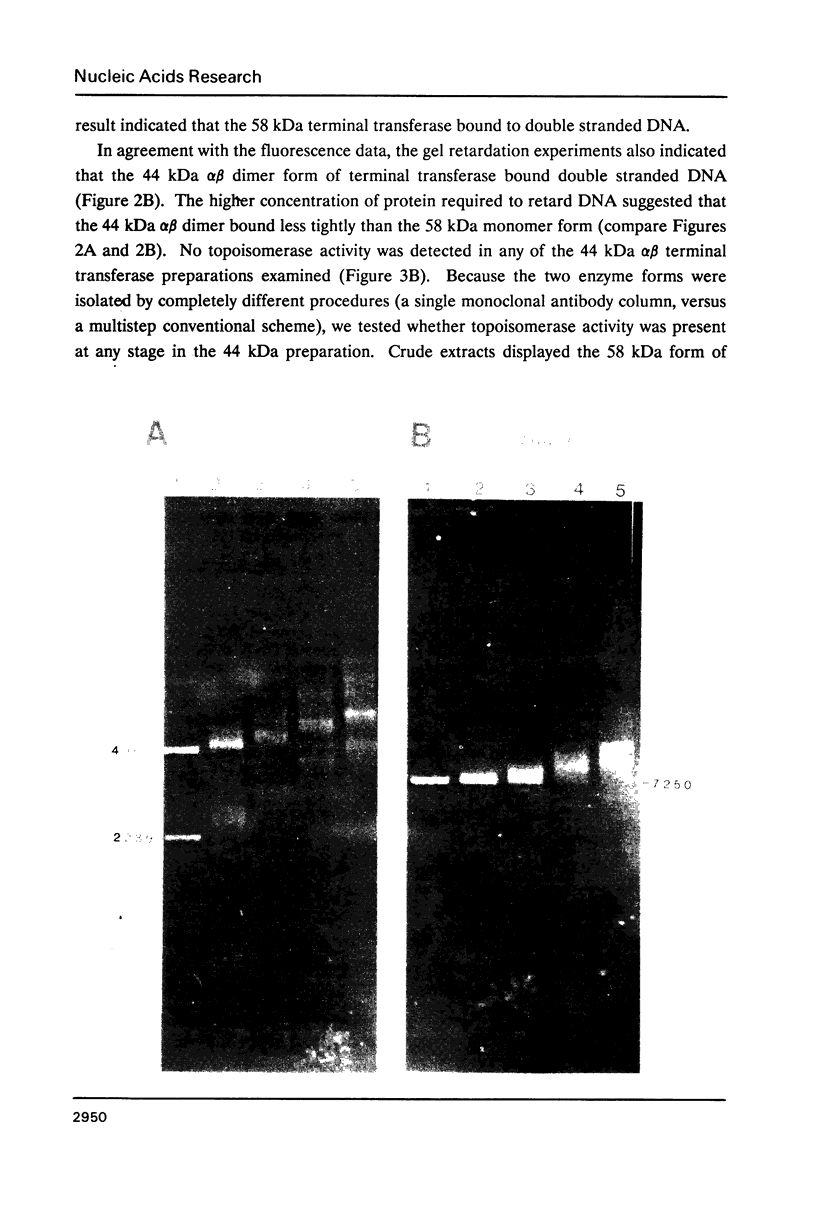
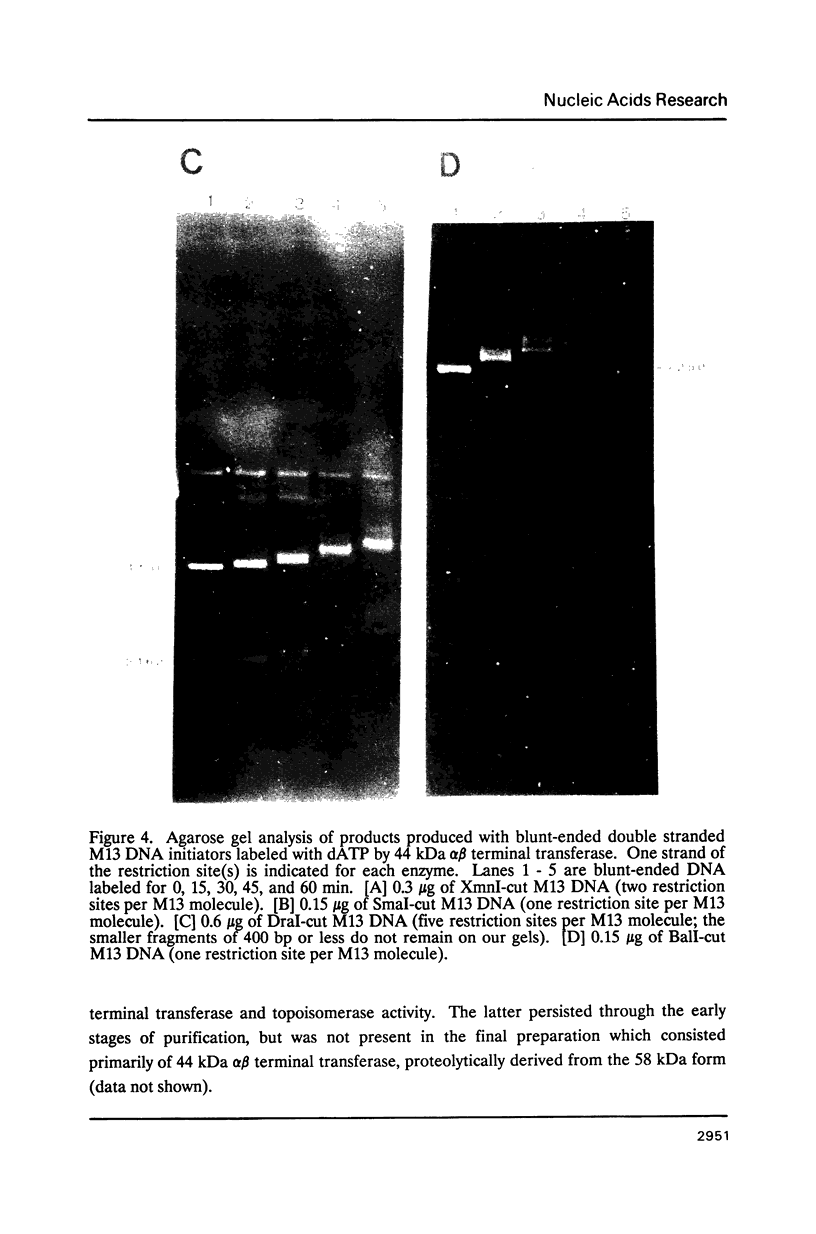
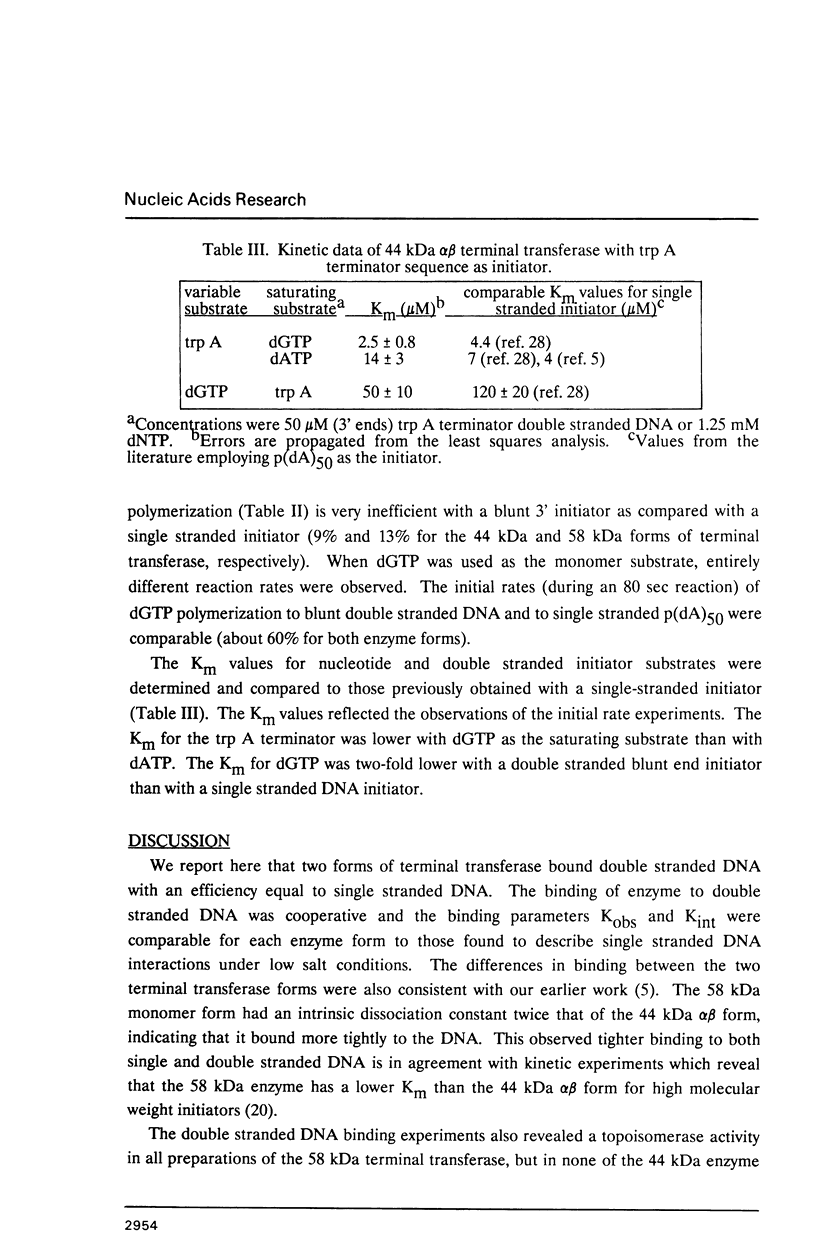
Binding of the 58 kDa monomer and 44 kDa alpha beta dimer forms of terminal deoxynucleotidyl transferase to double stranded DNA was demonstrated by gel retardation and tryptophan fluorescence quenching. The dissociation constants and cooperativity parameters were similar to those that have been determined for binding of these two forms of terminal transferase to single stranded DNA. However, the double stranded DNA binding site size of 10 nucleotides was half the size expected. The efficacy of blunt ended DNA as an initiator in the polymerization reaction catalyzed by terminal transferase was demonstrated by radiometric assays and product analyses on agarose gels. The initial reaction kinetics indicated that dGTP but not dATP was added efficiently to a blunt double stranded DNA 3' end. These results are correlated with current models for in vivo terminal transferase function.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessandrini A., Pierce J. H., Baltimore D., Desiderio S. V. Continuing rearrangement of immunoglobulin and T-cell receptor genes in a Ha-ras-transformed lymphoid progenitor cell line. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1799–1803. doi: 10.1073/pnas.84.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Coleman M. S. Terminal deoxynucleotidyl transferase: characterization of extraction and assay conditions from human and calf tissue. Arch Biochem Biophys. 1977 Aug;182(2):525–532. doi: 10.1016/0003-9861(77)90533-1. [DOI] [PubMed] [Google Scholar]
- Deibel M. R., Jr A specific endonuclease which co-purifies with calf thymus terminal deoxynucleotidyl transferase. Biochem Biophys Res Commun. 1983 Sep 30;115(3):909–916. doi: 10.1016/s0006-291x(83)80021-7. [DOI] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Biochemical properties of purified human terminal deoxynucleotidyltransferase. J Biol Chem. 1980 May 10;255(9):4206–4212. [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Limited proteolysis of calf thymus terminal deoxynucleotidyl transferase. Arch Biochem Biophys. 1980 Jul;202(2):414–419. doi: 10.1016/0003-9861(80)90445-2. [DOI] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Liu C. G., Barkley M. D. Fluorimetric assay for terminal deoxynucleotidyl transferase activity. Anal Biochem. 1985 Feb 1;144(2):336–346. doi: 10.1016/0003-2697(85)90126-5. [DOI] [PubMed] [Google Scholar]
- Deng G., Wu R. An improved procedure for utilizing terminal transferase to add homopolymers to the 3' termini of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4173–4188. doi: 10.1093/nar/9.16.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Goverman J., Minard K., Shastri N., Hunkapiller T., Hansburg D., Sercarz E., Hood L. Rearranged beta T cell receptor genes in a helper T cell clone specific for lysozyme: no correlation between V beta and MHC restriction. Cell. 1985 Apr;40(4):859–867. doi: 10.1016/0092-8674(85)90345-9. [DOI] [PubMed] [Google Scholar]
- Hagiya M., Davis D. D., Shultz L. D., Sakano H. Non-germ-line elements (NGE) are present in the T cell receptor beta-chain genes isolated from the mutant mouse, motheaten (me/me). J Immunol. 1986 Apr 1;136(7):2697–2700. [PubMed] [Google Scholar]
- Heidecker G., Messing J. Sequence analysis of zein cDNAs obtained by an efficient mRNA cloning method. Nucleic Acids Res. 1983 Jul 25;11(14):4891–4906. doi: 10.1093/nar/11.14.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K. I., Gonçalves J. M., Houts G. E., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. II. Properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967 Jun 10;242(11):2780–2789. [PubMed] [Google Scholar]
- Liu L. F., Miller K. G. Eukaryotic DNA topoisomerases: two forms of type I DNA topoisomerases from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3487–3491. doi: 10.1073/pnas.78.6.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. Characterization of the homopolymer tailing reaction catalyzed by terminal deoxynucleotidyl transferase. Implications for the cloning of cDNA. J Biol Chem. 1982 Dec 25;257(24):14773–14782. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. J., Barkley M. D., Coleman M. S. Interaction of terminal transferase with single-stranded DNA. J Biol Chem. 1987 Jul 15;262(20):9494–9502. [PubMed] [Google Scholar]
- Robbins D. J., Deibel M. R., Jr, Barkley M. D. Tryptophan fluorescence of terminal deoxynucleotidyl transferase: effects of quenchers on time-resolved emission spectra. Biochemistry. 1985 Dec 3;24(25):7250–7257. doi: 10.1021/bi00346a034. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
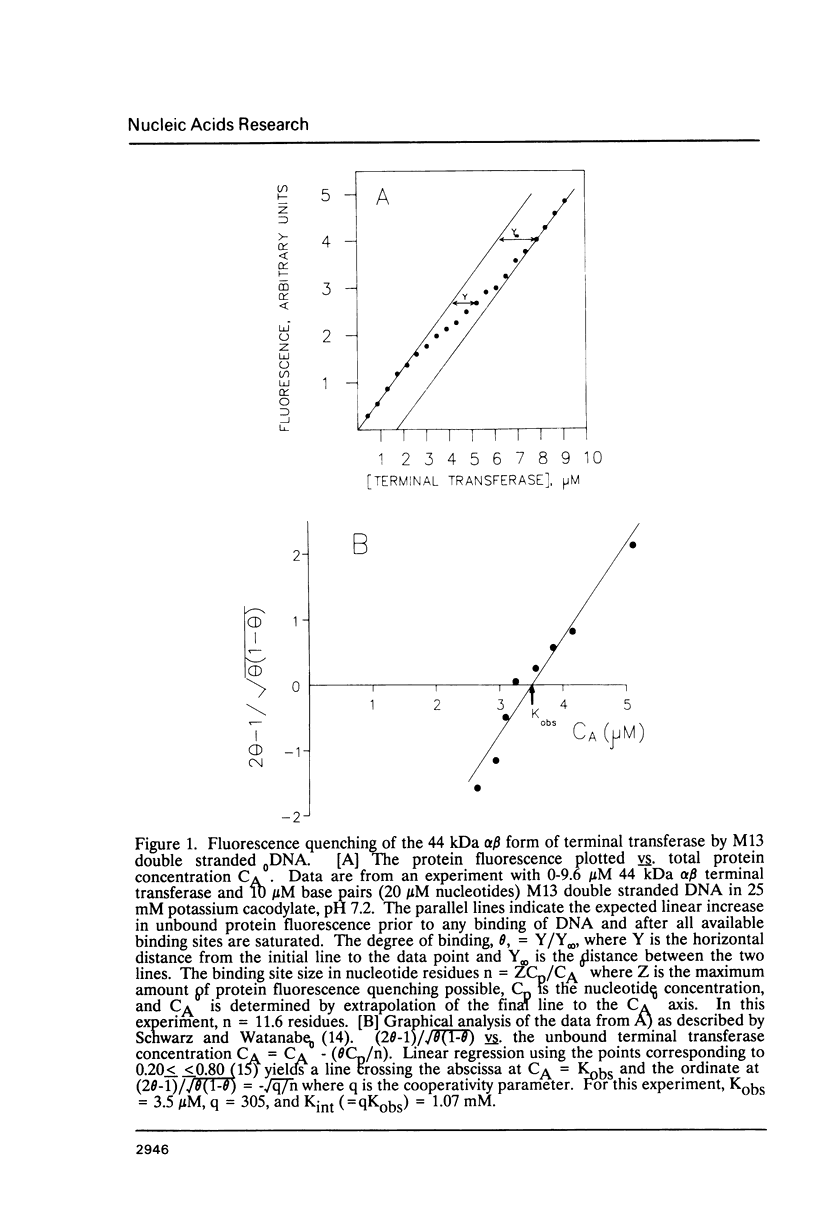
- Schwarz G., Watanabe F. Thermodynamics and kinetics of co-operative protein-nucleic acid binding. I. General aspects of analysis of data. J Mol Biol. 1983 Jan 25;163(3):467–484. doi: 10.1016/0022-2836(83)90069-4. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Watanabe F., Schwarz G. Thermodynamics and kinetics of co-operative protein-nucleic acid binding. II. Studies on the binding between protamine and calf thymus DNA. J Mol Biol. 1983 Jan 25;163(3):485–498. doi: 10.1016/0022-2836(83)90070-0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]