Abstract
Purpose
We evaluated the differences in calculi characteristics and their prevalence according to the presence of lower urinary tract symptoms between adult patients examined at the Urology Department and those examined at the Health Promotion Center (HPC).
Materials and Methods
The prevalence of prostatic calcification, characteristics of calculi (number, size, and location), and differences in lower urinary tract symptoms were compared and analyzed for 479 subjects who underwent transrectal ultrasonography at the HPC and the Urology Outpatients Department at our hospital from October 2009 to October 2010.
Results
Of 479 subjects, 268 patients were examined at the HPC, and 211 were examined at the Urology Outpatients Department. Between the two groups, age, prostate-specific antigen levels, prostate volume transrectal ultrasonography, International Prostate Symptom Score (total, voiding, and storage), quality of life, and the prostatic calcification rate were significantly higher in the patients who visited the Urology Outpatients Department. The prevalence of prostatic calcification was 41.5% (199/479), with 36.1% (97/268) from the HPC and 48.3% (102/211) from the Urology Outpatients Department. When the characteristics of prostatic calcification were compared, there were no significant differences in the appearance, size, or location of the calculi between the two groups.
Conclusions
The prevalence of prostatic calcification was high in patients complaining of lower urinary tract symptoms; however, there were no significant differences in the characteristics of the calculi. This finding leads us to believe that prostatic calcification can aggravate lower urinary tract symptoms but does not result in differences according to the number, size, or appearance of the calculi.
Keywords: Calcification, Prostate, Symptoms
INTRODUCTION
Most prostatic calcifications are found when urologic symptoms are present; however, they can also be fortuitously discovered during radiological screening for other internal diseases. Prostatic calcifications are often found in men with benign prostatic hypertrophy or prostate cancer [1]. Nevertheless, whether such fortuitously discovered prostatic calcifications have clinical significance or whether they are relevant factors for urologic disease remains unknown [2]. Moreover, the definition and classification of prostatic calcification has yet to be well established, and only a few studies have been conducted on the prevalence of these calcifications. In the studies that have been conducted, the frequency of prostatic calcification was found to vary. Fox [3] identified prostatic calcifications in 484 of 3,510 people (13.8%) who underwent radiological examination in the pelvic region. Conversely, Sondergaard et al. [4] reported prostatic calcifications in 99% of 300 autopsy cases, whereas Horio [5] reported prostatic calcifications in 100% of biopsies of patients over 30 years of age. However, research on the prevalence of these calcifications in Korean men is even more scarce.
We examined the frequency of prostatic calcifications in patients who visited our hospital and received a prostate screening among other health examinations and in patients who received a prostate screening after visiting the outpatient department of our hospital for lower urinary tract symptoms as their primary complaint. Furthermore, we evaluated the relationship and clinical significance between the presence of prostatic calcifications and lower urinary tract symptoms.
MATERIALS AND METHODS
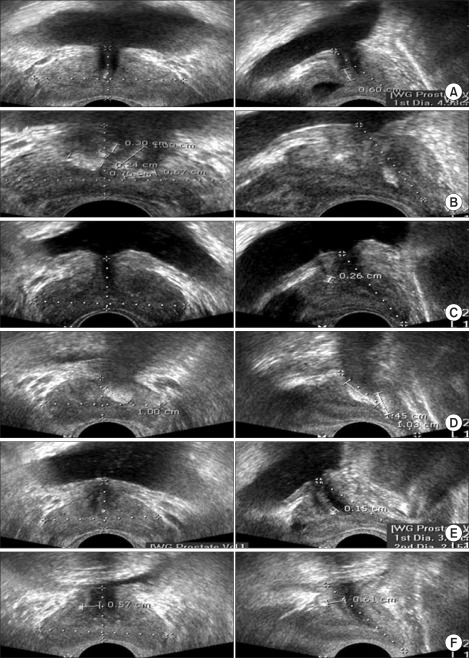
The exams were conducted by one urologist on a total of 479 men who visited the Urology Outpatients Department and the Health Promotion Center (HPC) at our hospital from January October 2009 to October 2010. Patients with acute urinary tract infections, such as pyelonephritis or prostatitis; patients who had undergone surgery or radiation therapy of the lower urinary tract organs; and patients with evidence of prostate cancer were excluded. A total of 268 healthy men visited the HPC for regular prostate screening and received examinations, and 211 patients with lower urinary tract symptoms visited the Urology Outpatients Department and received urological examinations. The histories, physical examinations, and International Prostate Symptom Scores (IPSSs) were checked for both groups. In addition, a blood test to evaluate the prostate-specific antigen (PSA) level, a digital rectal exam, and transrectal ultrasonography (TRUS) were performed. Prostatic calcification and prostate size (prostate volume) were measured during the TRUS. Fine and small hyperechoic calcifications (<2 mm along the major axis) and calcifications with no echoic mass in the ultrasound were not considered prostatic calcifications in this study. When the distribution of calcification existed across multiple locations, they were added together. In these situations, the group with a calcification size greater than 2 mm was included in the group with prostatic calcification; these were further classified into a group with one prostatic calcification and a group with two or more prostatic calcifications (Fig. 1). Based on the above ultrasonographic classifications for prostatic calcification, the group of men who visited the HPC and the group of patients who visited the Urology Outpatients Department were again further divided into two groups: one group without prostatic calcifications and the other group with prostatic calcifications. In addition, subgroups were created based on the locations of the calcifications with respect to the distance from the urethra: when calcifications were present within 0.2 cm of the urethra, the patients were placed in the central calcification group, and the patients with calcifications greater than 0.2 cm from the urethra were placed in the peripheral calcification group. All patient data were collected retrospectively. Data were analyzed via a Student's t-test and chi-square test using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). A p-value <0.05 was considered significant.
FIG. 1.
(A) Prostate calcification. (B) Multiple prostate calcifications. (C) Measurement of the size of the prostate calcification. (D) Measurement of the size of the prostate calcification when multiple calcifications were present. (E) Central prostate calcification. (F) Peripheral prostate calcification (left, coronal view; right, sagittal view).
RESULTS
1. Overall patient characteristics
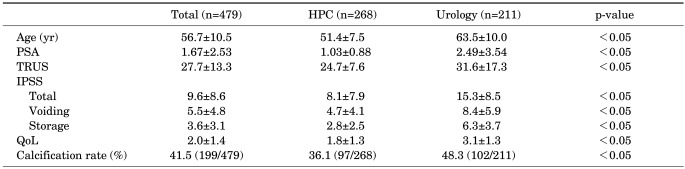
A total of 479 patients participated in this study. Of these, 268 healthy men visited the HPC at our hospital for regular prostate screenings, and 211 patients visited the Urology Outpatients Department at our hospital owing to lower urinary tract symptoms and received urological examinations. The average ages for the two groups were 51.4±7.5 and 63.5±10.0 years, respectively; thus, the average age of the group of patients who visited the Urology Outpatients Department was significantly higher (p<0.05). For the patients who visited the HPC and those who visited the Urology Outpatients Department, the overall IPSSs were 8.1±7.9 and 15.3±8.5, the voiding IPSSs were 4.7±4.1 and 8.4±5.9, and the storage IPSSs were 2.8±2.5 and 6.3±3.7 (p<0.05 for all), respectively, indicating that all IPSSs were significantly higher in the group of patients who visited the Urology Outpatients Department. The PSA levels were also significantly higher in the patients who visited the Urology Outpatients Department (1.03±0.88 vs. 2.49±3.54; p<0.05) (Table 1).
TABLE 1.
Initial patients characteristics and incidence of prostate calcifications
Values are presented as mean±SD.
HPC, health promotion center; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography; IPSS, International Prostate Symptom Score; QoL, quality of life.
2. Comparison of prostatic calcification rates and characteristics between the group visiting the hpc and the group visiting the urology outpatients department
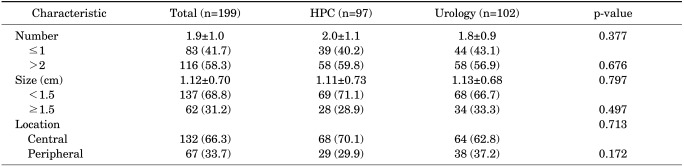
Of the 479 patients, prostatic calcifications were found in 199 (41.5%) by use of TRUS. Prostatic calcifications were found in 97 (36.1%) of the 268 men who visited the HPC for regular screenings; however, they were found in a significantly higher percentage of 102 (48.3%) of the 211 patients who visited the Urology Outpatients Department with lower urinary tract symptoms (p<0.05). A comparison of the numbers of prostatic calcifications did not reveal a significant difference (p=0.377), with 39 of 97 (40.2%) and 44 of 102 patients (43.1%) having one calcification. Moreover, there was no significant difference between the two groups even in the location of the calcification: 68 of 97 patients (69%) had a calcification in the central location, whereas 64 of 102 patients (62.7%) had a calcification in the peripheral location (p=0.172). In addition, no significant differences were observed regarding the size of the calcifications in the two groups (Table 2).
TABLE 2.
Characteristics of prostate calcifications in patients
HPC, Health Promotion Center.
DISCUSSION
Many authors have shown that prostatic calcifications are often found in men with benign prostatic hypertrophy or prostate cancer [1]. Nevertheless, whether such fortuitously discovered prostatic calcifications have clinical significance or whether they are relevant factors to urologic disease remains unclear [2]. Leader and Queen [6] claimed that a small prostatic calcification is not clinically significant and occurs physiologically during the aging process. In addition, Klimas et al. [7] also stated that prostatic calcification is formed as the corpora amylacea is calcified, which is one of the pathophysiological phenomena that occur during the aging process. To date, prostatic calcification has thus been accepted to occur as a matter of course during the aging process without resulting in specific symptoms [8].
However, the pathogenesis of prostatic calcification remains unclear. Some reports have indicated that prostatic calcification is associated with lower urinary tract symptoms. Kirby et al. [9] also stated that chronic prostatitis occurs due to the reflux of urine into the prostate, which can affect the formation of prostatic calcification. Geramoutsos et al. [1] reported that the majority of large calculi among the prostatic calcifications are associated with chronic prostatitis and lower urinary tract symptoms, whereas Shoskes et al. [10] claimed that prostatic calcification in patients with chronic pelvic pain syndrome is related to infection, bacterial colonization, and the duration of symptoms.
Based on these studies, we evaluated the differences in the frequency of prostatic calcifications between patients who did not complain of lower urinary tract symptoms and patients who did. As a result, prostatic calcification was found in 97 of 268 men (36.1%) who visited the HPC for a regular screening. Conversely, calcifications were found in 102 of 211 (48.3%) patients who visited the Urology Outpatients Department complaining of lower urinary tract symptoms (p<0.05). The HPC and Urology Outpatients Department groups also had differences in IPSSs, with overall IPSSs of 8.1±7.9 and 15.3±8.5, voiding IPSSs of 4.7±4.1 and 8.4±5.9, and storage IPSSs of 2.8±2.5 and 6.3±3.7 (p<0.05 for all), respectively. Based on these findings, the patient group that visited the HPC was characterized as not having lower urinary tract symptoms, whereas the group that visited the Urology Outpatients Department was characterized as having lower urinary tract symptoms. We used these group classifications to evaluate the correlation between lower urinary tract symptoms and prostatic calcifications.
This study had several limitations. Given that the group who visited the HPC did not visit because of urologic discomfort for the purpose of undergoing an examination, additional examinations, such as uroflowmetry and post-voiding residual urine sonogram and the use of a voiding diary, were not performed. In addition, the subject group was restricted because the prostate exam was available as an optional exam at the HPC. Despite these limitations, we were able to infer a correlation between lower urinary tract symptoms and prostatic calcifications through our findings. The lower urinary tract symptoms for the group visiting the HPC were mild, with an overall IPSS of 8.1±7.9. In addition, their voiding symptoms and storage symptoms were all significantly low. Despite these findings, their prostatic calcification rate was significantly low compared with that of the group who visited the Urology Outpatients Department, which exhibited moderate to severe lower urinary tract symptoms and had an overall IPSS of 15.3±8.5. Last, a restricted amount of data and the difference in the age distribution between the 2 groups may have led to the imbalance in the average ages of the 2 groups, which may have been a factor affecting lower urinary tract symptoms. We expect that the limitation above could be resolved by accumulating data in the long term from more patients.
Kim et al. [11] reported that the presence of central calcification in the prostate transition zone is associated with prognosis after prostatectomy, and Cha et al. [12] claimed that the presence of prostatic calcification in the prostate transition zone near the urethra can aggravate lower urinary tract symptoms. Furthermore, Park et al. [2] stated that prostatic calcification would probably affect the relaxation of the prostate, which would be an interfering factor during urination. Accordingly, we evaluated the differences in the characteristics of prostatic calcifications between our two groups. To compare our two groups according to location of the calcification as described by the aforementioned studies, we arbitrarily divided the location into central and peripheral locations, with 0.2 cm as the cutoff for peripheral classification.
Peeling and Griffiths [13] claimed that small-sized calculi are scattered according to the echogenicity of prostatic calcifications by ultrasound. They referred to calculi that were mainly spread across the prostate gland and in the prostatic cyst as "A-type." Kim et al. [14] commented that the A-type of prostatic calcifications do not affect lower urinary tract symptoms. The fact that there were also many incidences of multiple calculi found in our patients who visited our HPC and given that there were more differences in the size of the prostatic calcifications between the two groups when the calculi were less than 1.5 cm suggest that future studies on larger patient groups are necessary. Nevertheless, no significant differences were identified in this study between those with lower urinary tract symptoms and the distribution characteristics, sizes, or numbers of prostatic calcifications. Other limitations of this study are that there was a lack of references to aid in setting the criteria for measuring the size or distinguishing the location of calcifications when TRUS was performed and that errors resulted from performing ultrasound by use of unestablished criteria.
CONCLUSIONS
The frequency of prostatic calcifications identified through TRUS was higher in the group of patients who visited the Urology Outpatients Department with moderate to severe lower urinary tract symptoms than in the group who visited our HPC with no or mild lower urinary tract symptoms. However, when the characteristics of the calcification were compared, there were no significant differences in their numbers, sizes, or locations. This result leads us to believe that although prostatic calcifications can aggravate lower urinary tract symptoms, there are no differences in the numbers, sizes, or locations of these calcifications. Given that this is a field in which few studies have been conducted, additional studies with more patients are necessary. Moreover, establishing a precise definition of the locations and sizes of prostatic calcifications and using a more accurate method to measure calcification should be encouraged.
ACKNOWLEDGEMENTS
We acknowledge the financial support of the Healthy Medical Treatment Research and Development Program of the Ministry of Health & Welfare (No. A090481).
Footnotes
The authors have nothing to disclose.
References
- 1.Geramoutsos I, Gyftopoulos K, Perimenis P, Thanou V, Liagka D, Siamblis D, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur Urol. 2004;45:333–337. doi: 10.1016/j.eururo.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Park SW, Nam JK, Lee SD, Chung MK. Are prostatic calculi independent predictive factors of lower urinary tract symptoms? Asian J Androl. 2010;12:221–226. doi: 10.1038/aja.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox M. The natural history and significance of stone formation in the prostate gland. J Urol. 1963;89:716–727. doi: 10.1016/S0022-5347(17)64633-0. [DOI] [PubMed] [Google Scholar]
- 4.Sondergaard G, Vetner M, Christensen PO. Prostatic calculi. Acta Pathol Microbiol Immunol Scand A. 1987;95:141–145. doi: 10.1111/j.1699-0463.1987.tb00021_95a.x. [DOI] [PubMed] [Google Scholar]
- 5.Horio Y. On the morphological alteration of the prostate gland in aging. I. A histopathologic study of the prostate gland and the testis in aging and the correlation of their senile changes. Nihon Hinyokika Gakkai Zasshi. 1967;58:783–813. doi: 10.5980/jpnjurol1928.58.8_783. [DOI] [PubMed] [Google Scholar]
- 6.Leader AJ, Queen DM. Prostatic calculous disease. J Urol. 1958;80:142–146. doi: 10.1016/S0022-5347(17)66149-4. [DOI] [PubMed] [Google Scholar]
- 7.Klimas R, Bennett B, Gardner WA., Jr Prostatic calculi: a review. Prostate. 1985;7:91–96. doi: 10.1002/pros.2990070110. [DOI] [PubMed] [Google Scholar]
- 8.Ho KL, Segura JW. Lower urinary tract calculi. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders; 2007. pp. 2663–2673. [Google Scholar]
- 9.Kirby RS, Lowe D, Bultitude MI, Shuttleworth KE. Intra-prostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br J Urol. 1982;54:729–731. doi: 10.1111/j.1464-410x.1982.tb13635.x. [DOI] [PubMed] [Google Scholar]
- 10.Shoskes DA, Lee CT, Murphy D, Kefer J, Wood HM. Incidence and significance of prostatic stones in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2007;70:235–238. doi: 10.1016/j.urology.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Kim SG, Han WC, Jeong HJ, Rim JS. The influence of prostatic calcification and lymphocyte infiltration on the result of TURP in patients with benign prostatic hyperplasia. Korean J Urol. 2003;44:82–86. [Google Scholar]
- 12.Cha WH, Kim KH, Seo YJ. The effect of periurethral prostatic calculi on lower urinary tract symptoms in benign prostatic hyperplasia. Korean J Urol. 2008;49:237–241. [Google Scholar]
- 13.Peeling WB, Griffiths GJ. Imaging of the prostate by ultrasound. J Urol. 1984;132:217–224. doi: 10.1016/s0022-5347(17)49571-1. [DOI] [PubMed] [Google Scholar]
- 14.Kim WB, Doo SW, Yang WJ, Song YS. Influence of prostatic calculi on lower urinary tract symptoms in middle-aged men. Urology. 2011;78:447–449. doi: 10.1016/j.urology.2010.12.056. [DOI] [PubMed] [Google Scholar]