Abstract
Purpose
The pathogenesis of lower urinary tract symptoms (LUTS) is uncertain. We investigated the potential role of inflammation in the development of LUTS, with the use of high-sensitivity C-reactive protein (hsCRP) as an inflammatory marker, in a population-based study of aging men in Korea.
Materials and Methods
Our study used a multistage stratified design to recruit a random sample of 1,510 men aged 45 years or older in Chuncheon, Korea, in 2003. Men with urologic or neurologic diseases that could cause voiding dysfunction were excluded. Also, men with medical conditions that could affect inflammation, such as infection or the use of nonsteroidal anti-inflammatory drugs, were excluded. LUTS were defined according to the International Prostate Symptom Score (IPSS). Various potential confounding factors were included in the analyses.
Results
A total of 330 subjects were included in the final analyses. There were 155 (47.0%) with an IPSS<8 and 175 (53%) with an IPSS≥8. The mean age of all subjects was 69.2±8.4 years. The mean hsCRP level of all subjects was 2.30±3.27 (median, 1.19) mg/l. The hsCRP levels in subjects with an IPSS≥8 differed significantly from those in subjects with an IPSS<8. Also, IPSS, storage symptom, voiding symptom, and quality of life (QoL) scores increased as hsCRP levels increased, respectively. The hsCRP level remained an independent risk factor of LUTS (IPSS≥8, storage symptom score≥4, incomplete voiding, intermittency, and QoL) after adjustment for variable possible confounding factors.
Conclusions
Our results suggest that inflammatory processes may play an important role in the pathogenesis of LUTS and that hsCRP levels may indicate the severity of LUTS in aging men.
Keywords: C-reactive protein, Inflammation, Lower urinary tract symptoms
INTRODUCTION
Lower urinary tract symptoms (LUTS), which are divided into storage, voiding, and postvoiding symptoms, can be caused by various pathologic conditions. The etiologies of LUTS are multiple, and the role of inflammation in the development of LUTS has been less well studied [1].
Many studies suggest that prostatic inflammation may play an important role in histologic benign prostatic hyperplasia (BPH) [1-4]. Nevertheless, because LUTS are not significantly associated with BPH [5-8] and may develop in the absence of histologic BPH, prostatic inflammation may not directly affect the development of LUTS. However, there still remains a possibility that inflammation in other urinary organs, such as the bladder, affects the development of LUTS.
C-reactive protein (CRP) is one of the most extensively studied nonspecific markers of systemic inflammation and is a well-established risk factor in coronary events [9]. CRP is regarded as a reliable tool for study on inflammation, although the distribution of CRP levels varies among different races [10]. Recently, high-sensitivity CRP (hsCRP) was introduced, and has more accurate value than conventional CRP in the general population [11].
To the best of our knowledge, there are only four epidemiologic studies of inflammation and LUTS with the use of CRP as an inflammatory marker [12-15]. Furthermore, most of those studies were conducted in the West. Hence, we investigated the potential role of inflammation in the development of LUTS, by use of hsCRP, in a population-based, cross-sectional study of aging men in Korea.
MATERIALS AND METHODS
1. Study population
In the Hallym Aging Study on the quality of life (QoL) in elderly Korean people, a cohort was composed of 2,519 people aged 45 years or older who were randomly selected in Seoul and Chuncheon, large cities of Korea. Of these, the Chuncheon-based cohort was considered in the current study. The selection of the study populations can be summarized as follows [16]:
Of the 1,408 study sectors, which were classified on the basis of the 2,000 population census, 200 were randomly selected. In accordance with the proportion of the population aged 45 years or older from the individual Korean neighborhood classifications, the number needed for the study population was determined. On the basis of a list of study populations that were selected for the current study, study participants were systematically sampled. At this time, the number of study populations was determined in such a manner that 30% of the study population should be aged between 45 and 64 years and 70% should be aged 65 years or older with consideration of the effects of a long-term follow-up study as well as the calculation of stable epidemiological parameters. Of these, 1,510 people who responded to the primary panel survey underwent a second in-depth study. The survey was completed within a relatively short period, so as to minimize variations in collected data. All participants provided written informed consent and the study received institutional review board approval.
Of these people, subjects with urologic or neurologic diseases that could cause voiding dysfunction, except for BPH, were excluded. Those with medical conditions that could affect inflammation, such as infection or the use of non-steroidal anti-inflammatory drugs, were also excluded. A total of 330 male subjects aged 45 years and older were finally enrolled in the current study.
2. Assessment of LUTS
LUTS were measured by use of the International Prostate Symptom Score (IPSS), a clinically validated 7-item questionnaire. QoL was also measured.
The prior 7 items had an ordered answer (from 0 to 5). The IPSS was used as both a continuous and a categorical variable in 2 groups with no or mild symptoms (IPSS<8) vs. moderate or severe symptoms (IPSS≥8). Using a similar approach, the storage symptom and voiding symptom scores were used both as continuous and categorical variables. The storage and voiding symptom scores were categorized into 2 groups as no or mild symptoms (<4 and <5, respectively) vs. moderate or severe symptoms (≥4 and ≥5, respectively).
The individual symptom scores were categorized as no or mild symptoms (<3) vs. moderate or severe symptoms (≥3). The QoL score concerning the satisfaction of the respondent and had an ordered answer (from 0 to 6) and was categorized as <4 vs. ≥4.
3. Exposure assessment
We analyzed age, body mass index (BMI), hypertension, diabetes mellitus, dyslipidemia, depression, smoking, alcohol drinking, exercise, and hsCRP as variables. All data were obtained by trained interviewers or physicians. Anthropometric measurements and laboratory tests were performed by trained physicians by use of a standardized protocol in our institution.
Serum CRP was measured with the hsCRP, fully automated particle-enhanced nephelometric immunoassay (Hitachi 7600 analyzer; Hitachi Ltd, Tokyo, Japan). The lower limit of detection was 0.01 mg/l and the interassay coefficient of variation was below 5%.
4. Statistical analysis
To compare the distributions of variables between no or mild symptoms and moderate or severe symptoms, Student's t-test and chi-square test were used in the comparison of continuous and categorical variables, respectively. Simple regression analysis was used to assess the linear association between hsCRP and LUTS. Then, hsCRP levels were categorized as 4 groups according to quartiles. Logistic regression models were used to assess the association between hsCRP and LUTS and to adjust for potential confounding factors. IBM SPSS ver. 18.0 (IBM Co., New York, NY, USA) was used for all statistical assessment. All p-values were two-sided, and a p<0.05 was considered significant.
RESULTS
Of the 330 subjects, there were 155 (47.0%) with no or mild symptoms and 175 (53%) with moderate or severe symptoms. The mean age of all subjects was 69.2±8.4 years. The mean serum CRP level of all subjects was 2.30±3.27 mg/l (median, 1.19 mg/l). When hsCRP levels were categorized according to quartiles, the first, second, third, and fourth hsCRP quartiles were ≤0.68 mg/l (Q1), 0.68<hsCRP≤1.19 mg/l (Q2), 1.19<hsCRP≤2.26 mg/l (Q3), and >2.26 mg/l (Q4).
The characteristics of the subjects are shown in Table 1. Subjects with moderate or severe symptoms differed significantly from those with no or mild symptoms on several parameters. Subjects with moderate or severe symptoms tended to be older (p<0.001), to have more dyslipidemia (p=0.011), to have a greater current smoking ratio (p=0.007), and to drink more alcohol (p=0.015). The mean hsCRP level was higher in persons with moderate or severe symptoms than in those with no or mild symptoms (2.91 vs. 1.61 mg/l, p<0.001). When subjects were stratified according to quartiles of hsCRP, there were also significant differences (18.3% vs. 32.3% in Q1, p=0.005; 31.4% vs. 17.4% in Q4, p=0.003).
TABLE 1.
Association of different variables with the severity of LUTS
Values are presented as mean±SD or number (%).
LUTS, lower urinary tract symptoms; IPSS, International Prostate Symptom Score; BMI, body mass index; hsCRP, high sensitivity c-reactive protein; Q1, first hsCRP quartile (hsCRP≤0.68 mg/l); Q2, second hsCRP quartile (0.68<hsCRP≤1.19 mg/l); Q3, third hsCRP quartile (1.19<hsCRP≤2.26 mg/l); Q4, fourth hsCRP quartile (hsCRP>2.26 mg/l).
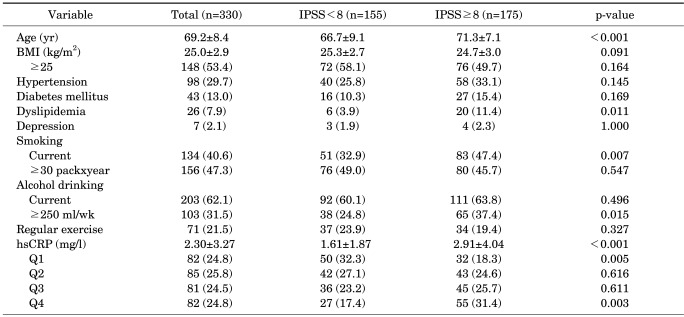
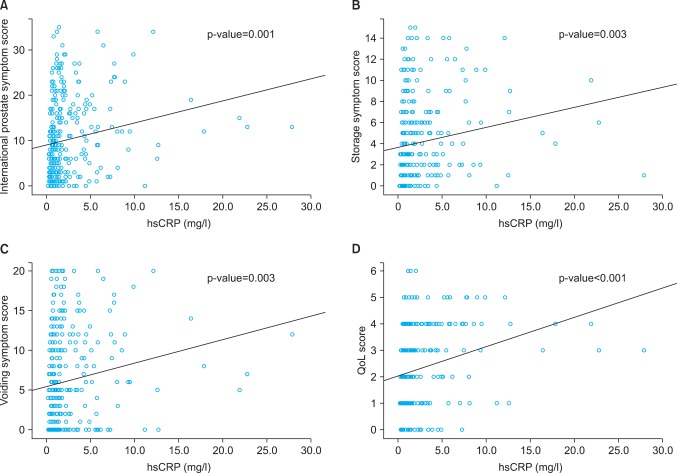
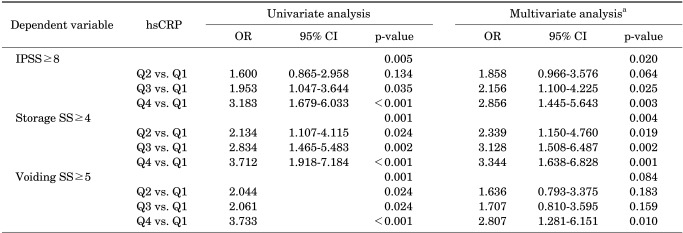
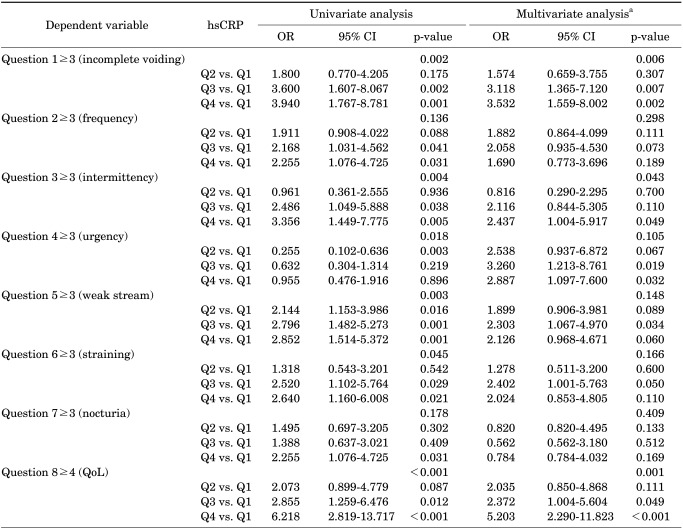
In linear association model analyses between hsCRP level and LUTS, IPSS increased as hsCRP levels increased (p=0.001) (Fig. 1A). Storage and voiding symptom scores and QoL scores also increased as hsCRP levels increased, respectively (p=0.003, 0.003, and <0.001) (Fig. 1B-D).
FIG. 1.
Linear association model analyses between high sensitivity c-reactive protein (hsCRP) level and International Prostate Symptoms Scores (A), storage symptom scores (B), voiding symptom scores (C), and quality of life (QoL) scores (D). As hsCRP levels increased, the symptoms scores increased.
We performed multivariate logistic regression analyses that were adjusted for age, BMI, hypertension, diabetes mellitus, dyslipidemia, depression, smoking, alcohol drinking, and regular exercise. When subjects were stratified according to IPSS≥8 and <8, quartiles of hsCRP level were an independent risk factor (p=0.020) (Table 2). The subjects in Q4 had an OR of 2.856 (p=0.003) (Table 2) compared with those in Q1. Also, when the subjects were stratified according to storage symptom score of ≥4 and <4, those in Q4 had an OR of 3.344 (p=0.001) (Table 2) compared with those in Q1. However, when the subjects were stratified according to voiding symptom score of ≥5 and <5, the hsCRP level did not remain an independent risk factor after multivariate adjustment (p=0.084) (Table 2).
TABLE 2.
Univariate and multivariate logistic regression analyses on predictors of the severity of LUTS
LUTS, lower urinary tract symptoms; hsCRP, high sensitivity c-reactive protein; OR, odds ratio; CI, confidence interval; IPSS, International Prostate Symptom Score Storage; SS, storage symptom score; Voiding SS, voiding symptom score; Q1, first hsCRP quartile (hsCRP≤0.68 mg/l); Q2, second hsCRP quartile (0.68<hsCRP≤1.19 mg/l); Q3, third hsCRP quartile (1.19<hsCRP≤2.26 mg/l); Q4, fourth hsCRP quartile (hsCRP>2.26 mg/l).
a: Adjusted for age, body mass index, hypertension, diabetes mellitus, dyslipidemia, depression, smoking, alcohol drinking, and regular exercise.
In the multivariate logistic analyses of the individual symptoms, hsCRP level was an independent risk factor of incomplete voiding (p=0.006), intermittency (p=0.043), and QoL (p=0.001) (Table 3). The risk of incomplete voiding (odds ratio [OR], 3.532; p=0.002), intermittency (OR, 2.437; p=0.049), and QoL (OR, 5.203; p<0.001) increased in Q4 compared with Q1.
TABLE 3.
Univariate and multivariate logistic regression analyses on predictors of the individual symptoms of the IPSS
IPSS, International Prostate Symptom Score; hsCRP, high sensitivity c-reactive protein; OR, odds ratio; CI, confidence interval; QoL, quality of life; Q1, first hsCRP quartile (hsCRP≤0.68 mg/l); Q2, second hsCRP quartile (0.68<hsCRP≤1.19 mg/l); Q3, third hsCRP quartile (1.19<hsCRP≤2.26 mg/l); Q4, fourth hsCRP quartile (hsCRP>2.26 mg/l).
a: Adjusted for age, body mass index, hypertension, diabetes mellitus, dyslipidemia, depression, smoking, alcohol drinking, and regular exercise.
DISCUSSION
Many studies have suggested that prostatic inflammation may have a prominent role in BPH, although the pathogenesis of BPH is still uncertain [1-4]. Regardless of its cause, the end effect of inflammation is an atypical cytokine-rich milieu that can lead to alterations in the microenvironment and chronic, repetitive wound healing ending in the development of BPH nodules [17]. Because most men with BPH have LUTS, LUTS and BPH remain intertwined in the treatment and study of urinary disorders in aging men [18]. Nevertheless, because LUTS are not significantly associated with BPH [5-8], inflammation may not directly affect the development of LUTS. However, there remains a possibility that inflammation affects the development of LUTS. It is possible that inflammation in the bladder might relate to an effect on bladder function, which might result from reduced bladder function, rather than increased outlet resistance [19]. This hypothesis is supported by investigations including women that had suggested a potential role of inflammation in OAB [20-22]. Another possibility is that the inflammation might affect the neuronal pathways involved in the innervation of the urinary tract, which could account for the increased storage voiding symptoms [23].
Our study showed that hsCRP level, a nonspecific inflammatory marker, was significantly associated with LUTS. The hsCRP levels in subjects with moderate or severe symptoms differed significantly from those in subjects with no or mild symptoms. IPSS, storage symptoms, voiding symptoms, and QoL scores also increased as hsCRP levels increased, respectively. The hsCRP level remained an independent risk factor of LUTS after adjustment for various confounding factors.
To the best of our knowledge, there are only four epidemiologic studies of inflammation and LUTS that used CRP [12-15]. Rohrmann et al. [12] first investigated the association between CRP and LUTS by using data from the third National Health and Nutrition Examination Survey (NHANES III). They did not find a significant association between CRP and LUTS. However, the NHANES III study had several weak points. Because LUTS were defined as only 4 urinary symptoms (incomplete voiding, weak stream, hesitancy, and nocturia), the analyses did not reflect LUTS exactly. Conventional CRP was also used instead of hsCRP in the analyses. Although conventional CRP levels have very high values associated with acute inflammation, chronic inflammation can result in near normal values and can produce clinically meaningless results. On the other hand, the clinical utility of the hsCRP level is significantly higher than that of conventional CRP in the general population, because the hsCRP test can measure lower and more exact values compared with the conventional CRP test [9]. In practice, numerous studies have consistently shown that hsCRP levels, not conventional CRP levels, provide a strong and independent indication of risk for cardiovascular diseases [11]. In this point of view, we used the complete IPSS including the satisfaction score and the hsCRP test measured to two decimal places.
Two reports were recently published in which CRP levels were significantly associated with LUTS in the United States [13,14]. One report from the Boston Area Community Health survey showed that CRP levels were associated with LUTS in men and suggested that the dose-response relationship between increased CRP levels and an increased odds of LUTS supported the hypothesized role of inflammatory processes in the etiology of LUTS [13]. The other report from the Olmsted County study showed that CRP levels were not associated with change in prostate volume but were associated with rapid increases in irritative LUTS, suggesting that inflammatory processes may play a role in the development of these outcomes [14]. The results from the above 2 studies were very similar to our results. Our study demonstrated that the IPSS, storage symptom scores, voiding symptom scores, and QoL scores increased as hsCRP levels increased, respectively. The hsCRP levels were also significantly associated with overall LUTS and storage symptoms after adjustment for various potential confounding factors. Note that those 2 studies were performed in the West. Several studies have suggested that significant race and gender differences exist in the population distribution of CRP levels [10,24]. In this respect, our study showed that CRP levels were significantly associated with LUTS, which suggests that inflammatory processes may play a role in the pathogenesis of LUTS in Korea as well as in westerners. Accordingly, our study is worthy of notice because it is the first such investigation in Korea.
More recently, Lu et al. [15] published a study to investigate the correlation between hsCRP levels and LUTS by using data from the Fangchenggang Area Male Health and Examination Survey (FAMHES) in China. They found that hsCRP levels were associated with overall LUTS, irritative symptoms, urgency, and nocturia, suggesting a positive correlation of hsCRP with LUTS. The results from the FAMHES study were also similar to those of our study. However, it should be noted that there was a distinct difference in age distribution between our study (mean, 69.2±8.4 years) and the FAMHES study (mean, 36.6±10.7 years), because age is one of the most well-known risk factors in the development of LUTS. Despite the difference in age distribution, hsCRP levels were still significantly associated with LUTS, which suggests that inflammatory processes may play a role in the pathogenesis of LUTS in aging men.
In our study, it was interesting that hsCRP levels were significantly associated with storage symptoms, not voiding symptoms, because LUTS in aging men are generally most likely related to BPH and present as voiding symptoms. Our results do not discount the possibility that prostatic inflammation is associated with histologic BPH. However, because LUTS are not significantly associated with BPH [5-8] and may develop in the absence of histologic BPH, our results suggest that the inflammatory process in LUTS might be more related to an effect on bladder function than on the prostate.
From the viewpoint of individual LUTS, hsCRP levels were significantly associated with incomplete voiding, intermittency, and QoL in our study. We first demonstrated the significant association between hsCRP levels and QoL. Because LUTS represent a cluster of chronic urinary disorders without identification of organ-specific or disease-specific causes, it is important for the diagnosis and treatment of LUTS that the degree of subjective QoL be defined, as well as the severity of LUTS. It may be a clue to the availability of the hsCRP test in real practice.
The results for individual symptoms in previous studies including our study were slightly different. Although the exact reasons for these differences are unclear, several explanations are possible. First, previous studies including our study did not determine all possible risk factors for LUTS. Also, the distribution of hsCRP levels varies among different races [10,24]. Also, because the categorical range of individual symptom scores (0 to 5) is relatively smaller than that of the overall IPSS (0 to 35), storage symptom score (0 to 15), and voiding symptom score (0 to 20), individual symptoms could be easily influenced by confounding factors.
There were a few limitations to our study. First, our study was limited geographically to the local area and had a relatively small population. However, our study had a systemically randomized multistage stratified design to reduce the bias. Second, our study did not determine all possible risk factors for LUTS. However, analyses from our study considered similar or more confounding factors than in previous studies. Finally, because our study was cross-sectional in design, we could not confirm whether inflammation was a risk factor for LUTS or not.
CONCLUSIONS
Our study demonstrated that hsCRP levels were significantly associated with overall LUTS, storage symptoms, incomplete voiding, intermittency, and QoL. Our results suggest that the inflammatory process may play an important role in the pathogenesis of LUTS and that hsCRP levels may indicate the severity of LUTS in aging men.
ACKNOWLEDGEMENTS
This research was supported by the Hallym University Research Fund, 2002 (HRF-2004-49).
Footnotes
The authors have nothing to disclose.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Di Silverio F, Gentile V, De Matteis A, Mariotti G, Giuseppe V, Luigi PA, et al. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43:164–175. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 3.Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra VC, Allen DJ, Nicolaou C, Sharif H, Hudd C, Karim OM, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100:327–331. doi: 10.1111/j.1464-410X.2007.06910.x. [DOI] [PubMed] [Google Scholar]
- 5.St Sauver JL, Jacobson DJ, Girman CJ, Lieber MM, McGree ME, Jacobsen SJ. Tracking of longitudinal changes in measures of benign prostatic hyperplasia in a population based cohort. J Urol. 2006;175(3 Pt 1):1018–1022. doi: 10.1016/S0022-5347(05)00408-8. [DOI] [PubMed] [Google Scholar]
- 6.Girman CJ, Jacobsen SJ, Guess HA, Oesterling JE, Chute CG, Panser LA, et al. Natural history of prostatism: relationship among symptoms, prostate volume and peak urinary flow rate. J Urol. 1995;153:1510–1515. doi: 10.1016/s0022-5347(01)67448-2. [DOI] [PubMed] [Google Scholar]
- 7.Bosch JL, Hop WC, Niemer AQ, Bangma CH, Kirkels WJ, Schroder FH. Parameters of prostate volume and shape in a community based population of men 55 to 74 years old. J Urol. 1994;152(5 Pt 1):1501–1505. doi: 10.1016/s0022-5347(17)32456-4. [DOI] [PubMed] [Google Scholar]
- 8.Bosch JL, Bangma CH, Groeneveld FP, Bohnen AM. The long-term relationship between a real change in prostate volume and a significant change in lower urinary tract symptom severity in population-based men: the Krimpen study. Eur Urol. 2008;53:819–825. doi: 10.1016/j.eururo.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 10.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Rifai N, Ridker PM. Inflammatory markers and coronary heart disease. Curr Opin Lipidol. 2002;13:383–389. doi: 10.1097/00041433-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rohrmann S, De Marzo AM, Smit E, Giovannucci E, Platz EA. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III) Prostate. 2005;62:27–33. doi: 10.1002/pros.20110. [DOI] [PubMed] [Google Scholar]
- 13.Kupelian V, McVary KT, Barry MJ, Link CL, Rosen RC, Aiyer LP, et al. Association of C-reactive protein and lower urinary tract symptoms in men and women: results from Boston Area Community Health survey. Urology. 2009;73:950–957. doi: 10.1016/j.urology.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Sauver JL, Sarma AV, Jacobson DJ, McGree ME, Lieber MM, Girman CJ, et al. Associations between C-reactive protein and benign prostatic hyperplasia/lower urinary tract symptom outcomes in a population-based cohort. Am J Epidemiol. 2009;169:1281–1290. doi: 10.1093/aje/kwp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Z, Gao Y, Tan A, Yang X, Zhang H, Mo L, et al. Increased high-sensitivity C-reactive protein predicts a high risk of lower urinary tract symptoms in Chinese male: Results from the Fangchenggang Area Male Health and Examination Survey. Prostate. 2012;72:193–200. doi: 10.1002/pros.21421. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Jeong JY, Choi YJ, Kim DH, Lee WK, Lee SH, et al. Association between lower urinary tract symptoms and vascular risk factors in aging men: The Hallym Aging Study. Korean J Urol. 2010;51:477–482. doi: 10.4111/kju.2010.51.7.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol. 2006;16:25–29. [PubMed] [Google Scholar]
- 18.Parsons JK. Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Curr Opin Urol. 2011;21:1–4. doi: 10.1097/MOU.0b013e32834100c9. [DOI] [PubMed] [Google Scholar]
- 19.Pinggera GM, Mitterberger M, Steiner E, Pallwein L, Frauscher F, Aigner F, et al. Association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: assessment using colour Doppler ultrasonography. BJU Int. 2008;102:470–474. doi: 10.1111/j.1464-410X.2008.07587.x. [DOI] [PubMed] [Google Scholar]
- 20.Tyagi P, Barclay D, Zamora R, Yoshimura N, Peters K, Vodovotz Y, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010;42:629–635. doi: 10.1007/s11255-009-9647-5. [DOI] [PubMed] [Google Scholar]
- 21.Comperat E, Reitz A, Delcourt A, Capron F, Denys P, Chartier-Kastler E. Histologic features in the urinary bladder wall affected from neurogenic overactivity--a comparison of inflammation, oedema and fibrosis with and without injection of botulinum toxin type A. Eur Urol. 2006;50:1058–1064. doi: 10.1016/j.eururo.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Apostolidis A, Dasgupta P, Denys P, Elneil S, Fowler CJ, Giannantoni A, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55:100–119. doi: 10.1016/j.eururo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Aronson WJ. Invited commentary: Lower urinary tract symptoms and inflammation--weighing the evidence. Am J Epidemiol. 2009;169:1291–1293. doi: 10.1093/aje/kwp084. [DOI] [PubMed] [Google Scholar]
- 24.Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]