Abstract
The novel centrally acting analgesic tapentadol [(−)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride] combines two mechanisms of action, μ-opioid receptor (MOR) agonism and noradrenaline reuptake inhibition (NRI), in a single molecule. Pharmacological antagonism studies have demonstrated that both mechanisms of action contribute to the analgesic effects of tapentadol. This study was designed to investigate the nature of the interaction of the two mechanisms. Dose-response curves were generated in rats for tapentadol alone or in combination with the opioid antagonist naloxone or the α2-adrenoceptor antagonist yohimbine. Two different pain models were used: 1) low-intensity tail-flick and 2) spinal nerve ligation. In each model, we obtained dose-effect relations to reveal the effect of tapentadol based on MOR agonism, NRI, and unblocked tapentadol. Receptor fractional occupation was determined from tapentadol's brain concentration and its dissociation constant for each binding site. Tapentadol produced dose-dependent analgesic effects in both pain models, and its dose-effect curves were shifted to the right by both antagonists, thereby providing data to distinguish between MOR agonism and NRI. Both isobolographic analysis of occupation-effect data and a theoretically equivalent methodology determining interactions from the effect scale demonstrated very pronounced synergistic interaction between the two mechanisms of action of tapentadol. This may explain why tapentadol is only 2- to 3-fold less potent than morphine across a variety of preclinical pain models despite its 50-fold lower affinity for the MOR. This is probably the first demonstration of a synergistic interaction between the occupied receptors for a single compound with two mechanisms of action.
Introduction
Monoamine reuptake inhibitors (tricyclics, nontricyclic serotonin-noradrenaline reuptake inhibitors) are among the first-line treatment options for chronic pain. These drugs are generally tolerated relatively well. However, analgesic efficacy of such drugs is often not satisfactory (Fishbain, 2000). Opioids also play an important role in the treatment of chronic pain and can produce potent analgesia (Kalso et al., 2004). However, opioids are often faced with tolerability problems. In particular, gastrointestinal side effects such as nausea, vomiting, and constipation can be troublesome with opioid treatment (Moore and McQuay, 2005).
Tapentadol[(−)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride] is a novel, centrally acting analgesic combining two mechanisms of action, μ-opioid receptor (MOR) agonism and noradrenaline reuptake inhibition (NRI), in a single molecule (Tzschentke et al., 2006, 2007, 2009). The rationale behind this combination is that beyond the fact that both mechanisms of action can produce analgesia in their own right they also interact synergistically at the spinal and supraspinal level. The NRI component can produce an “opioid-sparing effect,” such that a moderate MOR-agonistic activity is sufficient (in concert with the moderate NRI activity) to produce potent analgesia, thus reducing opioid-induced side effects. Indeed, despite a 50-fold lower affinity for the MOR, tapentadol is only 2- to 3-fold less potent than morphine across a variety of preclinical pain models (Tzschentke et al., 2006), clearly indicating that 1) the NRI component of tapentadol contributes to its analgesic effect, and 2) it does so in a supra-additive/synergistic manner. Consistent with the rationale above, tapentadol has demonstrated potent analgesia in acute and chronic pain along with a substantially improved gastrointestinal side-effect profile in clinical studies (Hale et al., 2009; Hartrick et al., 2009; Hartrick, 2009).
There is preclinical and clinical evidence that opioid analgesia can indeed be augmented by noradrenergic compounds. For example, MOR agonists and noradrenaline reuptake inhibitors or α2-adrenoceptor agonists additively or synergistically produced analgesia after systemic or intrathecal administration in models of acute and chronic pain. The noradrenaline reuptake inhibitor desipramine increased morphine analgesia after systemic and intrathecal administration (Ossipov et al., 1982; Reimann et al., 1999), and systemic as well as spinal combination of morphine with the α2-adrenoceptor agonist clonidine resulted in synergistic antinociception (Ossipov et al., 1990; Fairbanks and Wilcox, 1999). In clinical settings, morphine analgesia was potentiated by systemic tricyclic antidepressants (Levine et al., 1986; Ventafridda et al., 1990) and spinal/epidural clonidine (Motsch et al., 1990; Anzai and Nishikawa, 1995).
Isobolographic analysis is a method traditionally used to establish whether an interaction of two agonist drugs is subadditive, additive, or supra-additive/synergistic. In such applications the individual compounds are administered in graded doses by themselves and subsequently in dose combinations that are often fixed-ratio combinations of the two compounds (Tallarida, 2001, 2006, 2007; Tallarida et al., 2003; Tallarida and Raffa, 2010). In the present case, we were concerned with a single compound whose effect is mediated through two distinct mechanisms. The analysis used therefore consisted of a two-pronged approach: 1) a comparison of the observed and (calculated) additive effect magnitudes and 2) the use of isoboles based on the fractional occupancy of each binding site. Pharmacological antagonism studies have shown that both mechanisms of action of tapentadol (MOR, NRI) contribute to its analgesic effect (Tzschentke et al., 2006, 2007; Schröder et al., 2010). Accordingly, receptor-specific antagonists were used to distinguish between the effects mediated by each component in relation to dose. Toward that end, dose-response curves were generated in the low-intensity tail-flick model of acute nociception and the spinal nerve ligation (SNL) model of chronic mononeuropathic pain in rats for tapentadol alone, tapentadol in combination with the MOR antagonist naloxone, and tapentadol in combination with the α2-adrenoceptor antagonist yohimbine. The data from these experiments have been published in part previously in a different form and context (Schröder et al., 2010). In additional experiments, for purposes of converting tapentadol doses to receptor occupation, brain concentrations of tapentadol associated with each intraperitoneal dose were determined in satellite groups of rats at the same time point as the analgesic effect was quantified in the low-intensity tail-flick test. This allowed a correlation of tapentadol brain concentrations with a given analgesic effect that was caused by both mechanisms, exclusively because of MOR agonism (under yohimbine antagonism) or exclusively because of NRI (under naloxone antagonism).
Materials and Methods
Behavioral Testing
Animals.
Male Sprague-Dawley rats (Janvier, Le Genest St Isle, France) were housed under a 12:12-h light-dark cycle (lights on at 6:00 AM), room temperature 20 to 24°C, relative air humidity 35 to 70%, 15 air changes per hour, and air movement less than 0.2 m/s. The animals had free access to standard laboratory food and tap water. There were at least 5 days between the delivery of the animals and behavioral testing. Average weights were 160 to 240 g. All experiments were conducted according to the Declaration of Helsinki and the guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and the German Animal Welfare Law.
Experimental Procedures
Animals were assigned randomly to treatment groups. Different doses and vehicle were tested in a randomized fashion. Although the operators performing the behavioral tests were not formally “blinded” with respect to the treatment, they were not aware of the study hypothesis or the nature of the differences between drugs.
Low-Intensity Tail-Flick Test.
The tail-flick test was carried out in rats using a modification of the method described by D'Amour and Smith (1941). The tail-flick latency, defined by the time (in seconds) to withdraw the tail from a radiant heat source, was measured using a semiautomated device (Rhema Labortechnik, Hofheim, Germany). The rat was placed in a Plexiglas restrainer, and a low-intensity radiant heat beam was focused onto the dorsal surface of the tail root. The stimulus intensity was adjusted to result in a mean predrug control latency of 7 s, thus also allowing a supraspinal modulation of the spinally mediated acute nociceptive reflex. A cutoff time of 30 s was applied to avoid tissue damage. The increase in tail-flick latency was defined as antinociception and calculated as the percentage of maximal possible effect (MPE) according to the following formula: MPE [%] = (tl − tc)/(tcutoff − tc) × 100%, where tl is withdrawal latency, tc is control latency, and tcutoff is cutoff time. Animals were tested before and 30 min after intravenous administration of tapentadol or vehicle. Naloxone (1 mg/kg) and yohimbine (2.15 mg/kg) or the respective vehicle was given intraperitoneally 10 min before tapentadol.
In additional experiments focusing to correlate receptor occupancy with analgesic effects, animals were tested before and 10, 20, and 30 min after intraperitoneal administration of tapentadol or vehicle. The antagonist naloxone (1 mg/kg), yohimbine (4.64 mg/kg), or the respective vehicle was given intraperitoneally 10 min before tapentadol.
In the first set of experiments involving intravenous tapentadol administration, the minimal yohimbine dose (2.15 mg/kg i.p.) that produced complete antagonism of a maximally effective dose of the reference agonist reboxetine in both the low-intensity tail-flick test and the SNL model was used to compare the relative contribution of the NRI component to the effect of tapentadol in these two models (Schröder et al., 2010). In the second set of experiments involving intraperitoneal tapentadol administration in the low-intensity tail-flick test, the dose of yohimbine was further increased to a maximum of 4.64 mg/kg i.p. that did not produce confounding effects (i.e., caused by behavioral side effects) to ensure complete antagonism of the NRI component of tapentadol.
Drugs.
Tapentadol (Grünenthal GmbH, Aachen, Germany) and naloxone and yohimbine (Sigma Chemie, Deisenhofen, Germany) were dissolved in saline (0.9% NaCl). The volume of administration was 5 ml/kg. The antagonists naloxone and yohimbine or the respective vehicle were given intraperitoneally 5 min (SNL) or 10 min (low-intensity tail-flick) before intravenous or intraperitoneal tapentadol treatment. Because mechanical hypersensitivity testing in the SNL model lasted approximately 5 min, whereas testing in the tail-flick assay was performed instantaneously, the administration time point of the antagonists relative to the agonists was adapted accordingly in the SNL model. Antagonist drug doses were carefully chosen to reach full antagonism of a maximally active dose of respective reference agonists (morphine, reboxetine) without confounding analgesic effect or behavioral side effects (Schröder et al., 2010). All doses refer to the respective salt form as indicated above.
Data Analysis.
Data were analyzed by means of a one- or two-factor analysis of variance with or without repeated measures, depending on the experimental design, with a post hoc Bonferroni test. Significance of treatment, time, or treatment × time interaction effects was analyzed by means of Wilks Λ statistics. In case of a significant treatment effect, pairwise comparison was performed at the time of maximal effect by the Fisher least significant difference test. Results were considered statistically significant if P < 0.05. Median effective dose (ED50) values and 95% confidence intervals (CIs) were calculated by linear regression using the percentage of MPE values obtained at 10 min after intraperitoneal tapentadol administration. Median effective dose values with nonoverlapping 95% CIs were considered to be significantly different. Each group included 10 rats.
Determination of Plasma and Brain Concentrations
Six satellite groups of Sprague-Dawley rats (five animals per dose group) were dosed with tapentadol in the same way as in the low-intensity tail-flick experiment. Intraperitoneal doses of 1, 4.64, 10, 21.5, 46.4, and 68.1 mg/kg were administered; the highest two doses were given 10 min after prior administration of naloxone (1 mg/kg i.p.), whereas the other doses of tapentadol were preceded by intraperitoneal saline instead. Blood was collected from the orbital plexus under isoflurane anesthesia 10 min after intraperitoneal tapentadol administration, and samples were immediately transferred to ammonium heparin tubes. Immediately after blood sampling the rats were decapitated and the brains were removed from the skull. After washing with 0.9% NaCl, the brains were swabbed dry with cellulose pulp, weighed, and homogenized in 5 ml of 100 mM potassium phosphate, pH 7.4 using a Pro 200 hand-held homogenizer (Harvard Apparatus Inc., Holliston, MA).
Ammonia [25 μl, 25% (w/v)], 25 μl of internal standard (1 μM), and 500 μl of tert-butyl-methyl ether were added to 50-μl aliquots of the tissue homogenate or plasma. Liquid-liquid extraction was performed by shaking robustly for 20 min at room temperature (Vibrax VXR basic, IKA-Werke GmbH & Co. KG, Staufen, Germany). Then the samples were centrifuged (10 min, 4°C, 16,000 RCF rating), the ether phase was transferred into an autosampler vial and dried under a stream of nitrogen. Samples were reconstituted in 125 μl of 50% acetonitrile + 0.1% formic acid, 50% H2O + 0.1% formic acid.
Aliquots of 25 μl of the extract were analyzed by liquid chromatography/tandem mass spectrometry. Chromatography was performed on an AQUA 3 μ C18 125A (2 × 75 mm) column (Phenomenex, Torrance, CA) operated at 55°C. The mobile phase was a gradient using 0.5% acetic acid in water and 0.5% acetic acid in methanol at a flow rate of 0.5 ml/min. Detection was by tandem mass spectrometry (API-3000; Applied Biosystems, Foster City, CA) equipped with TurboIon Spray operated in the positive mode. Analytes and internal standards were monitored at mass transitions m/z 222.2 to 107.0 and 228.2 to 109.0 for tapentadol and its deuterium-labeled internal standard, respectively. Calibration and quality-control samples were prepared in rat plasma.
Theory
Isoboles.
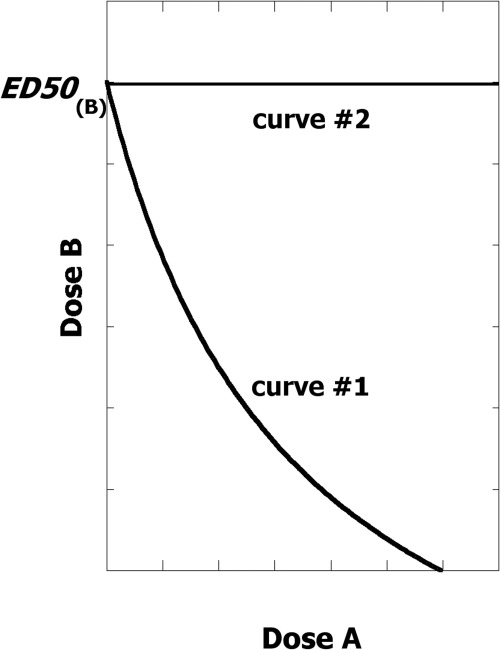
Isobolographic analysis, introduced and used by Loewe (1953, 1957), has a traditional application in describing the combination of two agonist drugs with overtly similar action (e.g., two analgesics). In this method the two agonist drugs (here denoted drug A and drug B) and their respective dose-effect relations allow a prediction of the combined effect from their individual potencies. From that relation one determines the combination dose pairs (a,b) that are calculated to give a specified level of effect (usually 50% of Emax, although other effect levels can be used). This set of dose pairs constitutes the isobole for the selected effect level. This plot is almost always a line or curve having a negative slope in a rectangular coordinate plot of dose B against dose A. If each drug alone is capable of attaining the specified effect (e.g., 50% of Emax), then the intercepts of the graph denote the individual drug doses that give the half-maximal effect (see Fig. 1). If drug A alone does not reach the 50% effect level, then there is no intercept on the axis for any dose of drug A. The isobole may be interpreted as a visual that shows the diminution in the dose of drug B caused by the presence of the dose of drug A, and it is this diminution that accounts for its negative slope.
Fig. 1.
The common isobole (which may be nonlinear or linear) is a decreasing curve (such as curve 1) when both drugs contribute based on their individual dose-effect curves. When both drugs achieve the desired effect (e.g., 50% level) then there are two intercepts representing the dose of drug A (denoted A) and the dose of drug B (denoted B) that individually give the specified effect level. When one of the drugs (e.g., drug A) does not contribute to the effect, then the isobole is horizontal (such as curve 2).
The exception to the negative slope is in that situation in which one of the drugs, say, drug A, lacks efficacy over some dose range. In this case there is no diminution in the needed dose of drug B; hence, in that case the isobole is a horizontal line (also shown in Fig. 1). The isobole has an historical use in defining unusual interactions (Loewe, 1953, 1957) and, in recent years, has witnessed a much expanded usage and application (Tallarida, 2001, 2006, 2007; Tallarida et al., 2003; Grabovsky and Tallarida, 2004; Braverman et al., 2008; Tallarida and Raffa, 2010). When experiments with actual combinations show that a dose pair below the isobole gives the specified effect, this means that lesser quantities were needed because of a synergistic interaction. In contrast, an experimental point above the isoble means an antagonistic interaction between the constituent drugs. Experimental points that lie on the isobole are the expected dose pairs under conditions of zero interaction and we refer to this case as an “additive interaction.” Details are given in several reviews (Tallarida, 2006, 2007; Tallarida and Raffa, 2010).
The theoretical basis of the isobole is the concept of dose equivalence for drugs A and B. Dose equivalence is determined from the individual dose-effect curves, i.e., a dose a of drug A will have a drug B-equivalent dose, beq(a). Thus, an actual dose b of drug B, when added to beq(a), is effectively the same as the ED50 of drug B: b + beq(a) = ED50. This mathematical relation defines the isobole and, in its most common form (when the relative potency is constant) the (a,b) dose pairs are given by the equation a/ED50 (A) + b/ED50 (B) = 1. (It is worthy of note that in these relations we have used the “dose,” but it is equally valid to analyze from the drug's concentration when that is known.)
Isoboles Based on Receptor Occupation.
While the common use of the isobole is for two drugs, we showed that the same concepts apply when analyzing a single drug that acts through two (or more) receptors (Braverman et al., 2008) and, therefore, this methodology is applicable to tapentadol. This approach begins with the conversion of concentrations to receptor occupations, thereby transforming concentration-effect data into occupation-effect data. The fraction of the receptors occupied is determined from mass action binding of the drug according to [C]/([C] + K), where K is the drug-receptor dissociation constant for that receptor and [C] is the drug concentration. When applied to the data for tapentadol we obtained the occupation-effect relations for both MOR and noradrenaline transporter (NAT) (fractional) occupation as described under Results. The fractional receptor occupation is ideally determined from the biophase concentration of the drug (as opposed to brain concentration). It is widely held that drug activity in the CNS is caused by the unbound brain concentration, because it is that concentration that determines the drug's occupation of the receptors. This widely accepted concept was confirmed by Liu et al. (2009) who showed that this unbound (biophase) concentration is approximately 1/100 of the total brain concentration for a number of agents, e.g., serotonin and dopamine transporter inhibitors (substances with molecular weights similar to tapentadol). Based on this, by using the brain concentration data (ng/g) for tapentadol (see Fig. 5), when adjusted for the brain composition (22% tissue and 78% H2O), we calculate that the fraction of drug in solution (free drug) is 0.0335 which leads to a free concentration that is (0.0335/0.78) = 0.043 of that in the whole brain. Therefore, a factor of 0.04 in determining biophase concentration was used and this same value was used for calculating both the observed and expected (additive) concentrations and the corresponding fractional occupancies. A different factor would lead to a different biophase concentration and fractional receptor occupancy, but the results of this analysis, which compares two receptors in the brain for interactions, are independent of the precise biophase values, i.e., this comparative analysis is sufficiently robust as to not require exact values of the occupancies.
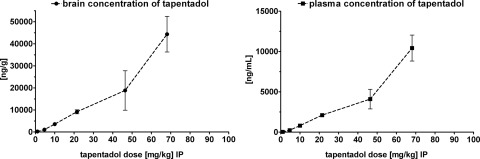
Fig. 5.
The brain (slope = 406.3) (left) and plasma (slope = 89.4) (right) concentration of tapentadol determined 10 min after intraperitoneal administration is seen to be linearly related to the intraperitoneal tapentadol dose over the main range of doses (up to 46.4 mg/kg). The error bars are S.D. based on n = 5.
Interactions Viewed on the Effect Scale: an Alternative to Isobolographic Analysis.
An alternative to isobolographic analysis uses drug combination data and derives the expected (additive) effect of the dose combination (a, b), i.e., an analysis on the effect scale. One might assume that the effect of the combination is a simple sum of the effects that each achieves alone, but that would be incorrect. For example, if the individual effects are, say, 70 and 55% of Emax, the addition of these percentages has no meaning. Thus, we used the concept of dose equivalence as follows: using symbols previously defined, we denote the effective dose of the combination as b + beq(a), and this quantity is used in the dose-effect relation for drug B as its effective dose, thereby giving the additive effect of the combination. In the special case in which dose a alone lacks efficacy, then beq(a) = 0, which means that this dose in the combination produces no change in the dose-effect relation of drug B.
Results
Interactions between the Two Mechanisms of Action Determined from the Effects of Tapentadol in Two Pain Models
One view of the interactions between the two mechanisms of action of tapentadol action is afforded from a comparison of the observed and (calculated) additive effect magnitudes. To this end, tapentadol was administered intravenously in varying doses as the sole agent as well as under conditions of yohimbine block (2.15 mg/kg i.p.) and naloxone block (1.0 mg/kg i.p.). The former case reveals tapentadol agonism caused by MOR stimulation, whereas the latter reveals agonism caused by NRI. Agonism in these two conditions was assessed in both an acute pain model (low-intensity tail-flick test) and a model of chronic mononeuropathic pain (SNL).
Low-Intensity Tail-Flick Test.
A graded dose-effect relationship was found in this model, as shown in Fig. 2A. It is evident from these relationships that tapentadol's MOR-mediated action was more potent than that caused by NRI in this test (Schröder et al., 2010). It is also seen (Fig. 2A) that the effect of NRI is not evident at tapentadol doses less than 4.64 mg/kg. Thus, in this low dose range the NRI component of action has no equivalent in terms of MOR agonism. It is therefore expected that, in this lower dose range, tapentadol's antinociceptive dose effect will be the same as that caused by MOR agonism if the interaction is additive. It is seen, however, that the tapentadol effects are elevated above the MOR-mediated effects. This elevation is shown numerically in Table 1, which shows the effect [with 95% confidence limit (CL)] for both conditions in the tapentadol dose range ≤4.64 mg/kg. From the concept of dose equivalence the effects are expected to be the same for a simply additive interaction. However, they are seen to be different, and the significant difference at each dose is a manifestation of synergism between these two mechanisms of action.
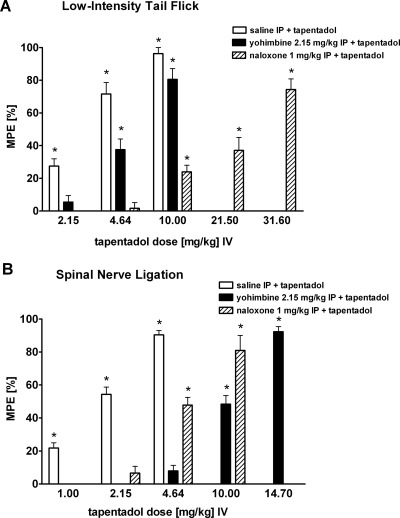
Fig. 2.
Dose-effect relations for tapentadol and its modifications caused by selective block of each of its two mechanisms of action are shown for two pain models [low-intensity tail-flick test (A) and SNL (B)] at 30 min after intravenous tapentadol administration. Data are presented as percentage of MPE (mean ± S.E.M.). *, P < 0.05 versus corresponding vehicle. Data are from Schröder et al., 2010.
TABLE 1.
Effects of tapentadol and its dual component in two pain models
Effect values are presented as percentage of MPE (with 95% confidence limits) at 30 min after intravenous tapentadol administration. In each pain model the tapentadol effect is expected to equal that of the indicated component if the interaction is simply additive. These significantly greater effects indicate synergism. Data are from Schröder et al., 2010.
| Intravenous Tapentadol Dose | Low-Intensity Tail Flick |
SNL |
||
|---|---|---|---|---|
| Tapentadol Effect | MOR Component Effect (Yohimbine Block) | Tapentadol Effect | NRI Component Effect (Naloxone Block) | |
| mg/kg | % of MPE | |||
| 1.0 | 21.8 (4.6–29.0) | 0 | ||
| 2.15 | 27.4 (17.5–37.3) | 5.47 (0–14.5) | 54.3 (44.4–64.2) | 6.6 (0–15.8) |
| 4.64 | 71.7 (55.9–87.5) | 7.5 (22.8–52.2) | ||
SNL.
The dose-effect relationships from this model are shown in Fig. 2B and reveal the interesting fact that tapentadol's NRI-mediated action is more potent than the MOR component of action (Schröder et al., 2010). This stands in contrast to the situation revealed in the acute pain model described above, where naloxone produced a greater rightward shift of the tapentadol dose-response relationship as yohimbine. It is also seen that in the lower tapentadol dose range (< 4.64 mg/kg) the MOR component of action is not evident; therefore, the NRI component is expected to be the same as that of unblocked tapentadol if there is no interaction. However, there is a prominent elevation in effect levels, e.g., 54.3% versus 6.6% at the 2.15 mg/kg tapentadol dose (Table 1). It is further seen that even at the 4.64 mg/kg dose, where MOR agonism is virtually undetectable in the SNL model, the tapentadol effect is significantly above that caused by NRI agonism, a finding indicative of synergism. The effect values (with 95% CLs) are given in Table 1.
Interactions between the Two Components of Tapentadol Action Determined from Receptor Occupation-Effect Relations in the Low-Intensity Tail-Flick Test
Another view of the interactions between the two mechanisms of action of tapentadol is afforded from the use of the fractional occupation of each receptor type as determined from the values of its brain concentration in an analysis based on receptor occupation. In an additional set of experiments, tapentadol was administered intraperitoneally in varying doses as the sole agent as well as after prior administration of either naloxone (1.0 mg/kg i.p.) or yohimbine (4.64 mg/kg i.p.).
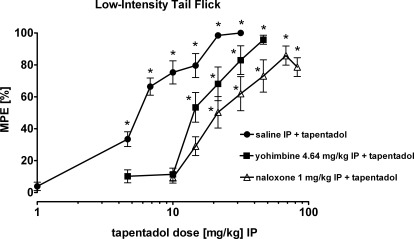
Tapentadol (alone) produced potent [ED50: 5.1 (4.4–5.8) mg/kg i.p.] dose- and time-dependent antinociception (treatment: F7,72 = 80.841, P < 0.001; time: F2,144 = 11.817, P < 0.001; interaction: F14,144) = 2.456, P < 0.001). Full efficacy, 10 min after intraperitoneal administration, was reached at 31.6 mg/kg (Figs. 3 and 4). Naloxone significantly shifted the dose-response curve of tapentadol to the right by a factor of 5.2 [ED50, 5.1 versus 26.3 (21.7–31.2) mg/kg; treatment: F7,69 = 25.184, P < 0.001; time: F2,138 = 0.113, P = 0.893; interaction: F14,138 = 1.475, P = 0.128] (Fig. 4). Statistical evaluation relates to the within-group effect of tapentadol, and differences between groups were assessed based on CI overlap (see Materials and Methods). Yohimbine significantly shifted the dose-response curve of tapentadol to the right by approximately a factor of 3 [ED50, 5.1 versus 15.2 (12.9–17.7) mg/kg; treatment: F5,54 = 29.124, P < 0.001; time: F2,108 = 7.023, P < 0.001; interaction: F10,108 = 2.127, P = 0.028] (Fig. 4). These ED50 values are based on effects at 10 min after intraperitoneal tapentadol administration. Administration of vehicle or antagonists alone did not produce antinociceptive effects (see legend to Fig. 4).
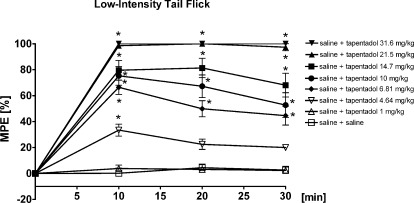
Fig. 3.
Dose- and time-dependent antinociceptive effect of tapentadol in the low-intensity tail-flick test in rats. All injections were made intraperitoneally. Data are presented as percentage of MPE (mean ± S.E.M.). *, P < 0.05 versus corresponding vehicle. Corresponding brain concentrations of tapentadol were determined in satellite groups 10 min after intraperitoneal administration of tapentadol (Fig. 5).
Fig. 4.
Naloxone shifted the dose-response curve of tapentadol farther to the right than yohimbine in the low-intensity tail-flick test in rats. Data are presented as percentage of MPE (mean ± S.E.M.) 10 min after intraperitoneal administration of tapentadol. *, P < 0.05 versus corresponding vehicle. Administration of vehicle and antagonists alone did not produce antinociceptive effects. The respective percentages of MPE (mean ± S.E.M.) 10 min after the second intraperitoneal administration were as follows: saline intraperitoneally + saline intraperitoneally, 0.2 ± 4.0; naloxone 1 mg/kg i.p. + saline intraperitoneally, 2.1 ± 2.9; yohimbine 4.64 mg/kg i.p. + saline intraperitoneally, −4.2 ± 3.0.
Brain Concentrations and Receptor Occupation of Tapentadol.
For use in the following analysis we show in Fig. 5 the relation between each intraperitoneal dose of tapentadol and the brain concentration determined 10 min after tapentadol administration. It is seen that the brain (and plasma) concentrations exhibit pronounced linearity up to doses of 46.4 mg/kg i.p. Generally, brain concentrations were approximately 4.5 times higher than in plasma. Effective plasma concentrations in humans are approximately 50 to 150 ng/ml, which is similar to the concentrations found in rat plasma at intraperitoneal doses of 1 to 4.64 mg/kg. Brain concentrations allow estimation of the biophase concentrations.
The fractional receptor occupation values were calculated from the biophase brain concentration values for each tapentadol dose and the previously determined dissociation constant of MOR (0.096 ± 0.009 μM) and functional inhibition constant of NAT (0.48 ± 0.11 μM) (Tzschentke et al., 2007).
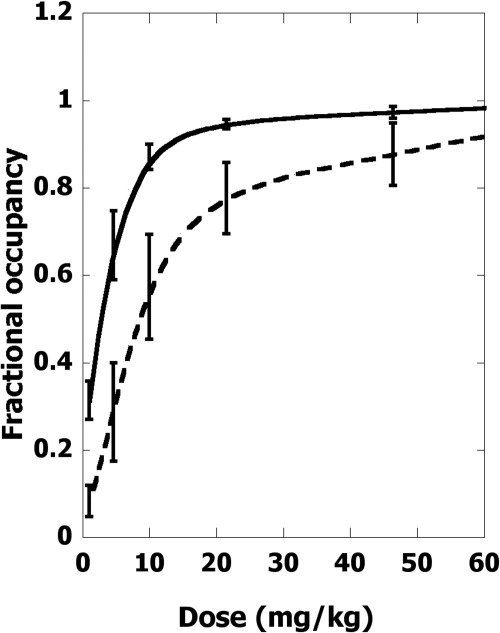
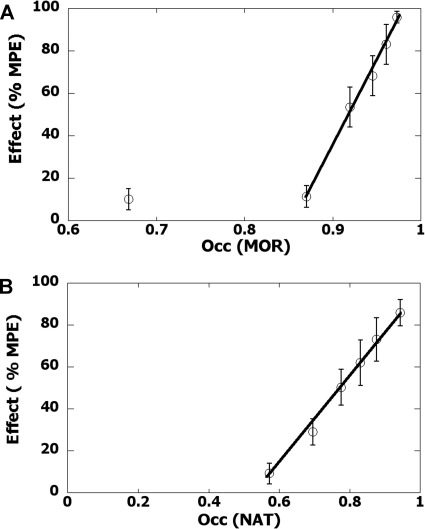
Figure 6 shows the relation between receptor occupation and intraperitoneal tapentadol dose. The receptor occupation values for each dose were coupled to the effect (here determined from the low-intensity tail-flick test), thereby yielding the occupation-effect curves of Fig. 7. These graphs show the occupation-effect relation for MOR fractional occupation (using the effect data with yohimbine block) and the corresponding NAT fractional occupation that uses effects that accompany the naloxone block. For example, in Fig. 7A the six points shown are derived from the dose-effect data of Fig. 4. The doses have been transformed to MOR fractional occupancy and plotted with the observed effect and, from these, we note that the 50% effect occurs at MOR occupancy = 0.92, the value that is used in constructing the subsequent isobole of additivity. Occupation-effect relations serve the same purpose as dose-effect relations for the detection of interactions. In this case, however, the interaction is not between two agonist drugs; it is, instead, between the two receptors occupied by the same drug. Just as dose equivalence is the basis of the common isobologram, it also follows that occupation equivalence is the basis of the isobologram in this case.
Fig. 6.
Fractional receptor occupation for MOR (solid curve) and NAT (broken curve) based on the published values of Ki for increasing intraperitoneal doses of tapentadol.
Fig. 7.
A, the occupation-effect relation for MOR fractional occupation was obtained from dose-effect data of tapentadol in the presence of yohimbine. The ED50 for MOR fractional occupation is 0.92 (corresponding to dose 15.3 mg/kg) with 95% CLs (0.86–0.94). B, occupation-effect relation for NAT (obtained with naloxone block) is plotted. This component of tapentadol's action is not evident for NAT fractional occupation less than 0.54.
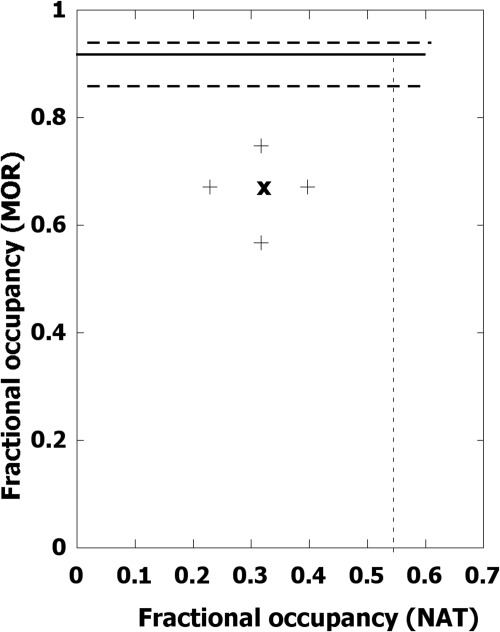
As seen in Fig. 8, the isobole of additivity (50% effect) is horizontal. This occurs because for all fractional occupancy values of the NAT that are less than 0.54 that occupied receptor yields no detectable effect in this low-intensity tail-flick test. (This is analogous to the situation in which one of the two drugs is devoid of efficacy, in which case the isobole of additivity is horizontal.) Thus, NAT occupation in the range of 0 to 0.54 is negligible, and therefore the expected additive isobole is horizontal at the 0.92 level (over the NAT domain up to 0.54) of the expected MOR fractional occupation for the half-maximal effect of the combined action (see also Fig. 7A). The experimentally determined occupation pair for this 50% effect, obtained from the tapentadol ED50 dose 5.1 mg/kg, is at a lower MOR occupancy value (0.67) with only 0.32 occupancy of NAT (X on Fig. 8), thereby showing synergism because occupancy 0.32 yields no effect. In other words, because this experimentally derived point is significantly below the additive isobole a synergistic interaction between these occupied receptors is indicated.
Fig. 8.
Isobologram for 50% of the MPE based on receptor occupancy showing synergism between the two components that contribute to tapentadol action in this test. Because NAT activity is not apparent up to fractional occupancy 0.54, the additive isobole (solid line) is horizontal (with 95% CLs shown as broken lines) and represents the occupation of MOR for this effect level. The experimental occupation pair is shown (X) with 95% CLs, and its position below the isobole indicates synergism.
Discussion
Tapentadol exerts its antinociceptive action through two mechanisms, MOR agonism and NRI, that have been well documented (Tzschentke et al., 2006, 2007, 2009). The current set of antinociceptive tests and accompanying analysis further confirm this dual mechanism and provide a quantitative analysis that shows that these two mechanisms interact in a synergistic way. This is probably the first demonstration of a synergistic interaction between the occupied receptors for a single compound with a dual mechanism of action. This synergistic interaction was derived from our two pronged analysis that included 1) an examination based on occupation isoboles and 2) the observed and predicted effect levels.
Viewed from the effect scale, the predicted (additive) effect of a drug dose combination uses the concept of dose equivalence, i.e., adding the drug B equivalent of drug A to the dose of drug B, to calculate the combination effect when there is no interaction. This same principle applies to isobolographic analysis but that method derives its conclusion from comparisons of observed and expected doses (or receptor fractional occupations) that give the specified effect magnitude. In this study the isobolographic analysis used occupation isoboles and is conceptually identical to that used in traditional isobolographic analysis with doses. When using occupation isoboles it is not dose equivalence; instead it is occupation equivalence that underlies the analysis. All other aspects of the traditional isobole apply to the occupation isobole in quantitatively characterizing the interaction which, as applied to tapentadol, is between the two occupied receptors (MOR and NAT). The synergistic interaction between the two mechanisms of action of tapentadol may well explain two remarkable observations. Tapentadol has a 50-fold lower affinity for the (rat) MOR than morphine (Ki = 0.096 versus 0.002 μM), yet tapentadol is only 2- to 3-fold less potent than morphine across a variety of preclinical pain models (Tzschentke et al., 2006), strongly suggesting that the NRI component of tapentadol contributes to its analgesic effect, and that it does so in a synergistic manner. Because the NRI activity of tapentadol is also only relatively moderate (Ki = 0.48 μM for rat synaptosomal uptake inhibition), a simple additive effect cannot explain the potent analgesia observed for tapentadol. Thus, through this synergistic interaction, two moderate pharmacological activities are sufficient to produce powerful analgesia, along with reduced MOR-related and without relevant NRI-related side effects.
The fact that the noradrenergic component contributes in a synergistic way may also explain why tapentadol produces potent analgesia in acute as well as in various chronic pain states. In acute pain, monoaminergic compounds are generally relatively ineffective (see Tzschentke, 2002), and in chronic pain, pure opioids, although still effective, are relatively less potent than in acute pain, necessitating dose escalation to obtain satisfactory analgesia. These high doses often cause intolerable opioid-typical side effects, limiting the usefulness of pure opioids in chronic pain (Portenoy, 1996; Kalso et al., 2004). It is noteworthy that whereas in acute pain models, the potency of tapentadol is (only) two to three times lower than that of morphine, in rat and mouse models of chronic (mononeuropathic and polyneuropathic) pain, the potency difference between tapentadol and morphine is even smaller, or tapentadol is even more potent than morphine (Tzschentke et al., 2009; Christoph et al., 2010; Schröder et al., 2010). This is probably related to the fact that noradrenergic mechanisms play a more relevant role in chronic as in acute pain states (Fishbain, 2000; Tzschentke et al., 2007; Schröder et al., 2010), such that the (synergistic) contribution of this mechanism is even more pronounced in chronic pain, leading to an even more pronounced potency advantage over pure opioids (relative to the MOR affinity).
A mechanistic/anatomical basis for this synergistic interaction may lie in the intricate interplay between the opioid system and monoaminergic systems (in particular the descending inhibitory noradrenergic system). Opioids act at several levels of the pain transmitting system. At the spinal level, opioids reduce the transmission of the pain signal from the primary afferents to the fibers of the spinothalamic tract via presynaptic and postsynaptic mechanisms (Millan, 1999). At the supraspinal level, in addition to various other effects, opioids activate the descending inhibitory pathways to the spinal cord. Within these pathways, noradrenaline is an important transmitter (Millan, 2002). Thus, a NRI mechanism of action contributes to analgesia by increasing noradrenergic activity at the spinal level by augmenting the influence of the descending inhibitory projection. By combining MOR and NRI mechanisms of action, analgesic potency is enhanced, not only through a summation of the individual effects at the supraspinal and the spinal level, but also through a mutual interaction of supraspinal and spinal effects. The effect of the opioid-induced supraspinal activation of the descending inhibitory noradrenergic pathways is further enhanced through the action of the NRI component at the spinal level. In other words, the MOR-agonistic component increases spinal levels of noradrenaline that in turn acts on spinal α2 adrenoceptors, and the NRI component also blocks the reuptake of this additionally released noradrenaline. Previously, it was shown that noradrenaline reuptake inhibitors are antinociceptive on their own and also potentiate the analgesic effect of both systemic and intrathecal morphine when administered spinally (Hwang and Wilcox, 1987). Furthermore, the complex supraspinal-spinal interaction between MOR and α2 adrenoceptors as described above was shown to underlie the antinociceptive synergism obtained with concurrent intrathecal and intracerebroventricular morphine administration in the mouse tail-flick test (Wigdor and Wilcox, 1987). Likewise, we were able to demonstrate a pronounced supraspinal-spinal synergism for tapentadol in heat hyperalgesia in a mouse model of streptozotocin-induced diabetic polyneuropathy and showed that this site-site synergism is mediated predominantly at the spinal level (T. Christoph, unpublished work).
Because of this intricate interaction and mutual augmentation of the individual effects, relatively moderate pharmacological activities are sufficient for both mechanisms of action of tapentadol to achieve a powerful analgesic effect. This, in turn, translates into a broad efficacy profile and clearly improved clinical tolerability (Hale et al., 2009; Hartrick et al., 2009; Hartrick, 2009).
In conclusion, a quantitative analysis based on additive isoboles of occupation and/or additive effects can be applied to study the interaction between two mechanisms of action located within a single molecule. The data presented here for tapentadol show that these mechanisms interact in a highly synergistic way. This may well explain why tapentadol is only 2- to 3-fold less potent than morphine across a variety of preclinical pain models despite a 50-fold lower affinity for the MOR. This is probably the first demonstration of a synergistic interaction between the occupied receptors for a single compound with a dual mechanism of action.
Supplementary Material
Acknowledgments
We thank M. Bräuer, H. Fischer, M. Kaminski, M. Langhans, H.-J. Leipelt, and H.-J. Weber for technical assistance.
This work was supported by Grünenthal GmbH, Aachen, Germany.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.175042.
- tapentadol
- (−)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride
- CI
- confidence interval
- CL
- confidence limit
- MPE
- maximal possible effect
- MOR
- μ-opioid receptor
- NAT
- noradrenaline transporter
- NRI
- noradrenaline reuptake inhibition
- SNL
- spinal nerve ligation.
Authorship Contributions
Participated in research design: Schröder, Tzschentke, Terlinden, De Vry, Jahnel, Christoph, and Tallarida.
Conducted experiments: Schröder and Christoph.
Performed data analysis: Schröder, Jahnel, and Tallarida.
Wrote or contributed to the writing of the manuscript: Schröder, Tzschentke, Terlinden, Jahnel, Christoph, and Tallarida.
References
- Anzai Y, Nishikawa T. (1995) Thoracic epidural clonidine and morphine for postoperative pain relief. Can J Anaesth 42:292–297 [DOI] [PubMed] [Google Scholar]
- Braverman AS, Tallarida RJ, Ruggieri MR., Sr (2008) The use of occupation isoboles for analysis of a response mediated by two receptors: M2 and M3 muscarinic receptor subtype-induced mouse stomach contractions. J Pharmacol Exp Ther 325:954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph T, De Vry J, Tzschentke TM. (2010) Tapentadol, but not morphine, selectively inhibits disease-related thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Neurosci Lett 470:91–94 [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79 [Google Scholar]
- Fairbanks CA, Wilcox GL. (1999) Spinal antinociceptive synergism between morphine and clonidine persists in mice made acutely or chronically tolerant to morphine. J Pharmacol Exp Ther 288:1107–1116 [PubMed] [Google Scholar]
- Fishbain D. (2000) Evidence-based data on pain relief with antidepressants. Ann Med 32:305–316 [DOI] [PubMed] [Google Scholar]
- Grabovsky Y, Tallarida RJ. (2004) Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther 310:981–986 [DOI] [PubMed] [Google Scholar]
- Hale M, Upmalis D, Okamoto A, Lange C, Rauschkolb C. (2009) Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, double-blind study. Curr Med Res Opin 25:1095–1104 [DOI] [PubMed] [Google Scholar]
- Hartrick C, Van Hove I, Stegmann JU, Oh C, Upmalis D. (2009) Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10-day, phase III, randomized, double-blind, active- and placebo-controlled study. Clin Ther 31:260–271 [DOI] [PubMed] [Google Scholar]
- Hartrick CT. (2009) Tapentadol immediate release for the relief of moderate-to-severe acute pain. Expert Opin Pharmacother 10:2687–2696 [DOI] [PubMed] [Google Scholar]
- Hwang AS, Wilcox GL. (1987) Analgesic properties of intrathecally administered heterocyclic antidepressants. Pain 28:343–355 [DOI] [PubMed] [Google Scholar]
- Kalso E, Edwards JE, Moore RA, McQuay HJ. (2004) Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 112:372–380 [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Smith R, McBryde R. (1986) Desipramine enhances opiate postoperative analgesia. Pain 27:45–49 [DOI] [PubMed] [Google Scholar]
- Liu X, Vilenski O, Kwan J, Apparsundaram S, Weikert R. (2009) Unbound brain concentration determines receptor occupancy: a correlation of drug concentration and brain serotonin and dopamine reuptake transporter occupancy for eighteen compounds in rats. Drug Metab Dispos 37:1548–1556 [DOI] [PubMed] [Google Scholar]
- Loewe S. (1953) The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 3:285–290 [PubMed] [Google Scholar]
- Loewe S. (1957) Antagonisms and antagonists. Pharmacol Rev 9:237–242 [PubMed] [Google Scholar]
- Millan MJ. (1999) The induction of pain: an integrative review. Prog Neurobiol 57:1–164 [DOI] [PubMed] [Google Scholar]
- Millan MJ. (2002) Descending control of pain. Prog Neurobiol 66:355–474 [DOI] [PubMed] [Google Scholar]
- Moore RA, McQuay HJ. (2005) Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 7:R1046–R1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motsch J, Gräber E, Ludwig K. (1990) Addition of clonidine enhances postoperative analgesia from epidural morphine: a double-blind study. Anesthesiology 73:1067–1073 [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Harris S, Lloyd P, Messineo E. (1990) An isobolographic analysis of the antinociceptive effect of systemically and intrathecally administered combinations of clonidine and opiates. J Pharmacol Exp Ther 255:1107–1116 [PubMed] [Google Scholar]
- Ossipov MH, Malseed RT, Goldstein FJ. (1982) Augmentation of central and peripheral morphine analgesia by desipramine. Arch Int Pharmacodyn Ther 259:222–229 [PubMed] [Google Scholar]
- Portenoy RK. (1996) Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage 11:203–217 [DOI] [PubMed] [Google Scholar]
- Reimann W, Schlütz H, Selve N. (1999) The antinociceptive effects of morphine, desipramine, and serotonin and their combinations after intrathecal injection in the rat. Anesth Analg 88:141–145 [DOI] [PubMed] [Google Scholar]
- Schröder W, Vry JD, Tzschentke TM, Jahnel U, Christoph T. (2010) Differential contribution of opioid and noradrenergic mechanisms of tapentadol in rat models of nociceptive and neuropathic pain. Eur J Pain 14:814–821 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. (2001) Drug synergism: its detection and applications. J Pharmacol Exp Ther 298:865–872 [PubMed] [Google Scholar]
- Tallarida RJ. (2006) An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther 319:1–7 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. (2007) Interactions between drugs and occupied receptors. Pharmacol Ther 113:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Cowan A, Raffa RB. (2003) Antinociceptive synergy, additivity, and subadditivity with combinations of oral glucosamine plus nonopioid analgesics in mice. J Pharmacol Exp Ther 307:699–704 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Raffa RB. (2010) The application of drug dose equivalence in the quantitative analysis of receptor occupation and drug combinations. Pharmacol Ther 127:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. (2002) NA and 5-HT reuptake inhibitors and α2-agonists, in Analgesics: From Chemistry and Pharmacology to Clinical Application (Buschmann H, Christoph T, Friderichs E, Maul C, Sundermann B. eds) pp 265–284, Wiley-VCH, Weinheim, Germany [Google Scholar]
- Tzschentke TM, Christoph T, Kögel B, Schiene K, Hennies HH, Englberger W, Haurand M, Jahnel U, Cremers TI, Friderichs E, et al. (2007) (−)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel μ-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 323:265–276 [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, De Vry J, Terlinden R, Hennies HH, Lange C, Strassburger W, Haurand M, Kolb J, Schneider J, Buschmann H, et al. (2006) Tapentadol hydrochloride. Drugs Future 31:1053–1061 [Google Scholar]
- Tzschentke TM, Jahnel U, Kogel B, Christoph T, Englberger W, De Vry J, Schiene K, Okamoto A, Upmalis D, Weber H, et al. (2009) Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today (Barc) 45:483–496 [DOI] [PubMed] [Google Scholar]
- Ventafridda V, Bianchi M, Ripamonti C, Sacerdote P, De Conno F, Zecca E, Panerai AE. (1990) Studies on the effects of antidepressant drugs on the antinociceptive action of morphine and on plasma morphine in rat and man. Pain 43:155–162 [DOI] [PubMed] [Google Scholar]
- Wigdor S, Wilcox GL. (1987) Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. J Pharmacol Exp Ther 242:90–95 [PubMed] [Google Scholar]
- Zimmermann M. (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.