Abstract
Chemotherapy is an important therapeutic approach for cancer treatment. However, drug resistance is an obstacle that often impairs the successful use of chemo-therapies. Therefore, overcoming drug resistance would lead to better therapeutic outcomes for cancer patients. Recently, studies by our own and other groups have demonstrated that there is an intimate correlation between the loss of the F-box and WD repeat domain-containing 7 (FBW7) tumor suppressor and the incurring drug resistance. While loss of FBW7 sensitizes cancer cells to certain drugs, FBW7-/- cells are more resistant to other types of chemotherapies. FBW7 exerts its tumor suppressor function by promoting the degradation of various oncoproteins that regulate many cellular processes, including cell cycle progression, cellular metabolism, differentiation, and apoptosis. Since loss of the FBW7 tumor suppressor is linked to drug resistance, FBW7 may represent a novel therapeutic target to increase drug sensitivity of cancer cells to conventional chemotherapeutics. This paper thus focuses on the new functional aspects of FBW7 in drug resistance.
Keywords: cancer, drug resistance, FBW7, Mcl-1, p53, tumor suppressor
Introduction
Although chemotherapy is a critically important treatment choice for most cancer patients, chemotherapy often fails to cure the disease due to unexpected drug resistance. Therefore, increasing drug sensitivity may open the way to novel approaches for successful treatment of cancer patients [1]. There are two categories of drug resistance: intrinsic (de novo) and acquired [2]. Tumor cells may be inherently resistant to certain drugs due to specific genetic mutations, which may lead to loss of tumor suppressors or gain-of-function expression of oncoproteins. In most instances, the tumor cells that initially respond well to anti-cancer drugs, gradually display a loss of response and acquire resistance during the course of treatment, subsequently leading to tumor recurrence [3].
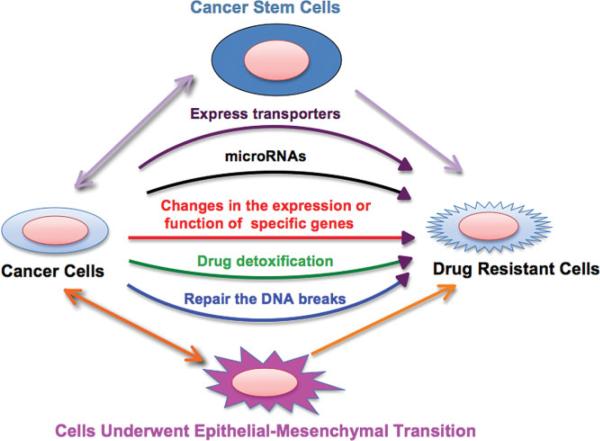
There are several reasons for the acquisition of drug resistance (Fig. 1) [4, 5]. First, cancer cells may acquire expression of energy-dependent transporters that eject the anti-cancer drugs from the tumor cells. For example, the ATP-binding cassette (ABC) drug transporters pump cytotoxic drugs from cancer cells, thus protecting tumor cells from certain chemotherapeutic drugs [5]. ABC drug transporters include many types of proteins, such as ABCB1 [P-glycoprotein (PGP)], ABCC1 [multidrug resistance-associated protein (MRP1)], and ABCG2 [breast cancer resistant protein (BCRP)], which are of particular importance, as they participate in the disposition of many clinically important drugs [5, 6]. Secondly, cancer cells may stop taking up chemotherapeutic drugs due to loss of function mutation in proteins that transport the drugs across the cell membrane [5]. Thirdly, cancer cells may acquire increased efficiency in repairing DNA breaks caused by chemotherapeutic drugs [5]. Fourthly, cancer cells may develop drug-detoxification mechanisms that render them insensitive to drug-induced apoptosis [7]. Fifthly, cancer cells may change cellular polarity and behavior, which could result in drug resistance. For example, epithelial-mesenchymal transition (EMT) has been found to be associated with acquired drug resistance [8]. Specifically, cancer cells with epithelial rather than mesenchymal gene signatures were reported to be more sensitive to epidermal growth factor receptor (EGFR) inhibitors, such as erlotinib, gefitinib, and cetuximab-mediated growth inhibition, in various human cancers including lung cancer, head and neck squamous cell carcinoma, and hepatocellular carcinoma [4]. In agreement with a possible role for EMT in drug resistance, studies have also revealed a strong link between EMT and drug-resistant cancer cells [4]. Sixthly, changes in the expression or function of specific genes involved in the control of cell growth, apoptosis, cell cycle regulation, and invasion could contribute to drug resistance [9]. Moreover, cancers develop a gatekeeper mutation after treatment with drugs, leading to acquired resistance. For example, β-tubulin mutant and EGFR T790M mutant show taxanes and gefitinib resistance, respectively [10, 11]. Lastly, cancer stem cells (CSCs) were recently discovered to be involved in drug resistance in various types of human cancers such as breast cancer, colorectal cancer, leukemia, glioblastoma, small cell lung cancer, ovarian cancer, prostate cancer, and pancreatic cancer [12]. CSCs constitute a small subset of cancer cells (sometimes referred to as side-population cells) that possess the ability to self-renew and maintain multi-potency. The drug-resistant features of CSCs result in difficulties in completely eradicating cancer by chemotherapies. Several groups have proposed that CSCs resist chemotherapeutic drugs by elevated expression of ABC drug transporters such as ABCG2 and ABCC1, which protect CSCs from chemotherapeutic agents [13]. In summary, although exciting and extensive research into potential molecular mechanism for drug resistance during chemotherapy has been carried out in the last few decades, the exact mechanisms have not yet been fully elucidated. It appears that no universal mechanism exists but rather different genetic alterations might account for the various drug-resistant pheno-types encountered in different types of cancers.
Figure 1.
Schematic illustration of the various mechanisms leading to cancer drug resistance.
Recently, many genes have been discovered to play important roles in the development of drug resistance. These genes include Akt [14], Bcl-2 [15], BRCA1/2 [16], cyclooxygenase-2 (COX-2) [17], cyclin D1 [18], extracellular signal-regulated kinase (ERK) [19], FoxM1 [20, 21], EGFR [22], fibroblast growth factor receptor (FGFR) [23], insulin-like growth factor (IGF) [24], mitogen-activated protein kinase (MAPK) [25, 26], mammalian target of rapamycin (mTOR) [27], nuclear factor-κB [28, 29], Notch [30, 31], platelet-derived growth factor (PDGF) [32], phosphatase and tensin homolog on chromosome 10 (PTEN) [33], and Survivin [34]. The roles of these genes in drug resistance have been studied and reviewed in considerable detail. Notably, recent studies by our and other groups have demonstrated that F-box and WD repeat domain-containing 7 (FBW7) is also involved in the regulation of drug resistance [35–37]. The role of FBW7 in drug resistance is just beginning to emerge. Therefore, the following sections summarize the function of FBW7 in the observed drug resistance, and how targeting FBW7 may be helpful for designing novel therapeutic strategies to improve drug sensitivity, which may result in achieving better treatment outcomes.
FBW7 is a tumor suppressor
Ubiquitination by the ubiquitin proteasome system (UPS) is a post-translational mechanism that regulates diverse cellular processes including cell proliferation, cell cycle progression, transcription, immune response, DNA damage repair, and apoptosis [38, 39]. It has been reported that the components of the UPS are commonly mutated in human cancers, leading to either the rapid degradation of certain tumor suppressors, or impaired destruction of certain oncoproteins, both of which can facilitate tumor development [40]. The UPS consists of the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2), and the ubiquitin-protein ligases (E3). The E1 enzyme activates ubiquitin and transfers it to the E2. The E2 enzyme then interacts with a specific E3 partner and transfers the ubiquitin to the target protein for degradation by the 26S proteasome [40]. In general, the E3 is responsible for adding polyubiquitin chain to specific substrate proteins. E3 ubiquitin ligases are categorized into multiple classes, such as single RING type, homologous to E6-AP C terminus (HECT) type and Ring/Cullin Ligase (RCL) type of E3 ligases [41]. Among the E3 ubiquitin ligase enzymes, the RCL type of E3 ligases contain the largest number of family members, and among them, the Skp1-Cullin1-F-box (SCF) E3 ligase complex is one of the best characterized. The F-box protein carries the specificity for recognition and binding of target proteins for subsequent ubiquitination. There are more than 70 putative F-box proteins encoded in the human genome [42]. The well-characterized F-box protein Skp2 and β-TRCP1 target p27 and Cdc25A, respectively, for ubiquitination and degradation. In most cases, proper phosphorylation of the substrates is required for interaction with the F-box proteins. FBW7, also known as Fbxw7, Ago, CDC4, or Sel10, is another well-studied SCF ubiquitin ligase that is reported to target various oncogenic proteins for ubiquitination (Fig. 2) [39]. However, the function and physiological substrates of the vast majority of F-box proteins have not yet been identified.
Figure 2.
Illustrated pathways of FBW7-mediated degradation of its major downstream targets. FBW7 recognizes the substrates, which are presented closely to the E2 enzyme to ensure consequent conjugation of ubiquitin. The addition of polyubiquitin targets proteins to the 26S-proteasome for degradation. Several proteins and miRNAs have been reported to regulate the expression of FBW7.
It has been determined that the specific substrates of FBW7 include cyclin E [43, 44], c-Myc [45, 46], c-Jun [47, 48], Notch [48–50], presenilin [51], Mcl-1 [36, 37], sterol regulatory element-binding proteins (SREBP) [52, 53], mTOR [54], Krüppel-like factors (KLFs) [55, 56], c-Myb [57], and Aurora A [58]. Because all these characterized substrates are well-known oncogenic proteins that are frequently overexpressed in a variety of human cancers, FBW7 is believed to be a tumor suppressor that contributes to the negative regulation of these oncogenic proteins. Indeed, it has been observed that FBW7-deficient mice are embryonic lethal at day 10.5 due to major developmental defects [59] and that conditional depletion of FBW7 in mouse T cells results in lymphomatogenesis [60]. Interestingly, FBW7 deletion also conferred a selective inhibition of p53 function, eventually resulting in development of T cell acute lymphoblastic leukemia (T-ALL) [61]. Consistent with these results, FBW7 is frequently inactivated by mutation, deletion, or promoter hypermethylation in multiple neoplasms, including breast cancer [56, 62], colon cancer [36, 63], and leukemia [64]. For example, ~30% mutation rates at the FBW7 genetic locus were identified in T-ALL [65]. In addition, FBW7 mutation was found in 16% of primary endometrial cancer and 11% of colorectal cancer, respectively [65–67]. Approximately 6% of all primary human tumors are estimated to harbor mutations in the FBW7 gene [65].
The next obvious question is how does FBW7 exert its anti-tumor activity? It is important to note that the exact molecular mechanisms by which FBW7 suppresses cancer formation remain unclear, even though the known substrates of FBW7 are well documented for their oncogenic roles in cancer development and progression. In this regard, several studies have shed light on the anti-tumor activity of FBW7. For instance, Minella et al. demonstrated that FBW7 could promote the turnover of cyclin E, and that complete loss of FBW7-mediated cyclin E degradation causes tissue-specific hyper-proliferation in vivo [44]. Nateri's group reported that loss of FBW7 induced development of gut adenomas through up-regulation of Notch and c-Jun expression [49]. Onoyama et al. observed that conditional inactivation of FBW7 caused the development of thymic lymphoma primarily due to c-Myc accumulation [60]. Moreover, FBW7 was also reported to target c-Myb in a Wnt-1 and Nemo-like kinase (NLK)-dependent, or glycogen synthase kinase (GSK)-dependent manner [68, 69]. Furthermore, we and others recently discovered that FBW7 targets Mcl-1, a pro-survival member of the Bcl-2 family of proteins, for ubiquitination and destruction [36, 37]. It is thus possible that deregulated destruction of individual substrates of FBW7 may only have a restricted role in the development of specific types of cancer. Without a doubt, another possibility is that accumulation of multiple substrates by loss of FBW7 may play a synergistic role in the overall transformation phenotype. Additional studies are required to confirm these hypotheses and their validities. While inhibition of these oncoproteins by FBW7 has been implicated, its contributions to suppress tumor growth still require further substantial characterization.
FBW7 is involved in drug resistance
Recently, a body of literature revealed that FBW7 is involved in drug resistance in human cancers [35–37]. Wertz et al. reported that FBW7 inactivation and increased Mcl-1 levels promoted resistance to anti-tubulin chemotherapeutic agents and accelerated tumorigenesis [37]. This group also reported that inhibition of Mcl-1 in FBW7-null cells restored their sensitivity to taxol- and vincristine-induced cell death [37]. Similarly, we found that FBW7-deficient T-ALL cells, which displayed higher levels of Mcl-1 expression, were more sensitive to the chemotherapeutic drug sorafenib, and acquired resistance to the Bcl-2 family inhibitor ABT-737 [36]. Moreover, accumulating evidence suggests that loss of FBW7 was also involved in resistance to gamma secretase inhibitor (GSI) treatment [70, 71]. These recent studies have shown an intimate relationship between drug resistance and FBW7 genetic status, demonstrating that targeting FBW7 may open a new therapeutic window for drug administration. Therefore, in the following paragraphs we present the biological functions of FBW7, which might help us to understand its involvement in drug resistance in various human malignancies.
In the absence of FBW7 sorafenib inhibits the Mcl-1 pro-survival factor, increasing apoptosis activation
Sorafenib is an inhibitor of multiple receptor tyrosine kinases involved in tumor growth and angiogenesis. This drug has been shown to improve survival rates in human cancer patients [72], and has been approved for systemic targeted therapy in clinical use. With regard to its downstream targets, sorafenib suppresses PDGF, vascular endothelial growth factor (VEGF) receptor kinase and Raf kinase [72]. Raf kinase is an enzyme capable of activating the MAPK signaling pathway. Panka et al. reported that sorafenib suppressed Mcl-1 levels by inactivating MAPK kinase [73]. Several other studies have also shown that sorafenib is an Mcl-1 antagonist [74]. In keeping with the critical role of the Mcl-1 pro-survival factor for FBW7-/- cells in evading the apoptotic pathway, we found that FBW7-deficient T-ALL cells were more sensitive to sorafenib compared to wild-type FBW7 T-ALL cells [36]. Work from several groups has shown that loss of FBW7 led to an elevated expression of the c-Jun, c-Myc, and Notch-1 oncoproteins, all of which are capable of promoting cell growth, although they can also provoke cellular apoptosis as a side effect [75]. Recent work from our laboratory and the Wertz group showed that elevated levels of the Mcl-1 pro-survival factor provided a protection mechanism. This mechanism allowed FBW7-deficient cells to take advantage of the pro-oncogenenic functions of c-Jun, c-Myc, and Notch-1, by balancing off their pro-apoptotic effects [36, 37]. However, because of the intrinsic balance of the cellular apoptotic pathway, FBW7-deficient T-ALL cells are dependent on higher levels of Mcl-1 expression, a phenotype termed “oncogene addiction” [76]. When the anti-apoptotic effect of Mcl-1 is inhibited by sorafenib, tumor cells undergo programmed cell death [36]. These findings suggest that FBW7-deficient T-ALL cells might require elevated levels of Mcl-1 to evade apoptosis. Therefore, down-regulation of Mcl-1 with sorafenib or specific Mcl-1 inhibitors could be a novel strategy to target FBW7-deficient cancer cells.
Inhibition of tumor growth by ABT-737 is impaired after loss of FBW7
ABT-737, a BH3 domain mimetic and a pan inhibitor of the Bcl-2 family, has been shown to inhibit anti-apoptotic proteins including Bcl-2, Bcl-XL, and Bcl-w, but not Mcl-1 [77]. It is known that Mcl-1 is an important member of the Bcl-2 family of proteins and suppresses the activities of pro-apoptotic molecules such as Bim, Bax, and Bak. Therefore, ABT-737 causes many cancer cell types to be refractory to treatment with this drug, primarily because ABT-737 fails to target and inactivate Mcl-1 due to its structural differences [77]. Indeed, van Delft et al. demonstrated that inactivation of Mcl-1 resulted in increased sensitivity to ABT-737 [78]. Our study has recently shown that FBW7 targets Mcl-1 for ubiquitination and degradation in a GSK3-dependent manner [36]. Specifically, we found that FBW7-deficient T-ALL cell lines were more resistant to ABT-737 [36]. We further showed that this is primarily due to the significant induction of Mcl-1 expression in FBW7-deficient cancer cells, and that FBW7 reconstitution can restore sensitivity to ABT-737 [36]. Moreover, specific depletion of Mcl-1 in FBW7-deficient TALL cell lines restored the sensitivity to ABT-737, suggesting that overexpression of Mcl-1 could be the reason for desensitization to ABT-737 [36]. Our data showed that loss of FBW7 elevated Mcl-1 expression, which led to resistance to ABT-737 [36]. On the one hand, our work indicates that FBW7-deficient patients will not respond well to ABT-737 treatment, but, on the other hand, suggests that re-introducing functional FBW7 might be a novel strategy to increase the sensitivity to ABT-737, although currently the gene therapy method is still in its infant stage.
Taxol-induced cell death is inhibited in the absence of FBW7
Taxol, also known as paclitaxel, is a cytotoxic chemotherapy drug, and is classified as an anti-microtubule agent that stabilizes microtubule structures within the cells, causing mitotic arrest and apoptosis [79]. Microtubules are an essential part of the cellular apparatus important for division and self-replication. Inhibition of these structures by Taxol arrests cells in mitosis and ultimately results in cell death [80]. Taxol has been used for the treatment of various types of solid cancers, including bladder, breast, esophageal, lung, melanoma, ovarian, and prostate cancers [79, 80]. Recently, the Wertz group reported that FBW7-null colon cancer cells were more resistant to Taxol-induced cell death than the wild-type cells [37]. Similar results were also observed in ovarian cancer cell lines with naturally occurring FBW7 mutations [37]. Similar trends were also found in human primary tumor samples. Specifically, tumors with mutant FBW7 were more resistant to Taxol compared to FBW7 wild-type parental tumors [37]. As expected, tumors expressing mutant FBW7 had higher levels of Mcl-1. Wertz et al. proposed that FBW7 mutations disrupted the association between FBW7 and Mcl-1 during mitotic arrest, leading to impaired Mcl-1 degradation and thus resistance to Taxol [37]. Further, inhibition of Mcl-1 in FBW7-null cells restored their sensitivity to Taxol-induced cell death [37], indicating that Mcl-1 is necessary for establishing resistance to Taxol in FBW7-deficient cells.
Vincristine-induced apoptosis is also inhibited in the absence of FBW7
Vincristine is also an anti-cancer chemotherapy drug that is classified as an anti-mitotic compound. Vincristine works as a microtubule de-stabilizer agent, inhibiting cellular microtubule structures and consequently inducing cell death [81]. Vincristine has been used for many human cancers such as acute leukemia, Hodgkin's and non-Hodgkin's lymphoma, neuroblastoma, rhabdomyosarcoma, Ewing's sarcoma, Wilms’ tumor, multiple myeloma, chronic leukemia, thyroid cancer, and brain tumors [81]. Recently, FBW7 was also found to be involved in sensitivity to Vincristine in human malignancies [37]. In ovarian and colon cancer cell lines, FBW7 wild-type cells were more sensitive to Vincristine-induced cell death than FBW7-deficient cells with elevated Mcl-1 expression due to failure of its degradation [37]. Additionally, inhibition of Mcl-1 expression in the FBW7-null cells enhanced Vincristine-induced apoptosis [37]. These studies demonstrated that restoration of FBW7 could open a new window to increase the sensitivity of tumor cells to Vincristine in human malignancies.
Inhibition of tumor growth by GSI is impaired following loss of FBW7
FBW7 has also been reported to be associated with resistance to GSI treatment [64, 70]. GSI has been used to inhibit the Notch signaling pathway in clinical trials [82]. The Notch signaling plays critical roles in many biological processes, including cell proliferation, apoptosis, migration, and invasion [83]. Notch genes encode transmembrane receptor proteins. Interacting with their ligands in the neighboring cells will activate Notch receptors. Subsequently, Notch can be cleaved through a cascade of proteolytic cleavages by the metalloprotease, tumor necrosis factor-α-converting enzyme (TACE) and gamma-secretase, leading to the release of the intracellular Notch (ICN) domain. GSI was shown to inhibit the Notch activity [83].
De-regulation of the Notch pathway has been found in various human cancers [84]. For example, activating mutations in Notch-1 were identified in ~50% of all human T-ALL cell lines as well as several mouse models of T cell leukemia [85]. Interestingly, most Notch-1 mutations often occur in the PEST domain, implying that the PEST domain may be required for proteasome-dependent degradation of Notch-1. Notch-1 mutation causes elevated ICN and subsequently activates its target genes such as Hes1 and c-Myc [86]. It has been found that FBW7 can target nuclear Notch-1 for ubiquitination and suppress Notch signaling [49, 70]. Recently, a study led by Nateri's group showed that loss of FBW7 in the gut resulted in the accumulation of Notch and c-Jun expression, and induced development of adenomas in mice [49]. FBW7 mutations are found in 30% of T-ALL clinical samples. Moreover, the majority of the FBW7 mutations are associated with Notch-1 mutations [87]. Furthermore, Thompson et al. [71] discovered that most of the T-ALL cell lines harboring FBW7 mutations were resistant to GSI treatment, and this resistance could be due to stabilization of the c-Myc protein, which is the Notch-1 target. Similarly, another group demonstrated that leukemic cell lines with FBW7 mutations were resistant to the GSI treatment [70]. Most of these resistant cell lines also failed to down-regulate Myc, one of the Notch targets, after GSI treatment, implying that the loss of FBW7 may function as a potential mechanism of drug resistance to GSI in T-ALL [70, 71]. Therefore, up-regulation of FBW7 could offer an attractive targeted therapy for tumors.
Targeting FBW7 to increase drug sensitivity
As we mentioned above, loss of FBW7 function in cancer cell lines affects their sensitivity to certain chemotherapeutic agents. Furthermore, reconstitution of FBW7 function can restore the cells’ sensitivity to the pharmacological agents, indicating that FBW7 loss, which is frequently observed in various types of human carcinomas, may be one of the causes for drug resistance changes [36]. Therefore, targeting FBW7 could be a novel strategy to increase the efficacy of chemo-therapeutic drugs. Although much is known about the targets of the FBW7 ubiquitin ligase pathway, there is limited knowledge about the regulatory mechanisms targeting FBW7 expression. Emerging evidence has demonstrated that multiple mechanisms contribute to the reduction or loss of FBW7 tumor suppressor function in vivo. The tumor suppressor gene p53 was first reported to directly promote the transcription of FBW7 mRNA. In other words, FBW7 is a direct transcriptional target of p53 [88, 89]. Recently, Welcker et al. showed that the EBNA1-binding protein 2 (Ebp2) directly binds to FBW7 like a substrate, and mediates FBW7 nucleolar targeting [90]. Furthermore, another group identified a new upstream gene called CCAAT/enhancer-binding protein-δ (C/EBPδ) that can directly inhibit the expression of FBW7 [91]. More recently, Lerner et al. discovered that microRNA (miRNA)-27a controls FBW7-dependent cyclin E degradation and cell cycle progression [92]. As FBW7 research continues to progress, we believe that more upstream regulatory proteins and miRNAs that regulate FBW7 expression will be identified. Therefore, targeting these proteins and miRNAs could be helpful in regulating FBW7, leading to increased drug sensitivity.
Targeting the p53 pathway to promote FBW7 expression
p53 is considered one of the most important tumor suppressor proteins as it is functionally compromised in a majority of human cancers. Central to p53 regulation is the Mdm2 oncoprotein, which is responsible for promoting the ubiquitination and destruction of p53 [93]. Therefore, enhanced Mdm2 destruction could contribute to the induction of p53 activity. FBW7 has been identified as a p53 target gene [88, 89]. In support of this notion, FBW7 was dramatically up-regulated by infection with adenovirus-mediated transfer of wild-type p53 into the p53-deficient cells. Moreover, p53-binding sites were discovered in the FBW7 exon, further strengthening FBW7 as a direct target of p53 [88]. Thus, targeting the p53 pathway could potentially regulate FBW7 expression. Because Mdm2 negatively regulates p53 and is found to be elevated in cancer cells, designing Mdm2 inhibitors to disrupt the Mdm2-p53 interaction, and consequently lead to up-regulation of FBW7, appears to be an attractive approach. Indeed, several Mdm2 inhibitors such as Nutlin-3a have been shown to sensitize cells to the effects of therapeutic drugs [93]. However, further study is warranted to investigate whether activating p53 could be a practical approach to promote FBW7 expression, thus enhancing the efficacy of certain chemotherapeutic drugs.
Targeting miRNAs to activate FBW7 expression
miRNAs, single-stranded 19–25-nucleotide RNAs, have recently been recognized as critical protein regulators that elicit their effects by binding to the 3′ untranslated region (3′UTR) of target mRNA, thereby inhibiting the translation of the encoded protein or destabilizing the target mRNA [94]. Some miRNAs that are overexpressed in human cancers have oncogenic activities. On the other hand, others have tumor suppressor activities and are down-regulated in cancers [95]. It is important to note that individual miRNAs could potentially regulate multiple target genes. Conversely, one given gene could be regulated by several different upstream miRNAs [95]. Recent studies have identified altered expression of specific miRNAs in drug-resistant tumor cells, indicating that targeting miRNAs could be useful to convert drug-resistant cells to drug-sensitive cells [94].
Specifically, one recent study provided the first evidence that two miRNAs, named miR-25 and miR-223, reduced mRNA levels and the activation of FBW7 [96]. Moreover, overexpression of miR-223 significantly increased expression and activity of cyclin E, an FBW target, while reduced miR-223 expression led to increased FBW7 expression and decreased cyclin E activity [96]. These observations imply that expression and activity of FBW7 can be modulated directly by the miRNA pathway [96]. Mavrakis et al. also found that Mcl-1 levels are increased in mouse leukemias expressing miR-223, which targets FBW7 [97]. A recent study led by the Sangfelt group [92] showed that miR-27a is a direct physiological regulator of FBW7. This group used a luciferase-based screening method in combination with existing miRNA expression profiling data and found that miR-27a is a major suppressor of FBW7 [92]. miR-27a interferes with FBW7-dependent ubiquitination and degradation of cyclin E, leading to dysregulation of cell cycle progression. Furthermore, miR-27a was overexpressed in pediatric precursor B cell acute lymphoblastic leukemia (B-ALL), implying that miR-27a has putative oncogenic function due to its negative regulation of FBW7 [92]. Several lines of evidence from the recent studies demonstrated that down-regulation of miR-27a could confer sensitivity to chemotherapeutic drugs such as Vincristine, Adriamycin, Cisplatin, and 5-Flurouracil in various human cancers [98, 99]. It is possible that down-regulation of miR-27a reverses drug resistance due to up-regulation of FBW7. However, further in-depth research is needed to fully understand how miRNA-27a regulates FBW7 and is involved in drug resistance. This evidence supports the hypothesis that down-regulation of miR-27a could be a new strategy to improve drug sensitivity. More importantly, these studies have created the rationale for identifying more miRNAs that could potentially regulate FBW7. Thus, it is our belief that additional miRNAs that regulate FBW7 will be discovered in the future. Furthermore, down- or overexpression of these miRNAs will reverse drug resistance by specifically regulating FBW7 functions.
Conclusions and perspectives
In conclusion, recent studies have demonstrated that loss of FBW7 tumor suppressor function in human cancer cell lines could promote resistance to certain therapeutic drugs such as Taxol and ABT-737, while increasing drug sensitivity to certain drugs such as sorafenib (Fig. 3). In parallel, reconstitution of FBW7 function was able to restore the sensitivity of tumor cells to the indicated pharmacological agents. Therefore, increasing FBW7 expression could be a novel treatment strategy to reverse drug resistance to many drugs including Taxol and ABT-737 for eliminating drug-resistant tumor cells that are the root cause of tumor recurrence. On the other hand, identification of additional drugs behaving similarly to sorafenib, which has increased drug sensitivity in FBW7-deficient cells, could also be used as a novel therapeutic approach for targeted (or personalized) therapies of FBW7-deficient patients.
Figure 3.
FBW7-deficient tumor cells with elevated levels of Mcl-1 are more sensitive to the Mcl-1 antagonist. The expression of c-Jun, c-Myc, and Notch-1 are increased due to loss of FBW7, which promotes cell growth in FBW7-deficient cells. The pro-apoptotic effects of c-Jun, c-Myc, and Notch-1, on the other hand, are balanced by the elevated expression of Mcl-1. Thus, FBW7-deficient tumor cells are “addicted” to high expression of the Mcl-1 oncoprotein. However, when FBW7-deficient tumor cells are treated with chemotherapeutic agents targeting Mcl-1, the pro-survival function of Mcl-1 is inhibited. In this scenario, loss of the protection of Mcl-1 causes the FBW7-deficient tumor cells to undergo programmed cell death.
So far, no compounds directly targeting FBW7 have been reported. Currently, only the proteasome inhibitor bortezomib has been approved by the FDA and successfully used in the treatment of myelomas [100]. Although, bortezomib has demonstrated a certain degree of cancer cell selectivity with measurable therapeutic index, bortezomib could cause unexpected toxicity in normal cells due to loss of specific target, leading to its inhibition of overall protein degradation [100]. Therefore, an alternative and ideal approach would be to target specific E3 ligases such as FBW7 to avoid unwanted side effects. However, results so far indicate that FBW7 mainly functions as a tumor suppressor, which attenuates the enthusiasm for developing specific FBW7 inhibitors for anti-cancer agents. On the other hand, it makes it appealing to develop specific inhibitors for upstream regulatory proteins serving to activate or restore the tumor suppressor function of FBW7. Alternatively, designing specific inhibitors to FBW7 downstream targets, many of which are well-characterized onco-proteins, may also be a viable therapeutic approach. Moreover, profiling the FBW7 status of individual tumors, such as mRNA levels and protein levels, could also be critical in guiding the optimization of personalized medicines to better sensitize the individual patient to specific drugs. We anticipate that in the future such approaches will benefit clinical development of compounds directly or indirectly targeting FBW7 for the development of more specific, less toxic, and more efficacious anti-cancer therapeutics.
Acknowledgments
We sincerely apologize to all those colleagues whose important work was not cited in this paper due to space limitations. The authors’ work cited in paper was funded by grants from the National Institute of General Medicines, NIH (GM089763) to W. W., and Massachusetts Life Science Center New Investigator award (W. W.), and Department of Defense Prostate New Investigator award to W. W.
Abbreviations
- ABC
ATP-binding cassette
- B-ALL
precursor B cell acute lymphoblastic leukemia
- BCRP
breast cancer resistant protein
- COX-2
cyclooxygenase-2
- CSC
cancer stem cell
- Ebp2
EBNA1-binding protein 2
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- FBW7
F-box and WD repeat domain-containing 7
- FGFR
fibroblast growth factor receptor
- GSI
gamma secretase inhibitor
- GSK
glycogen synthase kinase
- HECT
homologous to E6-AP C terminus
- ICN
intracellular Notch
- IGF
insulin-like growth factor
- KLF
Krüppel-like factor
- MAPK
mitogen-activated protein kinase
- miRNA
microRNA
- MRP1
multidrug resistance-associated protein
- mTOR
mammalian target of rapamycin
- NLK
Nemo-like kinase
- PDGF
platelet-derived growth factor
- PGP
P-glycoprotein
- RCL
Ring/Cullin Ligase
- SCF
Skp1-Cullin1-F-box
- SREBP
sterol regulatory element-binding proteins
- T-ALL
T cell acute lymphoblastic leukemia
- UPS
ubiquitin proteasome system
- 3′UTR
3′ untranslated region
- VEGF
vascular endothelial growth factor
References
- 1.Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat. 2011;14:150–63. doi: 10.1016/j.drup.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Li Y, Ahmad A, Banerjee S, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 3.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Li Y, Ahmad A, Azmi AS, et al. Targeting miRNAs involved in cancer stem cell and EMT regulation: an emerging concept in overcoming drug resistance. Drug Resist Updat. 2010;13:109–18. doi: 10.1016/j.drup.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 7.Burger H, Loos WJ, Eechoute K, Verweij J, et al. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat. 2011;14:22–34. doi: 10.1016/j.drup.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin JJ, Castano B, Blazquez AG, Rosales R, et al. Strategies for overcoming chemotherapy resistance in enterohepatic tumours. Curr Mol Med. 2010;10:467–85. doi: 10.2174/156652410791608261. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Cabral F. Paclitaxel resistance in cells with reduced beta-tubulin. Biochim Biophys Acta. 2005;1744:245–55. doi: 10.1016/j.bbamcr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Ercan D, Zejnullahu K, Yonesaka K, Xiao Y, et al. Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor. Oncogene. 2010;29:2346–56. doi: 10.1038/onc.2009.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 13.Ding XW, Wu JH, Jiang CP. ABC G2: a potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010;86:631–7. doi: 10.1016/j.lfs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16:12–9. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 15.Karnak D, Xu L. Chemosensitization of prostate cancer by modulating Bcl-2 family proteins. Curr Drug Targets. 2010;11:699–707. doi: 10.2174/138945010791170888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhillon KK, Swisher EM, Taniguchi T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011;102:663–9. doi: 10.1111/j.1349-7006.2010.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku JL, Shin YK, Kim DW, Kim KH, et al. Establishment and characterization of 13 human colorectal carcinoma cell lines: mutations of genes and expressions of drug-sensitivity genes and cancer stem cell markers. Carcinogenesis. 2010;31:1003–9. doi: 10.1093/carcin/bgq043. [DOI] [PubMed] [Google Scholar]
- 18.Zwart W, Rondaij M, Jalink K, Sharp ZD, et al. Resistance to antiestrogen arzoxifene is mediated by overexpression of cyclin D1. Mol Endocrinol. 2009;23:1335–45. doi: 10.1210/me.2008-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22:708–22. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 20.Carr JR, Park HJ, Wang Z, Kiefer MM, et al. FoxM1 mediates resistance to herceptin and paclitaxel. Cancer Res. 2010;70:5054–63. doi: 10.1158/0008-5472.CAN-10-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Ahmad A, Li Y, Banerjee S, et al. Forkhead box M1 transcription factor: a novel target for cancer therapy. Cancer Treat Rev. 2010;36:151–6. doi: 10.1016/j.ctrv.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Fiore F, Sesboue R, Michel P, Sabourin JC, et al. Molecular determinants of anti-EGFR sensitivity and resistance in metastatic colorectal cancer. Br J Cancer. 2010;103:1765–72. doi: 10.1038/sj.bjc.6606008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kono SA, Marshall ME, Ware KE, Heasley LE. The fibroblast growth factor receptor signaling pathway as a mediator of intrinsic resistance to EGFR-specific tyrosine kinase inhibitors in non-small cell lung cancer. Drug Resist Updat. 2009;12:95–102. doi: 10.1016/j.drup.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hixon ML, Paccagnella L, Millham R, Perez-Olle R, et al. Development of inhibitors of the IGF-IR/PI3K/Akt/mTOR pathway. Rev Recent Clin Trials. 2010;5:189–208. doi: 10.2174/157488710792007329. [DOI] [PubMed] [Google Scholar]
- 25.Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res. 2010;16:3329–34. doi: 10.1158/1078-0432.CCR-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haagenson KK, Wu GS. The role of MAP kinases and MAP kinase phosphatase-1 in resistance to breast cancer treatment. Cancer Metastasis Rev. 2010;29:143–9. doi: 10.1007/s10555-010-9208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11:63–76. doi: 10.1016/j.drup.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167–80. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Li Y, Ahmad A, Azmi AS, et al. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta. 2010;1806:258–67. doi: 10.1016/j.bbcan.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Li Y, Kong D, Banerjee S, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–7. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Ahmad A, Li Y, Kong D, et al. Emerging roles of PDGF-D signaling pathway in tumor development and progression. Biochim Biophys Acta. 2010;1806:122–30. doi: 10.1016/j.bbcan.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Yu D. PI(3)king apart PTEN's role in cancer. Clin Cancer Res. 2010;16:4325–30. doi: 10.1158/1078-0432.CCR-09-2990. [DOI] [PubMed] [Google Scholar]
- 34.Cheung CH, Cheng L, Chang KY, Chen HH, et al. Investigations of survivin: the past, present and future. Front Biosci. 2011;16:952–61. doi: 10.2741/3728. [DOI] [PubMed] [Google Scholar]
- 35.Inuzuka H, Fukushima H, Shaik S, Liu P, et al. Mcl-1 ubiquitination and destruction. Oncotarget. 2011;2:239–44. doi: 10.18632/oncotarget.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inuzuka H, Shaik S, Onoyama I, Gao D, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–9. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wertz IE, Kusam S, Lam C, Okamoto T, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–4. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 38.Crusio KM, King B, Reavie LB, Aifantis I. The ubiquitous nature of cancer: the role of the SCF(Fbw7) complex in development and transformation. Oncogene. 2010;29:4865–73. doi: 10.1038/onc.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 40.Bedford L, Lowe J, Dick LR, Mayer RJ, et al. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–44. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- 42.Jin J, Cardozo T, Lovering RC, Elledge SJ, et al. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–80. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koepp DM, Schaefer LK, Ye X, Keyomarsi K, et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–7. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- 44.Minella AC, Welcker M, Clurman BE. Ras activity regulates cyclin E degradation by the Fbw7 pathway. Proc Natl Acad Sci USA. 2005;102:9649–54. doi: 10.1073/pnas.0503677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yada M, Hatakeyama S, Kamura T, Nishiyama M, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23:2116–25. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welcker M, Orian A, Jin J, Grim JE, et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA. 2004;101:9085–90. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei W, Jin J, Schlisio S, Harper JW, et al. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Hoeck JD, Jandke A, Blake SM, Nye E, et al. Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nat Neurosci. 2010;13:1365–72. doi: 10.1038/nn.2644. [DOI] [PubMed] [Google Scholar]
- 49.Babaei-Jadidi R, Li N, Saadeddin A, Spencer-Dene B, et al. FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation. J Exp Med. 2011;208:295–312. doi: 10.1084/jem.20100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tetzlaff MT, Yu W, Li M, Zhang P, et al. Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci USA. 2004;101:3338–45. doi: 10.1073/pnas.0307875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rocher-Ros V, Marco S, Mao JH, Gines S, et al. Presenilin modulates EGFR signaling and cell transformation by regulating the ubiquitin ligase Fbw7. Oncogene. 2010;29:2950–61. doi: 10.1038/onc.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundqvist A, Bengoechea-Alonso MT, Ye X, Lukiyanchuk V, et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab. 2005;1:379–91. doi: 10.1016/j.cmet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Punga T, Bengoechea-Alonso MT, Ericsson J. Phosphorylation and ubiquitination of the transcription factor sterol regulatory element-binding protein-1 in response to DNA binding. J Biol Chem. 2006;281:25278–86. doi: 10.1074/jbc.M604983200. [DOI] [PubMed] [Google Scholar]
- 54.Fu L, Kim YA, Wang X, Wu X, et al. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009;69:8967–76. doi: 10.1158/0008-5472.CAN-09-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu N, Li H, Li S, Shen M, et al. The Fbw7/human CDC4 tumor suppressor targets proproliferative factor KLF5 for ubiquitination and degradation through multiple phosphodegron motifs. J Biol Chem. 2010;285:18858–67. doi: 10.1074/jbc.M109.099440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao D, Zheng HQ, Zhou Z, Chen C. The Fbw7 tumor suppressor targets KLF5 for ubiquitin-mediated degradation and suppresses breast cell proliferation. Cancer Res. 2010;70:4728–38. doi: 10.1158/0008-5472.CAN-10-0040. [DOI] [PubMed] [Google Scholar]
- 57.Thompson BJ, Jankovic V, Gao J, Buonamici S, et al. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J Exp Med. 2008;205:1395–408. doi: 10.1084/jem.20080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finkin S, Aylon Y, Anzi S, Oren M, et al. Fbw7 regulates the activity of endoreduplication mediators and the p53 pathway to prevent drug-induced polyploidy. Oncogene. 2008;27:4411–21. doi: 10.1038/onc.2008.77. [DOI] [PubMed] [Google Scholar]
- 59.Tsunematsu R, Nakayama K, Oike Y, Nishiyama M, et al. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J Biol Chem. 2004;279:9417–23. doi: 10.1074/jbc.M312337200. [DOI] [PubMed] [Google Scholar]
- 60.Onoyama I, Tsunematsu R, Matsumoto A, Kimura T, et al. Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J Exp Med. 2007;204:2875–88. doi: 10.1084/jem.20062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuoka S, Oike Y, Onoyama I, Iwama A, et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 2008;22:986–91. doi: 10.1101/gad.1621808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akhoondi S, Lindstrom L, Widschwendter M, Corcoran M, et al. Inactivation of FBXW7/hCDC4-beta expression by promoter hypermethylation is associated with favorable prognosis in primary breast cancer. Breast Cancer Res. 2010;12:R105. doi: 10.1186/bcr2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sancho R, Jandke A, Davis H, Diefenbacher ME, et al. F-box and WD repeat domain-containing 7 regulates intestinal cell lineage commitment and is a haploinsufficient tumor suppressor. Gastroenterology. 2010;139:929–41. doi: 10.1053/j.gastro.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 64.O'Neil J, Look AT. Mechanisms of transcription factor deregulation in lymphoid cell transformation. Oncogene. 2007;26:6838–49. doi: 10.1038/sj.onc.1210766. [DOI] [PubMed] [Google Scholar]
- 65.Akhoondi S, Sun D, von der Lehr N, Apostolidou S, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–12. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 66.Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, et al. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- 67.Spruck CH, Strohmaier H, Sangfelt O, Muller HM, et al. hCDC4 gene mutations in endometrial cancer. Cancer Res. 2002;62:4535–9. [PubMed] [Google Scholar]
- 68.Kitagawa K, Hiramatsu Y, Uchida C, Isobe T, et al. Fbw7 promotes ubiquitin-dependent degradation of c-Myb: involvement of GSK3-mediated phosphorylation of Thr-572 in mouse c-Myb. Oncogene. 2009;28:2393–405. doi: 10.1038/onc.2009.111. [DOI] [PubMed] [Google Scholar]
- 69.Kitagawa K, Kotake Y, Hiramatsu Y, Liu N, et al. GSK3 regulates the expressions of human and mouse c-Myb via different mechanisms. Cell Div. 2010;5:27. doi: 10.1186/1747-1028-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Neil J, Grim J, Strack P, Rao S, et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007;204:1813–24. doi: 10.1084/jem.20070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson BJ, Buonamici S, Sulis ML, Palomero T, et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J Exp Med. 2007;204:1825–35. doi: 10.1084/jem.20070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yau T, Pang R, Chan P, Poon RT. Molecular targeted therapy of advanced hepatocellular carcinoma beyond sorafenib. Expert Opin Pharmacother. 2010;11:2187–98. doi: 10.1517/14656561003724705. [DOI] [PubMed] [Google Scholar]
- 73.Panka DJ, Cho DC, Atkins MB, Mier JW. GSK-3beta inhibition enhances sorafenib-induced apoptosis in melanoma cell lines. J Biol Chem. 2008;283:726–32. doi: 10.1074/jbc.M705343200. [DOI] [PubMed] [Google Scholar]
- 74.Huber S, Oelsner M, Decker T, zum Buschenfelde CM, et al. Sorafenib induces cell death in chronic lymphocytic leukemia by translational downregulation of Mcl-1. Leukemia. 2011;25:838–47. doi: 10.1038/leu.2011.2. [DOI] [PubMed] [Google Scholar]
- 75.Minella AC, Clurman BE. Mechanisms of tumor suppression by the SCF(Fbw7). Cell Cycle. 2005;4:1356–9. doi: 10.4161/cc.4.10.2058. [DOI] [PubMed] [Google Scholar]
- 76.Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214–31. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- 77.Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009;9:321–6. doi: 10.1038/nrc2615. [DOI] [PubMed] [Google Scholar]
- 78.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baird RD, Tan DS, Kaye SB. Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nat Rev Clin Oncol. 2010;7:575–82. doi: 10.1038/nrclinonc.2010.120. [DOI] [PubMed] [Google Scholar]
- 80.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 81.Moore A, Pinkerton R. Vincristine: can its therapeutic index be enhanced? Pediatr Blood Cancer. 2009;53:1180–7. doi: 10.1002/pbc.22161. [DOI] [PubMed] [Google Scholar]
- 82.Rizzo P, Osipo C, Foreman K, Golde T, et al. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–31. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z, Li Y, Sarkar FH. Notch signaling proteins: legitimate targets for cancer therapy. Curr Protein Pept Sci. 2010;11:398–408. doi: 10.2174/138920310791824039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–51. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 85.Demarest RM, Ratti F, Capobianco AJ. It's T-ALL about Notch. Oncogene. 2008;27:5082–91. doi: 10.1038/onc.2008.222. [DOI] [PubMed] [Google Scholar]
- 86.Real PJ, Ferrando AA. NOTCH inhibition and glucocorticoid therapy in T-cell acute lymphoblastic leukemia. Leukemia. 2009;23:1374–7. doi: 10.1038/leu.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maser RS, Choudhury B, Campbell PJ, Feng B, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–71. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mao JH, Perez-Losada J, Wu D, Delrosario R, et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432:775–9. doi: 10.1038/nature03155. [DOI] [PubMed] [Google Scholar]
- 89.Yokobori T, Mimori K, Iwatsuki M, Ishii H, et al. p53-Altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res. 2009;69:3788–94. doi: 10.1158/0008-5472.CAN-08-2846. [DOI] [PubMed] [Google Scholar]
- 90.Welcker M, Larimore EA, Frappier L, Clurman BE. Nucleolar targeting of the fbw7 ubiquitin ligase by a pseudosubstrate and glycogen synthase kinase 3. Mol Cell Biol. 2011;31:1214–24. doi: 10.1128/MCB.01347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balamurugan K, Wang JM, Tsai HH, Sharan S, et al. The tumour suppressor C/EBPdelta inhibits FBXW7 expression and promotes mammary tumour metastasis. EMBO J. 2010;29:4106–17. doi: 10.1038/emboj.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lerner M, Lundgren J, Akhoondi S, Jahn A, et al. miRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle. 2011;10:2172–83. doi: 10.4161/cc.10.13.16248. [DOI] [PubMed] [Google Scholar]
- 93.Cheok CF, Verma CS, Baselga J, Lane DP. Translating p53 into the clinic. Nat Rev Clin Oncol. 2011;8:25–37. doi: 10.1038/nrclinonc.2010.174. [DOI] [PubMed] [Google Scholar]
- 94.Sarkar FH, Li Y, Wang Z, Kong D, et al. Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resist Updat. 2010;13:57–66. doi: 10.1016/j.drup.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 96.Xu Y, Sengupta T, Kukreja L, Minella AC. MicroRNA-223 regulates cyclin E activity by modulating expression of F-box and WD-40 domain protein 7. J Biol Chem. 2010;285:34439–46. doi: 10.1074/jbc.M110.152306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mavrakis KJ, Van Der Meulen J, Wolfe AL, Liu X, et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL). Nat Genet. 2011;43:673–8. doi: 10.1038/ng.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao X, Yang L, Hu J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. J Exp Clin Cancer Res. 2011;30:55. doi: 10.1186/1756-9966-30-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng DD, Zhang H, Zhang P, Zheng YS, et al. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukemia. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01213.x. in press, DOI: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duensing S, Duensing A. Bortezomib: killing two birds with one stone in gastrointestinal stromal tumors. Oncotarget. 2010;1:6–8. doi: 10.18632/oncotarget.103. [DOI] [PMC free article] [PubMed] [Google Scholar]