Abstract
Essential tremor (ET) is among the most prevalent neurological diseases. A substantial increase in the number of Purkinje cell axonal swellings (torpedoes) has been identified in ET brains. We recently demonstrated that torpedoes in ET contain an over-accumulation of disorganized neurofilament (NF) proteins. This now raises the question whether NF protein composition and/or phosphorylation state in cerebellar tissue might differ between ET cases and controls.
We used a Western blot analysis to compare the levels and phosphorylation state of NF proteins and α-internexin in cerebellar tissue from 47 ET cases vs. 26 controls (2:1 ratio). Cases and controls did not differ with respect to the cerebellar levels of NF-light (NF-L), NF-medium (NF-M), NF-heavy (NF-H), or α-internexin. However, SMI-31 levels (i.e., phosphorylated NF-H) and SMI-32 levels (i.e., non-phosphorylated NF-H) were significantly higher in ET cases than controls (1.28 ± 0.47 vs. 1.06 ± 0.32, p = 0.02; and 1.38 ± 0.75 vs. 1.00 ± 0.42, p = 0.006). Whether the abnormal phosphorylation state that we observed is a cause of defective axonal transport and/or function of NFs in ET is not known. NF abnormalities have been demonstrated in several neurodegenerative diseases. Regardless of whether these protein aggregates are the cause or consequence of these diseases, NF abnormalities have been shown to be an important factor in the cellular disruption observed in several neurodegenerative diseases. Therefore, further analyses of these NF abnormalities and their mechanisms are important to enhance our understanding of disease pathogenesis in ET.
Keywords: essential tremor, Purkinje cell, pathophysiology, neurofilament proteins, neurodegenerative, Western blot
Introduction
Essential tremor (ET) is among the most prevalent age-related neurological diseases [17]. The precise pathogenesis is not understood, although recent postmortem studies have identified several structural, degenerative changes within the cerebellum of ET patients [15]. Prominent among these changes is a substantial increase in the number of torpedoes (i.e., swellings of the proximal Purkinje cell axon) [16]. Electron microscopic studies have revealed that torpedoes contain an excess and disorganization of neurofilaments (NFs) within these axonal swellings [18].
In general, abnormal accumulations of NFs, and the ensuing axonal swellings (i.e., torpedoes), can either be a cause or a consequence of impaired axonal transport within neurons that are compromised [1, 13]. In mice that over-express NFs, these filaments self-assemble to form disorganized structures within axonal swellings, and this is thought it result in neuronal dysfunction and neuronal death [1, 13]. A decrease in number of Purkinje cells has been reported in ET, and ET cases with more torpedoes are also characterized by more Purkinje cell loss [16].
Neuronal intermediate filaments (IF) in the central nervous system are composed of the NF triplet proteins (NFTPs) and α-internexin [12], and provide the main scaffolding for axons. NFs are composed of three distinct molecular weight proteins: NF-light (NF-L), NF-medium (NF-M), and NF-heavy (NF-H) [9]. These proteins are under tight stoichiometric balance during the assembly of the NF network [23]. α-Internexin is the first IF protein expressed in both central and peripheral neurons during development, but is less abundant in mature neurons than the NFTPs.
NF-H is a large protein whose tail domain (amino acid residues 502 – 823) includes an estimated 38 – 43 phosphorylation sites, which include but are not limited to 33 non-contiguous lysine-serine-proline repeats [6, 8, 9, 22, 23]. Monoclonal antibodies to SMI-31 react with phosphorylated epitopes on NF-H, although the number and precise distribution of these sites in the tail region of NF-H has not been mapped. Furthermore, while each of these sites can be phosphorylated by a number of kinases, there is considerable heterogeneity in phosphorylation, and the percentage that are phosphorylated at any given time in a normal individual is not known [8]. Monoclonal antibodies to SMI-32 react with nonphosphorylated epitopes of NF-H; here as well, the precise distribution of these sites in the tail region of NF-H has not been mapped. Western blotting with SMI31 or SMI32 antibodies identifies a range of ~160 – 210 kDa molecular mass proteins in SDS gels, which reflects this variable phosphate content of the NF-H protein [29].
Many mechanisms can lead to the accumulation of NFs, including mutation, dysregulation of NF protein synthesis, defective axonal transport, and decreased proteolysis [10, 14]. Neurofilament phosphorylation is known to affect all of these processes [10]. Neurofilament phosphorylation may be abnormal, with evidence of either increase, decrease, or both [23]. The question is “open to speculation” as to whether increased or decreased phosphorylation indicates axonal injury [23]. Abnormal phosphorylation may also affect NF function [23].
Abnormal phosphorylation of neuronal cytoskeletal proteins is one of the major pathological hallmarks of several neurodegenerative disorders, including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and Parkinson’s disease (PD) [24].
Capitalizing on the resources of the Essential Tremor Centralized Brain Repository (ETCBR), which stores the largest collection of ET brains in the world, we used Western blot analysis to quantify the levels of NF proteins in cerebellar tissue. Using mouse monoclonal SMI-31 and SMI-32 antibodies, which are among several antibodies that identify phosphorylated and non-phosphorylated states of NF-H, we explored the phosphorylation state of NF-H. We tested the hypothesis that NF protein composition and/or phosphorylation state in cerebellar tissue might differ between ET cases and controls.
Methods
Subjects
The study was conducted at the ETCBR of the New York Brain Bank (NYBB), Columbia University Medical Center. All ET cases were diagnosed by their treating neurologist and the ET diagnosis was confirmed using published ETCBR criteria by a second neurologist specializing in movement disorders (E.D.L.) [16]. Frozen cerebellar tissue was available on 47 ET cases (mean age = 87.5 ± 7.3 years).
Control brains were normal elderly control subjects from the NYBB, derived from the Alzheimer’s Disease Research Center and the Washington Heights Inwood Columbia Aging Project; they were free of clinical diagnoses of AD, ET, or PD and without neuropathological diagnoses of neurodegenerative disease. We selected those controls who had available frozen cerebellar tissue and, in order to approximate the age of the cases, those who were ≥ 65 years of age at the time of death. This resulted in an approximate 2:1 ratio of cases to controls (i.e., 47:26). The NYBB operates under approval of the Institutional Review Board (IRB) of Columbia University Medical Center.
Clinical Evaluation
During life, clinical data were collected through a series of semi-structured questionnaires. Heavy ethanol use was defined previously as consumption of an average of ≥4 standard drinks/day (men) or ≥3/day (woman), at any point in their lives [7]. Data on lifetime exposure to medications known to cause cerebellar damage (e.g., lithium, diphenylhydantoin) were collected. Most ET cases underwent a standardized, videotaped neurological examination, including a detailed assessment of tremor [19].
Neuropathological Assessment
All brains underwent a complete neuropathological assessment at the NYBB [16], and had standardized measurements of brain weight (grams), postmortem interval (PMI, hours between death and placement of brain in a cold room or upon ice), Braak and Braak AD staging for neurofibrillary tangles [3], Braak PD staging of Lewy bodies [4], and Consortium to Establish a Registry for AD (CERAD) ratings for neuritic plaques [20].
As described, a standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum [16]; the block included the cerebellar cortex, white matter and dentate nucleus. A senior neuropathologist (P.L.F.) who was blinded to all clinical information counted torpedoes throughout one entire Luxol Fast Blue counterstained with Hematoxylin and Eosin (LH&E) 7-um thick section and one entire Bielschowsky stained 7-um thick section, and counted and averaged Purkinje cells in five 100× fields (LH&E) [16]. As described [5], a semiquantitative (0 – 3) rating of the appearance of the basket cell plexus surrounding Purkinje cell bodies throughout Bielschowsky preparations was carried out by the same neuropathologist.
Western Blot Analysis
Frozen cerebellar tissue (250 mg) from lateral cerebellar hemisphere was homogenized in 2 mL of 62.5 mM Tris HCl, pH 6.8, 5mM EDTA, 1% SDS, with protease and phosphatase inhibitors (Roche Diagnostics), and centrifuged at 14,000 × g, 10 minutes at 4°C. Proteins (20 mg; Pierce BCA assay) were separated on 8% or 10% SDS polyacrylamide gels and transferred to polyvinylidene difluoride membrane. Blots were probed overnight at 4°C with antibody to NF-L (N5139, mouse, 1:1000, Sigma, St. Louis, MO), NF-M (NB 300-133, rabbit, 1:20,000, Novus Biologicals, Littleton, CO), NF-H (NB 300-135, rabbit, 1:3000, Novus Biologicals, Littleton, CO), α-internexin (AB 40758, rabbit, 1:5000, Abcam, Cambridge, MA), phosphorylated NF-H (SMI-31R-100, mouse, 1:1000, Covance, Princeton, NJ) or non-phosphorylated NF-H (SMI-32R-100, mouse, 1:1000, Covance, Princeton, NJ), and antibody to b-actin (rabbit, ab8227; mouse, ab6267, 1:5000; Abcam, Cambridge, MA). LICOR secondary antibodies were visualized with a LI-COR Infrared Odyssey Scanner (LI-COR Biosciences, Lincoln, NE). Signal intensities were analyzed with Image Quant (Amersham Biosciences, Piscataway, NJ), normalized to the b-actin signal and expressed relative to the average control value, arbitrarily set at 1.0.
Immunohistochemistry
Paraffin sections (7 mm) were heated in Trilogy Solution (Cell Marque, Rocklin, CA), followed by 1% hydrogen peroxide and serum blocking solution (10% normal goat serum, 1% bovine serum albumin, 1% Triton, in PBS). Rabbit anti-NF-H (1:500), mouse monoclonal SMI-31 (1:200) or mouse monoclonal SMI-32 antibody (1:300) were applied overnight at 4°C. Secondary antibody (SMI-31, peroxidase-goat-anti-mouse, [Thermo Scientific, Waltham, MA]; SMI32, biotinylated goat-anti-mouse [Vector Laboratories, Burlingame, CA]; NF-H, biotin-SP goat-anti-Rabbit [Fisher Scientific, Waltham, MA], followed by Streptavidin-HRP (AbD Serotec, Raleigh, NC) for biotinlyated antibodies) was developed with DAB Chromogen Solution (Dako, Carpinteria, CA). Staining was not detected with NF-L, NF-M or a-internexin antibodies.
Statistical Analyses
Statistical analyses were performed in SPSS (version 18.0). Clinical and postmortem findings were compared in cases and controls using Student’s t tests, chi-square tests, and non-parametric tests (Kruskal-Wallis test, Mann-Whitney test), when required. Pearson’s correlation coefficients were used to assess linear correlations, and Spearman’s correlation coefficients for non-linear correlations.
Results
There were 47 ET cases and 26 controls (Table 1). Cases and controls had similar gender distributions, brain weights and CERAD plaque scores, but cases had a shorter PMI (Table 1). While cases and controls were of advanced age (mean age > 80 in each group), cases were on average 6.3 years older than controls (Table 1). None of the cases or controls was a heavy ethanol user or had been exposed to medications known to cause cerebellar damage.
Table 1.
Clinical Characteristics of 47 ET Cases and 26 Controls
| ET Cases | Controls | Significance | |
|---|---|---|---|
| Age (years) | 87.5 ± 7.3 | 81.2 ± 8.3 | p = 0.002 a |
| Female gender | 30 (63.8) | 14 (53.8%) | p = 0.40 b |
| Tremor duration (years) | 48.4 ± 22.6 | Not applicable | Not applicable |
| PMI (hours) | 3.3 ± 4.8 | 6.1 ± 4.7 | p = 0.01 a |
| Brain weight (grams) | 1191 ± 147 | 1192 ± 129 | p = 0.98 a |
| CERAD plaque score | p = 0.14 c | ||
| 0 | 18 (38.3) | 14 (53.8) | |
| A | 19 (40.4) | 9 (34.6) | |
| B | 6 (12.8) | 3 (11.5) | |
| C | 4 (8.5) | 0 (0.0) | |
| Braak AD stage | p = 0.001 c | ||
| 0 | 0 (0.0) | 6 (23.1) | |
| 1 | 12 (25.5) | 9 (34.6) | |
| 2 | 12 (25.5) | 7 (26.9) | |
| 3 | 19 (40.4) | 3 (11.5) | |
| 4 | 3 (6.4) | 1 (3.8) | |
| 5 | 1 (2.1) | 0 (0.0) | |
| 6 | 0 (0.0) | 0 (0.0) | |
| Torpedo count (LH&E) | 16.3 ± 15.7 [12] | 4.0 ± 6.0 [3] | p < 0.001 d |
| Torpedo count (Bielschowsky) | 26.7 ± 26.5 [16] | 3.2 ± 3.2 [3] | p < 0.001 d |
| Purkinje cell count (LH&E) | 6.6 ± 1.7 [6.8] | 9.1 ± 2.4 [7.9] | p < 0.001 d |
| Basket cell axonal plexus densitye | p = 0.005 c | ||
| 0 | 1 (2.4) | 1 (5.9) | |
| 0.5 | 2 (4.9) | 1 (5.9) | |
| 1 | 4 (9.8) | 7 (41.2) | |
| 1.5 | 7 (17.1) | 1 (5.9) | |
| 2 | 9 (22.0) | 6 (35.3) | |
| 2.5 | 7 (17.1) | 1 (5.9) | |
| 3 | 11 (26.8) | 0 (0.0) | |
AD (Alzheimer’s disease), CERAD (Consortium to Establish a Registry for AD), LH&E (Luxol Fast Blue counterstained with Hematoxylin and Eosin), PMI (Postmortem interval).
All values are mean ± standard deviation [median] or number (percentage).
Student’s t test.
Chi-square test.
Kruskal-Wallis test.
Mann-Whitney test.
Some subjects did not have available data.
Cases had higher torpedo counts, lower Purkinje cell counts, higher Basket cell axonal plexus density, and higher Braak AD stage compared with controls (Table 2). One case and two controls had incidental brainstem Lewy bodies (Braak Stage 1 or 2).
Table 2.
Quantitative Western Blot Data on Neuronal Intermediate Filaments in Cerebellar Cortex
| ET Cases (n = 47) | Controls (n = 26) | Significance | |
|---|---|---|---|
| NF-L | 1.08 ± 0.28 | 1.01 ± 0.19 | p = 0.26 a |
| NF-M | 1.08 ± 0.59 | 1.02 ± 0.25 | p = 0.56 a |
| NF-H | 1.01 ± 0.21 | 1.00 ± 0.10 | p = 0.89 a |
| α-internexin | 1.08 ± 0.29 | 1.00 ± 0.21 | p = 0.21 a |
| SMI-31 | 1.28 ± 0.47 | 1.06 ± 0.32 | p = 0.02 a |
| SMI-32 | 1.38 ± 0.75 | 1.00 ± 0.23 | p = 0.006 a |
NF-L (NF-light), NF-M (NF-medium), NF-H (NF-heavy).
The protein level for each neuronal IF is normalized to β-actin within each sample. The -fold change in the protein level is expressed relative to that in control brains, which was arbitrarily set at 1.0.
All values are mean ± standard deviation.
Student’s t test
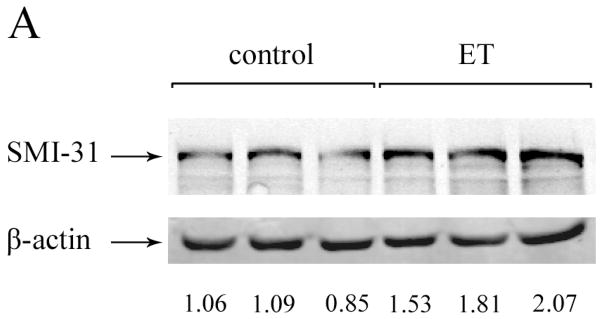
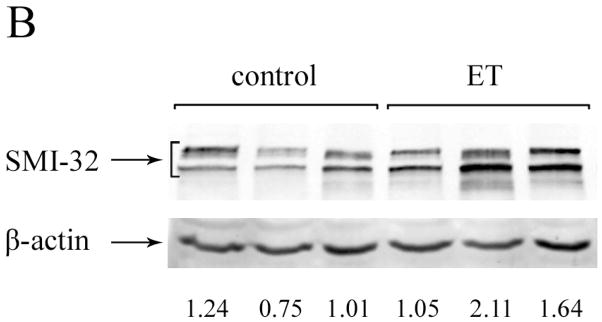
We performed Western blot analysis to quantify the protein level of neuronal IFs in cerebellar cortex of ET cases versus controls. Quantitative analysis of blots from cases and controls did not differ with respect to the cerebellar levels of NF-L, NF-M, NF-H or α-internexin. However, SMI-31 levels (i.e., phosphorylated NF-H) and SMI-32 levels (i.e., non-phosphorylated NF-H) were both significantly higher in ET cases than controls (Table 2, Figure 1).
Figure 1.
Western blots of NF from cerebellar cortex in ET cases and controls, using SMI-31 antibody (A) or SMI-32 antibody (B). Representative blots including three control lanes (left) and three ET case lanes (right) are shown. The NF protein level is normalized to a β-actin loading control. The –fold change in NF protein level, expressed relative to control brains, is shown below each lane. An arrow to the side of the blots indicate the band positions for these proteins.
There was no association between age and any of the neuronal IF levels (e.g., for SMI-32, Pearson’s r = 0.17, p = 0.25 [in cases] and Pearson’s r = 0.23, p = 0.25 [in controls]). Therefore, age could not have accounted for the observed case-control difference in SMI-31 or SMI-32 levels. Similarly, there was no association between PMI or Braak AD stage and any of the NF levels.
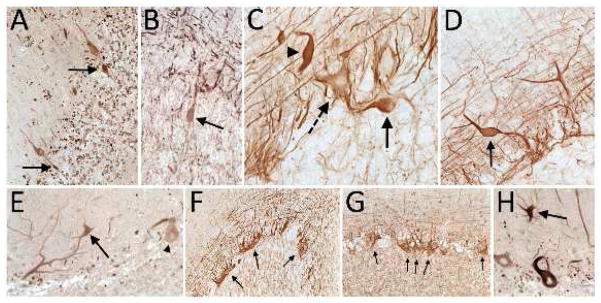
Sections from the cerebellar neocortex demonstrated immunoreactivity to antibodies directed against NF-H, SMI-31 and SMI-32. As previously observed in light microscopic studies of ET cerebellum [15, 26], there was a greater preponderance in ET cases than controls of Purkinje cell axonal swellings (i.e., torpedoes), Purkinje cell dendritic swellings, hypertrophic changes in the basket cell plexus, and in several cases, atypical Purkinje cell dendritic morphology (Figure 2). NF-H and SMI-31 immunostains had similar staining patterns, with prominent axonal staining in all cerebellar cortical layers, including torpedoes and hypertrophic changes in the basket cell plexus (Figure 2, B–D, F, G) and in white matter (not shown); in contrast, NF-H additionally stained the cell body cytoplasm of Purkinje cells (Figure 2C) and proximal portions of Purkinje cell dendrites, including dendrite swellings (Figure 2, C and D). SMI-32 staining was predominantly seen in the cell body cytoplasm of Purkinje cells and their proximal dendrites, and similarly revealed abnormalities in Purkinje cell dendrite morphology (Figure 2E). While SMI-32 only weakly stained cerebellar white matter (not shown), there was labeling of torpedoes and the adjacent Purkinje cell initial axonal segment (Figure 2A).
Figure 2.
(A – H): Cerebellar neocortex sections from ET cases immunostained with SMI-32 (A, E, H), SMI-31 (B, G), or NF-H (C, D, F). A: Two torpedoes (arrows) in close proximity to one another (SMI-32, 200×). B: One torpedo (arrow) (SMI-31, 400×). C: A torpedo (arrow) and a Purkinje cell dendritic swelling (arrow head) adjacent to a Purkinje cell body (dashed arrow) (NF-H, 400×). D: Purkinje cell dendritic swelling (arrow) (NF-H, 400×). E: Purkinje cell dendritic swelling (arrow) (SMI-32, 400×). F: Hypertrophic changes in the basket cell plexus (rating = 3) (arrows) (NF-H, 200×). G: Hypertrophic changes in the basket cell plexus (rating = 3) (arrows) (SMI-31, 200×). H: Atypical Purkinje cell dendritic morphology (arrow) (SMI-32, 400×).
In ET cases, SMI-31 level determined by Western blot was correlated with age of tremor onset (Pearson’s r = 0.32, p = 0.037) but there was no correlation between SMI-32 levels and age of onset (Pearson’s r = 0.13, p = 0.43). There was no correlation in ET cases between SMI-31 or SMI-32 levels and torpedo counts, Purkinje cell counts, or Basket cell axonal plexus density (all p values > 0.05).
Discussion
Using quantitative Western blot analysis in postmortem cerebellar cortex, we have demonstrated an ET case-control difference in the phosphorylation state of NF-H, with significantly higher levels of both SMI-31 (phosphorylated NF) and SMI-32 (non-phosphorylated NF) epitopes in ET cases. We did not detect significant quantitative differences in protein levels of NH-L, NH-M, NF-H or α-internexin between ET cases and controls. To our knowledge, this is the first study to examine NF proteins in ET, a progressive, late-life disorder [2]. Our analyses directly compared brain tissue of cases and controls, capitalizing on the resources of the largest, prospectively-collected number of ET brains in the world (the ETCBR), and focusing on the brain region that has been implicated in ET (i.e., the cerebellum) in prior postmortem studies [16].
We used mouse monoclonal SMI-31 and SMI-32 antibodies, which are among several antibodies that identify the multiple phosphorylated and non-phosphorylated states of NF-H. Other antibodies used in this study identify neuronal IFs independent of phosphorylation. Indeed, the extent of NF-H phosphorylation varies within neurons, as there are many phosphorylation sites on NF-H [11]. Our studies demonstrate that phosphorylation-dependent epitopes in NF-H significantly differ in cerebellum of ET cases versus controls.
Abnormal phosphorylation of neuronal cytoskeletal proteins is one of the major pathological hallmarks of neurodegenerative disorders such as AD, ALS, and PD [24]. Whether an increase or decrease in NF phosphorylation indicates axonal injury is not known [23]. Thus, in ALS, some studies have shown increased staining for phosphorylated NF in anterior horn cells [21], while others have demonstrated increased staining for non-phosphorylated NF in cultured spinal neurons after exposure to cerebrospinal fluid from ALS patients [25]. In studies of AD, both phosphorylated and non-phosphorylated NF have been linked to neurofibrillary tangles [23].
Many mechanisms can lead to the accumulation of NFs, [10, 14], and abnormal phosphorylation is known to affect all of these processes [10]. Abnormal phosphorylation may also affect NF function itself [23]. Whether the abnormal phosphorylation status that we observed is a cause of defective axonal transport and/or function of NFs in ET is not known.
The life-time of NFs has been estimated to be on the order of 1 – 2 years [23]. Because of this long life, NFs are prone to accumulate injuries caused by oxidative stress, inflammation or other mechanisms; this damage is thought to lead to disturbed NF assembly, accumulation and axonal pathology [23].
NF-H becomes highly phosphorylated post-translationally, after being transported from the neuronal cell soma to the axon. Immunohistochemical staining for SMI-31 from ET and control cerebellae in this study demonstrated a predominance of axonal staining, which further highlighted axonal pathology in ET cerebellum, including torpedoes and hypertrophic changes in the basket cell plexus, as previously described [15]. Both NF-H and SMI-32 antibodies stained cytoplasm of Purkinje cell bodies and proximal dendrites, and revealed abnormalities in Purkinje cell dendrite morphology (Figure 2), which are more prominent in ET cerebellum [26]. Interestingly, non-phosphorylated NFs detected by SMI-32 immunostain strongly labeled axonal torpedoes and the initial Purkinje cell axon segment proximal to the torpedo (Figure 2A), which correlates with axonal damage and neuronal dysfunction in Purkinje cells.
We did not observe a correlation in ET cases between SMI-31 or SMI-32 levels and torpedo counts, Purkinje cell counts, or Basket cell axonal plexus density. There are several possible explanations. First, we used a Western blot approach, where cerebellar cortex was homogenized. This may not optimally reflect NF protein alterations in Purkinje cells, as the Purkinje cells are only one among five types of neurons within the cerebellar cortex. A second possibility is that light microscopic structural markers (i.e., torpedoes) may be an end-stage or terminal neuropathological event, and thus not serve as the optimal biomarker of a more upstream disruption on NF protein phosphorylation and axonal dysfunction. A third possibility is that some of the changes (e.g., Purkinje cell loss) may be degenerative, some may be compensatory/regenerative (Basket cell changes), and some may be both (torpedoes), so that the correlation with other markers (e.g., changes in phosphorylation status) are likely to be complex, non-linear, and even multi-directional.
In summary, we demonstrated differences in the phosphorylation state of NFs in the ET cerebellum. Whether the abnormal phosphorylation status is a cause of defective axonal transport and/or function of NFs in ET is not known. NF abnormalities have been demonstrated in many neurodegenerative diseases, where they are an important factor in cellular disruption and clearly linked with neuronal dysfunction. Therefore, an understanding of the characteristics of these NF abnormalities and their mechanisms is central to an understanding of these diseases [14].
Highlights.
Studies have identified structural changes in the cerebellum in essential tremor.
Among these changes is an increase in neurofilament-filled axonal swellings.
We performed a quantitative Western blot analysis of postmortem cerebellar cortex.
The phosphorylation state of neurofilament proteins differed in cases vs. controls.
These changes could be of mechanistic importance.
Acknowledgments
This work was supported by R01 NS042859 (National Institutes of Health, Bethesda, MD). The sponsor had no role in the conduct of the research or the preparation of the manuscript.
Footnotes
Conflicts of Interest: The authors had no conflicts of interest. Each of the authors participated in a substantive manner with the research and in the preparation of the manuscript and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beaulieu JM, Nguyen MD, Julien JP. Late onset of motor neurons in mice overexpressing wild-type peripherin. J Cell Biol. 1999;147:531–544. doi: 10.1083/jcb.147.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermejo-Pareja F. Essential tremor - a neurodegenerative disorder associated with cognitive defects? Nat Rev Neurol. 2011;7:273–282. doi: 10.1038/nrneurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 5.Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figlewicz DA, Krizus A, Martinoli MG, Meininger V, Dib M, Rouleau GA, Julien JP. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:1757–1761. doi: 10.1093/hmg/3.10.1757. [DOI] [PubMed] [Google Scholar]
- 7.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–235. [PubMed] [Google Scholar]
- 8.Jaffe H, Veeranna Shetty KT, Pant HC. Characterization of the phosphorylation sites of human high molecular weight neurofilament protein by electrospray ionization tandem mass spectrometry and database searching. Biochemistry. 1998;37:3931–3940. doi: 10.1021/bi972518u. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Chang R, Teunissen C, Gebremichael Y, Petzold A. Neurofilament stoichiometry simulations during neurodegeneration suggest a remarkable self-sufficient and stable in vivo protein structure. J Neurol Sci. 2011;307:132–138. doi: 10.1016/j.jns.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Kushkuley J, Metkar S, Chan WK, Lee S, Shea TB. Aluminum induces neurofilament aggregation by stabilizing cross-bridging of phosphorylated c-terminal sidearms. Brain Res. 2010;1322:118–123. doi: 10.1016/j.brainres.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 11.Lees JF, Shneidman PS, Skuntz SF, Carden MJ, Lazzarini RA. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988;7:1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liem RK. Molecular biology of neuronal intermediate filaments. Curr Opin Cell Biol. 1993;5:12–16. doi: 10.1016/s0955-0674(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 13.Liem RK, Leung CL. Neuronal intermediate filament overexpression and neurodegeneration in transgenic mice. Exp Neurol. 2003;184:3–8. doi: 10.1016/s0014-4886(03)00291-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Xie F, Alvarado-Diaz A, Smith MA, Moreira PI, Zhu X, Perry G. Neurofilamentopathy in neurodegenerative diseases. Open Neurol J. 2011;5:58–62. doi: 10.2174/1874205X01105010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9:613–622. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, Pahwa R, Lyons KE, Ross GW, Borden S, Moskowitz CB, Lawton A, Hernandez N. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Yi H, Erickson-Davis C, Vonsattel JP, Faust PL. Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett. 2009;450:287–291. doi: 10.1016/j.neulet.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–396. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18:S91–94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 21.Munoz DG, Greene C, Perl DP, Selkoe DJ. Accumulation of phosphorylated neurofilaments in anterior horn motoneurons of amyotrophic lateral sclerosis patients. J Neuropathol Exp Neurol. 1988;47:9–18. doi: 10.1097/00005072-198801000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31:383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233:183–198. doi: 10.1016/j.jns.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Rudrabhatla P, Grant P, Jaffe H, Strong MJ, Pant HC. Quantitative phosphoproteomic analysis of neuronal intermediate filament proteins (NF-M/H) in Alzheimer's disease by iTRAQ. FASEB J. 2010;24:4396–4407. doi: 10.1096/fj.10-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tikka TM, Vartiainen NE, Goldsteins G, Oja SS, Andersen PM, Marklund SL, Koistinaho J. Minocycline prevents neurotoxicity induced by cerebrospinal fluid from patients with motor neurone disease. Brain. 2002;125:722–731. doi: 10.1093/brain/awf068. [DOI] [PubMed] [Google Scholar]
- 26.Yu M, Ma K, Faust PL, Honig LS, Cortes E, Vonsattel JP, Louis ED. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol. 2012;19:625–630. doi: 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]