Abstract
We report the metallo-responsive high fidelity switching between hexadecameric and octameric supramolecular G-quadruplexes triggered by a change in the metal cation promoter from potassium to strontium, respectively.
Further advances in the development of molecular machinery require the construction of components that alternate their configuration between, at least, two distinct states. Most current examples of artificial supramolecular machinery rely on interconversions between specific configurations of mechanically linked systems (e.g., catennanes, rotaxanes) or within contorted structures (e.g., cis-trans isomers, foldamers).1-3 A complementary, and comparatively underdeveloped, strategy will take advantage of changes in the oligomeric state (molecularity) of self-assembled supramolecules. In biological systems, fluctuations in the oligomeric state of assemblies provide an efficient way to alter their structure and, thus, their function. For example, changes in the oligomeric state for the protective antigen of the anthrax toxin (heptamer-octamer) provide a means of controlling cytotoxicity.4 Analogous changes in RuvA enzymes (tetramer-octamer) enable control of the dynamics of processing DNA in Holliday junctions.5
Most responsive multimeric assemblies rely on an all or none strategy where the two available states are assembled or fully disassembled.6 An additional challenge is the development of systems where two well-defined oligomeric states (in particular those with molecularities larger than trimers or tetramers) interconvert with high fidelity.7, 8 The interconversion can be achieved by tuning intrinsic parameters via covalent modification of the monomeric building blocks with responsive functional groups (e.g., photo-, redox- or pH-active) which is a commonly used approach. Alternatively, such tuning can be achieved through the modulation of extrinsic parameters by altering the surroundings of the supramolecule such as the solvent,9-11 temperature,12 or metal cations.13,14 Herein, we present a metallo-responsive supramolecular G-quadruplex (GQ) that switches between a hexadecameric and octameric states in processes triggered by changes in the metal cation from potassium to strontium, respectively (Fig. 1).15
Fig. 1.
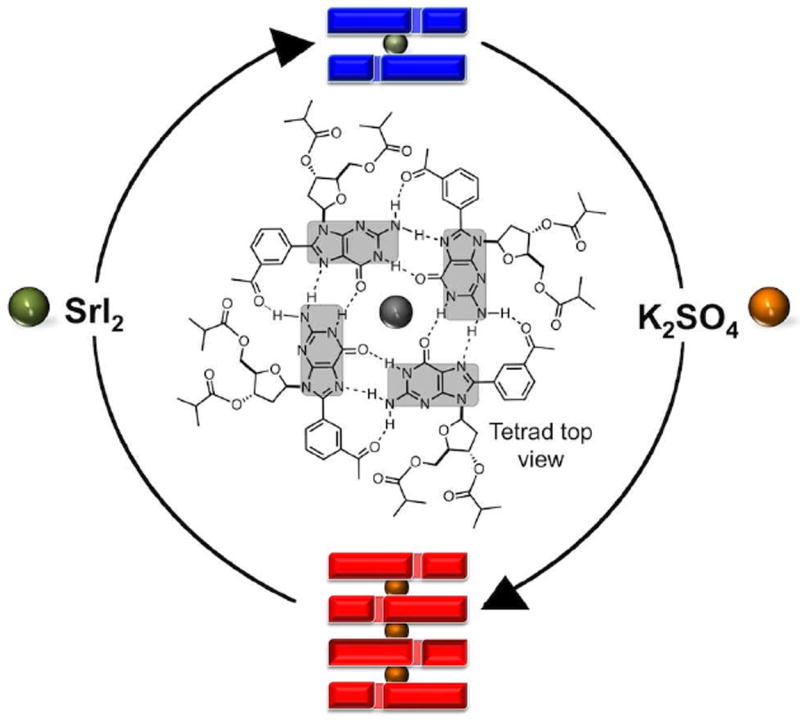
Schematic representation of the cycle of the metallo-responsive supramolecules formed by 1, a tetrad of which is represented in the center. Side view depictions of the octamer 18•Sr2+ (top, blue) and the hexadecamer 116•3K+ (bottom, red). Strontium and potassium cations are represented by green and orange spheres, respectively.
Supramolecular GQs are formed by the self-assembly of guanine, or related derivatives, that form stacks of planar hydrogen-bonded tetramers in the presence of cations of appropriate size (e.g., Na+, K+, NH4+).16,17 In recent years we have studied the self-assembly of 8-aryl-2′-deoxyguanosine derivatives (8ArGs) and surveyed the effects of modulating intrinsic and extrinsic parameters, on the structure and properties of their corresponding assemblies.18,19 Properties such as their selectivity for binding20 and transporting metal cations21 can also be modulated by such parameters. Specifically, the 8-(m-acetylphenyl)-2’-deoxyguanosine (mAG) scaffold induces the reliable formation of hexadecamers, in both organic19 and aqueous media,22 even when containing large, and sterically demanding groups such as dendrons.23,24 Furthermore, modulation of these parameters has enabled the development of solvo-9 and thermo-12 responsive systems, paving the way to more elaborate supramolecular machinery.
We have shown that titrating a solution of 1 in CD3CN with KI, initially leads to the formation of the D4-symmetric octamer 18•K+ (from 0.0313 to 0.125 equiv of KI), leading to the formation of the hexadecamer 116•3K+ after the addition of 0.5 equiv of KI.9 Similarly, the addition of SrI2 (0.125 equiv) to a solution of 1 (30 mM) in CD3CN reveals a single set of signals corresponding to the octamer 18•Sr2+. In contrast, higher concentration of Sr2+ decreases the fidelity and appears to destabilize the octamer (as previously reported for other GQs25), but does not induce the formation of a hexadecamer. DOSY experiments in CD3CN are consistent with the formation of 18•Sr2+, giving a D = (7.0 ± 0.2) × 10-10 m2 s-1 at 298 K, which corresponds to a hydrodynamic radius (RH) of 9.2 Å. The constitution of 18•Sr2+, is further supported by vapour pressure osmometry (VPO) and HR-ESI MS (CD3CN with 0.125 equiv of SrI2). The former provides a molecular weight in solution of 4,566 Da (ESI†, Table S2), whereas the latter reveals a clean spectrum with a base peak at m/z = 2145.7058 amu corresponding to 18•Sr2+ (ESI†, Fig. S3).
Surprisingly, in CD3CN with 0.5 equiv of KI, increasing proportions of SrI2 shifts the equilibrium from the hexadecamer 116•3K+ towards 18•Sr2+ (Fig. 2). The octamer 18 is detectable after the addition of just 0.03 equiv of SrI2 (Fig. 2) and it becomes the main species after the addition of 0.125 equiv of such salt. Although the cations of potassium and strontium have similar ionic radii (1.51 and 1.26 Å, respectively),26 in acetonitrile, their distinct charge densities seem to dictate the resulting molecularity of the assemblies of 1. Whereas, a hexadecamer can accommodate three consecutive potassium cations in its internal channel, the corresponding arrangement with divalent strontium cations is untenable due to the increased electrostatic repulsion.27,28
Fig. 2.
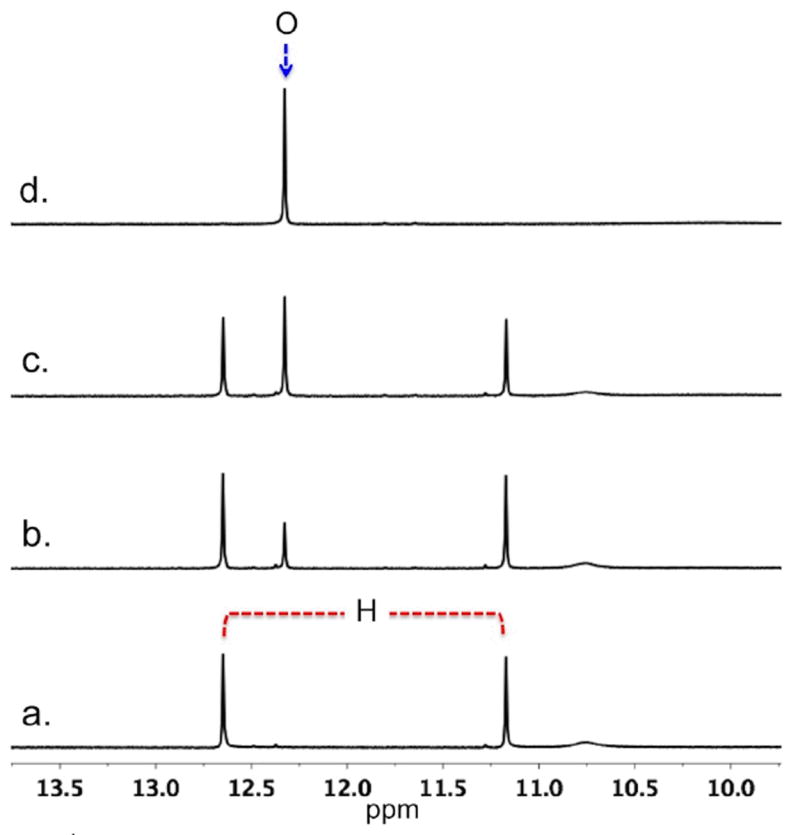
1H NMR (500 MHz, 298.2 K) spectra for 1 (30 mM) in (a) CD3CN with 0.5 equiv of KI, 97 % of hexadecamer (H) 116•3K+; (b) 0.0313 equiv of SrI2, 79% H and 19% octamer (O) 18•Sr2+; (c) 0.0625 equiv of SrI2 60% H and 39 % O; (d) 0.125 equiv of SrI2 100% O.
The addition of KI to 18•Sr2+ does not lead to the formation of 116•3K+ (ESI†, Fig. S7), and the persistence of the former, even after adding an excess of KI, is consistent with its higher thermal stability (Tm ≥ 333.2 K). This stabilization effect of strontium cations have been attributed to a stronger coordination between C6-carbonyl groups.25,29 In order to achieve a reversible metallo-responsive behaviour, and revert the cycle from an octamer to a hexadecamer, we decided to use sulphate as a counter anion for potassium capable of sequestering the strontium cations from 18•Sr2+. The addition of 0.125 equiv of K2SO4 to the octamer 18•Sr12+ induces it to switch back to the hexadecamer 16•3K+ (ESI†, Fig. S8). A precipitate of SrSO4 is observed due to the strong ionic pair formation. Further addition of SrI2 reverts once again the cycle inducing the switch of the hexadecamer to the octamer (ESI†, Fig. S9). Three full cycles were performed, as illustrated in Fig. 3 with no obvious signs of fatigue in the system.
Fig. 3.
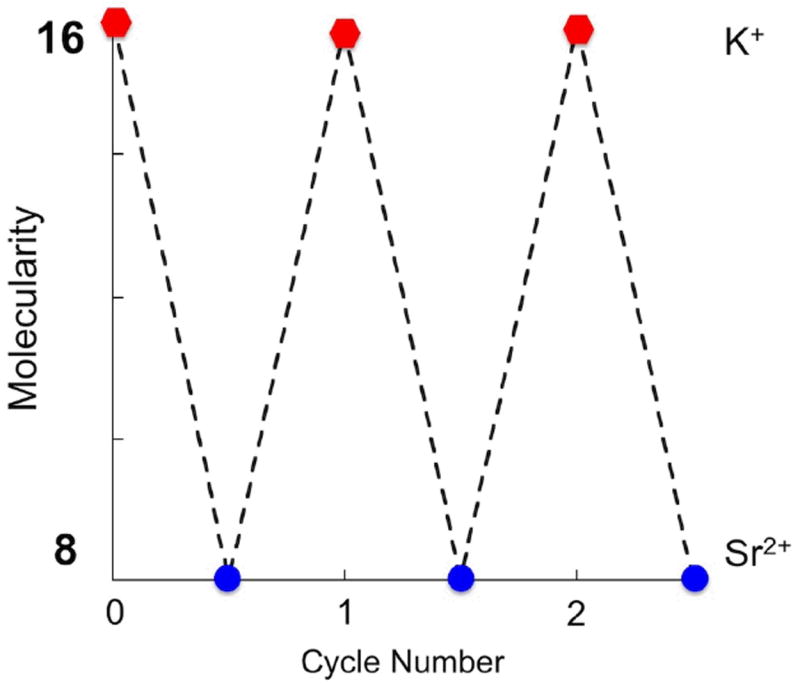
Changes in the molecularity of the assemblies of 1 induced by alternating the metal cation between potassium (red hexagons) and strontium (blue circles).
Direct addition of K2SO4 to a solution of 1 in acetonitrile leads to the formation of 18•K+; (ESI†, Fig. S10), but even an excess of this salt does not promote the formation of 116•3K+. Potassium sulfate enables the interconversion of 18•SrI2 to 116•3KI by increasing the chemical potential and eventual net outward flow of strontium cations from within the octamer to the bulk solution (where it precipitates as SrSO4). This process, however, needs the presence of a soft counter anion like iodide to increase the activity of the cation in solution leading to the high fidelity self-assembly of GQs.30 Precipitation of SrSO4 enables completion of the cycle by leaving potassium as the only cation available to promote the formation of the hexadecamer 116•3K+.
Conclusions
Here we have shown for the first time how alternating the metal cation promoter enables the control, and reversible switching, between two well-defined supramolecular GQs. This metallo-responsive system represents our latest addition to a toolbox of active supramolecular GQs that already contains solvo-4 and thermo-7 responsive systems. We expect these to be valuable in the development of versatile supramolecular machinery.
Supplementary Material
Acknowledgments
This research was financially supported by the NIH-SCoRE (Grant No. 5SC1GM093994-02). M.M.H. thanks the NSF-IFN-EPSCoR (01A-0701525) and the Alfred P. Sloan Foundation for graduate fellowships.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details, NMR, VPO, and ESI-MS. See DOI: 10.1039/b000000x/
Notes and references
- 1.Klajn R, Stoddart JF, Grzybowski BA. Chem Soc Rev. 2010;39:2203–2237. doi: 10.1039/b920377j. [DOI] [PubMed] [Google Scholar]
- 2.Balzani V, Credi A, Venturi M. Molecular devices and machines. Wiley-VCH; Weinheim: 2008. [Google Scholar]
- 3.Feringa BL. Molecular Switches. Wiley-VCH; Weinheim: 2001. [Google Scholar]
- 4.Kintzer AF, Thoren KL, Sterling HJ, Dong KC, Feld GK, Tang II, Zhang TT, Williams ER, Berger JM, Krantz BA. J Mol Biol. 2009;392:614–629. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YC, Flora R, McCafferty JA, Gor J, Tsaneva IR, Perkins SA. J Mol Biol. 2003;333:677–682. doi: 10.1016/j.jmb.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 6.(a) Moon K, Kaifer AE. J Am Chem Soc. 2004;126:15016–15017. doi: 10.1021/ja045587m. [DOI] [PubMed] [Google Scholar]; (b) Lena S, Neviani P, Masiero S, Pieraccini S, Spada GP. Angew Chem Int Ed. 2010;49:3657–3660. doi: 10.1002/anie.201000805. [DOI] [PubMed] [Google Scholar]
- 7.Ghoussoub A, Lehn JM. Chem Comm. 2005:5763–5765. doi: 10.1039/b512527h. [DOI] [PubMed] [Google Scholar]
- 8.(a) Pieraccini S, Masiero S, Pandoli O, Samori P, Spada GP. Org Lett. 2006;8:3125–3128. doi: 10.1021/ol061115w. [DOI] [PubMed] [Google Scholar]; (b) Ciesielski A, Lena S, Masiero S, Spada GP, Samori P. Angew Chem Int Ed. 2010;49:1963–1966. doi: 10.1002/anie.200905827. [DOI] [PubMed] [Google Scholar]
- 9.(a) Betancourt JE, Martí-Hidalgo M, Gubala V, Rivera JM. J Am Chem Soc. 2009;131:3186–3188. doi: 10.1021/ja809612d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pieraccini S, Bonacchi S, Lena S, Masiero S, Montalti M, Zaccheroni N, Spada GP. Org Biomol Chem. 2010;8:774–781. doi: 10.1039/b920220j. [DOI] [PubMed] [Google Scholar]
- 10.Provent C, Rivara-Minten E, Hewage S, Brunner G, Williams A. Chem Eur J. 1999;5:3487–3494. [Google Scholar]
- 11.Suzuki K, Kawano M, Fujita M. Angew Chem Int Ed. 2007;46:2819–2822. doi: 10.1002/anie.200605084. [DOI] [PubMed] [Google Scholar]
- 12.Betancourt JE, Rivera JM. J Am Chem Soc. 2009;131:16666–16668. doi: 10.1021/ja9070927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harano K, Hiraoka S, Shionoya M. J Am Chem Soc. 2007;129:5300–5301. doi: 10.1021/ja0659727. [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka S, Yi T, Shiro M, Shionoya M. J Am Chem Soc. 2002;124:14510–14511. doi: 10.1021/ja028659n. [DOI] [PubMed] [Google Scholar]
- 15.For a related example where strontiun cations promote the assembly of 5’-GMP into G-quartet-based nanowires, and their ensuing EDTA triggered dissasembly, see: Hu D, Ren J, Qu X. Chem Sci. 2011;2:1356–1361.
- 16.Davis JT, Spada GP. Chem Soc Rev. 2007;36:296–313. doi: 10.1039/b600282j. [DOI] [PubMed] [Google Scholar]
- 17.Davis JT. Angew Chem Int Ed. 2004;43:668–698. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- 18.Rivera-Sánchez MdC, Andujar-de-Sanctis I, Garcia-Arriaga M, Gubala V, Hobley G, Rivera JM. J Am Chem Soc. 2009;131:10403–10405. doi: 10.1021/ja9040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gubala V, Betancourt JE, Rivera JM. Org Lett. 2004;6:4735–4738. doi: 10.1021/ol048013v. [DOI] [PubMed] [Google Scholar]
- 20.Gubala V, De Jesus D, Rivera JM. Tetrahedron Lett. 2006;47:1413–1416. [Google Scholar]
- 21.Martín-Hidalgo M, Camacho-Soto K, Gubala V, Rivera JM. Supramol Chem. 2010;22:862–869. doi: 10.1080/10610278.2010.510263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-Arriaga M, Hobley G, Rivera JM. J Am Chem Soc. 2008;130:10492–10492. doi: 10.1021/ja8039019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera LR, Betancourt JE, Rivera JM. Langmuir. 2011;27:1409–1414. doi: 10.1021/la103961m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betancourt JE, Rivera JM. Org Lett. 2008;10:2287–2290. doi: 10.1021/ol800701j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.In oligonucleotide-based G-quadruplexes the increased stability imparted by strontium cations has also been attributed to the enhanced screening of the electrostatic repulsion between the phosphates in the backbone. For more information see: Neidle S, Balasubramanian S. Quadruplex Nucleic Acids. The Royal Society of Chemistry; Cambridge: 2006.
- 26.Shannon RD. Acta Crystallogr, Sect A. 1976;32:751–767. [Google Scholar]
- 27.Davis and coworkers have reported the assembly of a lipophilic guanosine derivative into a hexadecamer containing strontiun cations. Such hexadecamer, however, only contained two strontiun cations (between the outer G-tetrads) with no cations between the inner G-tetrads. The assembly is in essence a dimer of octamers where the two octamers are held together by a belt of four picrate anions that interact strongly with the guanosine subunits via the formation of hydrogen bonds. For more details see: Shi X, Fettinger JC, Davis JT. Angew Chem Int Ed. 2001;40:2827–2831. Shi XD, Mullaugh KM, Fettinger JC, Jiang Y, Hofstadler SA, Davis JT. J Am Chem Soc. 2003;125:10830–10841. doi: 10.1021/ja035267n.
- 28.Wu and coworkers recently reported a complete NMR and ESI-MS study of a lipophilic guanosine derivative in which strontium promotes the formation of an octamer. For details see: Kwan ICM, She Y-M, Wu G. Can J Chem. 2011;89:835–844.
- 29.Nikan M, Sherman JC. J Org Chem. 2009;74:5211–5218. doi: 10.1021/jo9001245. [DOI] [PubMed] [Google Scholar]
- 30.This agrees with our previous report (see reference 9a) where we showed that the counter anion, although not necessarily engages in specific interactions with the assemblies of 8ArGs, could tune the chemical potential of such supramolecules by regulating the activity of the potassium cations. For a related work, read: González-Rodríguez D, van Dongen JLJ, Lutz M, Spek AL, Schenning APHJ, Meijer EW. Nature Chem. 2009;1:151–155. doi: 10.1038/nchem.177.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


