Abstract
Although glycogen synthase kinase-3 beta (GSK-3β) was originally named for its ability to phosphorylate glycogen synthase and regulate glucose metabolism, this multifunctional kinase is presently known to be a key regulator of a wide range of cellular functions. GSK-3β is involved in modulating a variety of functions including cell signaling, growth metabolism, and various transcription factors that determine the survival or death of the organism. Secondary to the role of GSK-3β in various diseases including Alzheimer's disease, inflammation, diabetes, and cancer, small molecule inhibitors of GSK-3β are gaining significant attention. This paper is primarily focused on addressing the bifunctional or conflicting roles of GSK-3β in both the promotion of cell survival and of apoptosis. GSK-3β has emerged as an important molecular target for drug development.
1. Introduction
Glycogen synthase kinase-3 is a ubiquitously expressed protein kinase that exists in two isoforms, α and β. Originally identified based on its role in glycogen biosynthesis based on its inactivating phosphorylation of glycogen synthase, it has since been found to regulate a myriad of functions through Wnt and other signaling pathways [1]. The two isoforms are strongly conserved within their kinase domain but differ greatly at the C-terminus, while the α isoform additionally contains a glycine-rich N-terminus extension [2]. Our paper will focus on the β isoform due to its more established role in cell survival and viability. Glycogen synthase kinase-3 beta (GSK-3β) is involved in the regulation of a wide range of cellular functions including differentiation, growth, proliferation motility, cell cycle progression, embryonic development, apoptosis, and insulin response [1–8]. It has emerged as an important regulator of neuronal, endothelial, hepatocyte, fibroblast, and astrocyte cell death in response to various stimuli [6, 7, 9].
GSK-3β is comprised of 12 exons in humans and 11 exons in mice with the ATG start codon located within exon 1 and the TAG stop codon found in the terminal exon. The gene product is a 46 kDa protein consisting of 433 amino acids in the human and 420 amino acids in the mouse. Figure 1 shows the overall structure of GSK-3β. It is similar to other Ser/Thr kinases [10, 11]. The N-terminal domain is comprised of the first 135 residues and forms a 7-strand β-barrel motif. A small linker region connects the N-terminal domain to the central α-helical domain formed by residues 139 through 342. The ATP-binding site lies at the interface of the N-terminal and α-helical domains. Residues 343 through 433 form the C-terminal domain, which is outside of the classical Ser/Thr kinase core fold. These residues form a helix/loop domain that interacts with the core α-helical domain. The N-terminal amino acids 78 through 92 are necessary for association with p53 (Figure 1). The activity of GSK-3β can be reduced by phosphorylation at Ser-9. Several kinases are able to mediate this modification, including p70S6 kinase, p90RSK, PKC, and Akt [12, 13]. In opposition to the inhibitory phosphorylation of GSK-3β at Ser-9, phosphorylation of GSK-3β at Tyr-216 by ZAK1 or Fyn increases its enzyme activity [14] (Figure 2).
Figure 1.
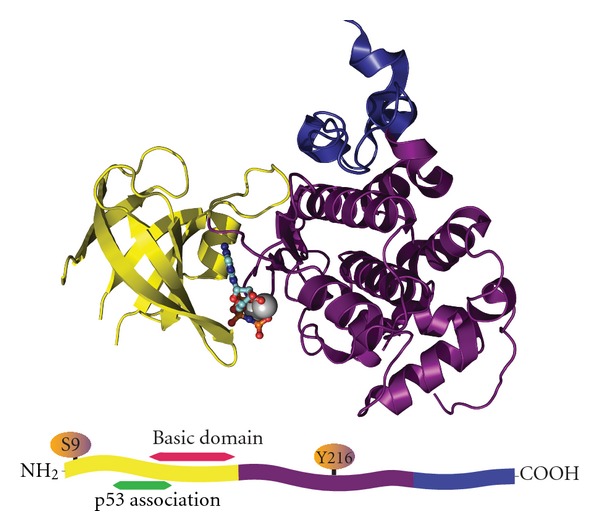
Glycogen synthase kinase-3β (GSK-3β) structure. GSK-3β is a 433 residue protein consisting of 3 distinct structural domains. The N-terminal domain (yellow) consists of the first 134 residues and forms a 7-strand β-barrel. A short linker from the N-terminal domain, residues 135–151 connect the N-terminal domain to the α-helical domain (magenta). The α-helical domain is composed of residues 152–342. Sandwiched between the N-terminal and α-helical domain is the ATP-binding site. The C-terminal domain consists of residues 343–433 (blue). A strand diagram of GSK-3β. Phosphorylation of Ser-9 inactivates the enzyme, while phosphorylation of Tyr-216 activates. The p53 association region and basic domain region are both located in the N-terminal domain. Image was made using PyMol Molecular Graphics Software version 1.3 with the PDB structure 1UV5.
Figure 2.
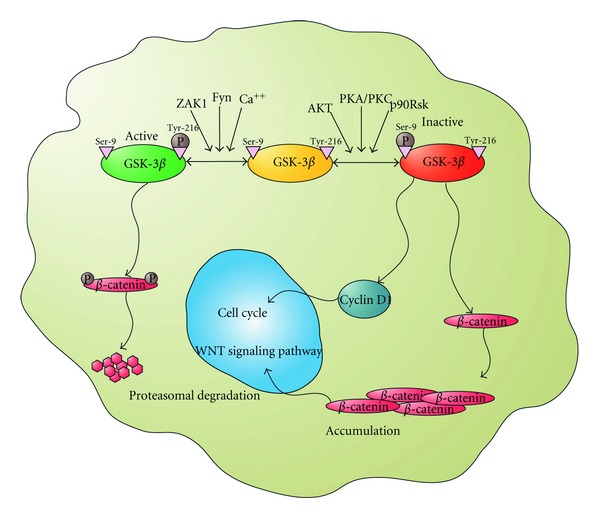
Regulation of GSK-3β. GSK-3β is a multifunctional kinase that has a role in various signaling pathways that regulate cell fate. ZAK1 or Fyn can phosphorylate Tyr-216 which increases the GSK-3β activity. GSK-3β can phosphorylate downstream targets like β-catenin and degrade it through the ubiquitin-proteasome system. Akt and PKC on the other hand can attenuate GSK-3β enzymatic activity by phosphorylating Ser-9. Inhibition of GSK-3β activity therefore leads to stabilization and accumulation of β-catenin in the cytosol, which is shuttled into the nucleus where it functions to regulate gene expression. GSK-3β is also involved in cell cycle regulation through the phosphorylation of cyclin D1, which results in the rapid proteolytic turnover of cyclin D1 protein.
Dysregulation of GSK-3β expression leads to many pathological conditions, including diabetes (or insulin resistance), neuronal dysfunction, Alzheimer's disease [15–18], schizophrenia [19], Dopamine-associated behaviors [20], bipolar disorders [21], Parkinson's disease [22], and cancer. Of special interest is the involvement of GSK-3β in cancer with data supporting a role as a tumor suppressor and tumor promoter, a discrepancy that at least in part depends on both cell type and signaling environment. For example, GSK-3β has been shown to inhibit androgen receptor-stimulated cell growth in prostate cancer, thus acting as a tumor suppressor [23]. In contrast, GSK-3β is highly expressed in colorectal cancer [24, 25] and has been shown to participate in nuclear factor-κB (NF-κB) mediated cell survival in pancreatic cancer [26], thus behaving as a tumor promoter. Moreover, the kinase has dual functions in the regulation of cell survival, where it can either activate or inhibit apoptosis [3, 27], further complicating its involvement in cancer. This paper will focus on how GSK-3β can both activate as well as protect from apoptosis with a focus on oncology.
Regulation of β-catenin levels is a critical step in Wnt signaling. β-Catenin is phosphorylated by GSK-3β and then degraded through the ubiquitin-proteasome system [28–30]. Inhibition of GSK-3β activity leads to stabilization and accumulation of β-catenin in the cytosol, which is shuttled into the nucleus and regulates gene expression (Figure 2). GSK-3β is also involved in cell cycle regulation through the phosphorylation of cyclin D1, which results in the rapid proteolytic turnover of cyclin D1 protein [1, 31] (Figure 2). Direct overexpression of wild-type GSK-3β is known to induce apoptosis in various cell types in culture, and specific inhibitors of GSK-3β are able to stop this apoptotic signaling [6, 7, 9, 32]. The detailed molecular mechanism of GSK-3β's proapoptotic effect is as yet unknown, but it involves regulation of metabolic and signaling proteins, transcription factors, and gene expression [4, 33].
GSK-3β is required for proper development [4] and is ubiquitously expressed in the animal kingdom. GSK-3β protein was originally isolated from skeletal muscle, but though widely expressed, the protein is most abundant in brain tissue, especially neurons. The high level of expression in brain tissue is likely due to its vital role in neuronal signaling. In neuronal cells, GSK-3β is required for dendrite extension and synapse formation in newborns.
2. Regulation of Apoptosis by GSK-3β
GSK-3β has been shown to induce apoptosis in a wide variety of conditions including DNA damage [34], hypoxia [35], endoplasmic reticulum stress [36], and Huntington's disease-associated polyglutamine toxicity [37]. In cell culture studies, apoptosis was either attenuated or fully abrogated by inhibiting GSK-3β in primary neurons [38], HT-22 cells [39], PC12 cells [40], and human SH-SY5Y neuroblastoma cells [36, 41].
GSK-3β promotes apoptosis by inhibiting prosurvival transcription factors, such as CREB and heat shock factor-1 [42], and facilitating proapoptotic transcription factors such as p53 [34]. A list of some alternative conditions where GSK-3β facilitates apoptosis is given in Table 1. A large number of proteins have been shown to interact with the tumor suppressor transcription factor p53 to regulate its actions [43, 44], which has been implicated in the proapoptotic actions of GSK-3β in several studies. Following DNA damage, the normally short-lived p53 protein is stabilized and modified by a complex array of posttranslational modifications, such as phosphorylation, acetylation, methylation, ubiquitination, sumoylation, glycosylation, and neddylation. One of these regulatory proteins is GSK-3β, which forms a complex with nuclear p53 to promote p53-induced apoptosis [34, 45, 46]. GSK-3β binds directly to p53, and the C-terminal region of p53 is necessary for this interaction [45]. GSK-3β was shown to directly phosphorylate p53 at Ser-33 [47] and to mediate p53 phosphorylation at Ser-315 and Ser-376 [48, 49]. GSK-3β also promotes p53-mediated transcription of specific genes and regulates the intracellular localization of p53 [45, 46, 49]. In addition to GSK-3β regulating p53, GSK-3β is also regulated by p53. The activity of GSK-3β is increased by a phosphorylation-independent mechanism of direct binding of p53 to GSK-3β [34]. Nuclear localization of GSK-3β may also be regulated by binding of activated p53 [50].
Table 1.
Conditions where GSK-3β facilitates apoptosis.
| System or stimulus | Mechanism |
|---|---|
| C(2) Ceramide-associated damage | Inhibits the phosphorylation of AKT and ERK pathways and through the dephosphorylation of GSK-3β [51]. GSK-3β inhibitors have been shown to inhibit apoptosis through inhibiting dephosphorylation of AKT and GSK-3β [52]. |
|
| |
| LPS-mediated endotoxic shock | While specific apoptotic studies have not been performed, LPS has been shown to stabilize apoptotic signal-regulating kinase-1 (ASK-1), a serine-threonine kinase associated with stress-induced apoptosis [53]. |
|
| |
| Immune system | Regulates in apoptosis of activated T-Cells [54]. |
|
| |
| HIV-mediated neuronal damage | Inhibits NF-κB [55–57]. |
|
| |
| Neurodegenerative disease-related toxicity and oxidative stress |
Neuronal or oligodendrocyte injury or toxicity (including prion peptide) is associated with increased activity of GSK-3β[51, 58–64]. |
| Negative regulators of GSK-3β are associated with increased survival factors [51, 58–64] and neuroprotection [9, 38]. | |
|
| |
| ER stress | ER stress can lead to dephosphorylation of pGSK-3β(S9), leading to stress-induced apoptosis through activated caspase-3 [12–14, 26, 28]. |
|
| |
| Hypoxia/ischemia | Activates mitochondrial death pathway [35, 65–68]. |
In addition to direct interaction, GSK-3β can regulate p53 levels through the phosphorylation of the p53-specific E3 ubiquitin ligase MDM2 [69]. Regulation of p53 by MDM2 is multifaceted. In the classical model, N-terminal phosphorylation of p53 at Ser-15 (mouse Ser-18) and Ser-20 (mouse Ser-23) inhibits the interaction with MDM2 and thereby prevents MDM2-mediated ubiquitination and the resulting proteasomal degradation of p53 [44] (Figure 3). Stabilized p53 then enters a complex regulatory network to induce DNA binding and transcriptional activation of p53 target genes, in part through the recruitment of coactivators and corepressors. This determines the specific cellular response, which can include survival, growth arrest, DNA repair, or apoptosis [44]. Inhibition of GSK-3β in hippocampal neurons protected it from radiation-induced apoptosis [9, 70]. Similar protection from GSK-3β inhibition has been seen in primary neurons [38]. The mechanism of protection from radiation-induced apoptosis in these cells involves subcellular localization and interaction of GSK-3β, p53, and MDM2. GSK-3β inhibition blocks radiation-induced accumulation of p53 by upregulating levels of MDM2 that subsequently result in decreased radiation-dependent apoptosis [71]. In addition to abrogation of radiation-induced p53 phosphorylation, accumulation, and nuclear translocation, GSK-3β inhibition results in the accumulation of MDM2 and sequestration of GSK-3β, p53, and MDM2 in the cytoplasm where p53 cannot act on its target genes [71]. The role of attenuated p53 function in the prosurvival effects of the GSK-3β inhibitors, has also been previously described [34, 46, 70, 72, 73].
Figure 3.
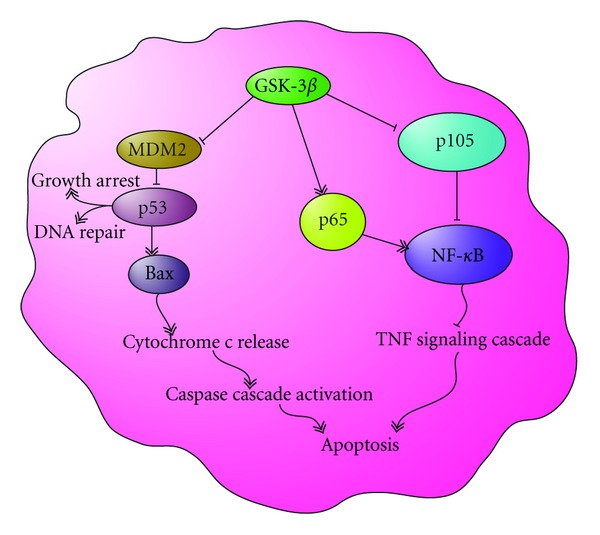
GSK-3β's role in apoptosis signaling. The above schematic shows the role of activated GSK-3β and its role in regulating apoptosis. Active GSK-3β inhibits MDM2 regulation of p53, leading to DNA repair and growth arrest, and in some cases the activation of the caspase cascade through Bax to promote apoptosis. Active GSK-3β also positively regulates NFκB by activating IKK, IκB, and p65, leading to the inhibition of TNF-mediated apoptosis. These actions inhibit the initiation of apoptosis through the TNF signaling cascade.
In regulation of the apoptotic response, mammalian cells employ multiple prosurvival proteins from the Bcl-2 family (Bcl-2, Bcl-XL, Bcl-w, Mcl1, and A1) that antagonize the proapoptotic function of Bax and Bak [34, 74]. Bax and Bak localize to the mitochondrial outer membrane and trigger death signals leading to cytochrome c release to the cytosol [74, 75]. Apoptosis requires a group of effector caspases to dismantle the cells. Cytochrome c activates caspase-9, which subsequently activates caspase-3 [76]. The activation of caspase-3 is an essential step leading to cleavage of the DNA repair enzyme, poly (ADP-ribose) polymerase (PARP), resulting in genomic DNA fragmentation. Bax protein levels and cleavage (activation) of caspase-3 were increased due to radiation and were abrogated by GSK-3β inhibitors [77] (Figure 3). GSK-3β was also found to be associated with mitochondrial apoptotic signaling. Inhibition of GSK-3β prevented mitochondrial release of cytochrome c, which is known to activate caspase-3 and initiate apoptosis [34]. Phosphatidylinositol 3-kinase (PI3-kinase) and its downstream effector, the protein-serine/threonine kinase Akt, a negative regulator of GSK-3β, play an important role in preventing apoptosis by blocking activation of the caspase cascade [78].
3. Survival-Promoting Effects of GSK-3β
GSK-3β is involved in multiple signaling pathways and has many phosphorylation targets. It should therefore not be surprising that GSK-3β has both pro- and antiapoptotic roles. The overall effect of GSK-3β on cell survival varies depending on cell type, transformation status, and the specific signaling pathway being activated. For example, despite evidence for a substantial proapoptotic role of GSK-3β, it is the inhibition of GSK-3β that promotes apoptosis and decreases viability in neuroblastoma cells [79]. Several examples of pro-survival roles of GSK-3β not mentioned here are summarized in Table 2 [80–84].
Table 2.
Other pro survival roles of GSK-3β.
| System | Mechanism |
|---|---|
| ER stress | Reduces expression of the proapoptotic transcription factor CHOP/GADD153 [87]. |
| Glioblastoma differentiation | Promotes self-renewal through interaction with Bmi1 [81]. |
| Death receptor complex | Inhibits apoptotic signaling and caspase activation [83]. |
| Chemotherapy | Targeted by death-inducing drugs suggesting an inhibitory role [84]. |
| Oncogenic activation | Inhibits apoptotic activation by c-myc [82]. |
| Glucose metabolism | Prevents apoptosis through mitochondrial stabilization [82]. |
Additionally, while GSK-3β has been typically identified as an activator of p53-mediated apoptosis [34], conflicting reports suggest an inhibitory effect of GSK-3β signaling on p53 activation. Inhibition of GSK-3β blocks activation of MDM2 by reducing Ser-254 phosphorylation. This prevents p53 degradation and promotes apoptosis despite the induction of p53 ubiquitination. Similarly, ionizing radiation was found to induce an inactivating phosphorylation at Ser-9 of GSK-3β, corresponding to hypophosphorylation of MDM2 and accumulation of p53 [69]. In contrast to its proapoptotic effects, this data suggests that GSK-3β inhibits apoptosis under basal conditions through MDM2-dependent degradation of p53. Overexpression of β-catenin, a downstream signaling factor negatively regulated by GSK-3β, was found to increase basal p53 levels by blocking both MDM2-dependent and independent degradation in neuroblastoma cells [85], providing additional supporting evidence for an inhibitory effect of GSK-3β on p53-mediated apoptosis. Interestingly, a negative feedback loop exists between β-catenin and p53; while β-catenin upregulates p53 levels, the activation of p53 results in degradation of β-catenin through GSK-3β [86]. While the majority of publications suggest a proapoptotic role for GSK-3β in p53 signaling, it is clear that more comprehensive studies are needed in order to fully understand the p53-GSK-3β relationship.
GSK-3β is specifically required for hepatocyte survival in normal embryos, and GSK-3β knockout mice are embryonically lethal between E13.15–14.5. Hepatocyte apoptosis in GSK-3β knockout mice and mouse embryonic fibroblasts results only after exposure to tumor necrosis factor (TNF), while inhibition of GSK-3β in wild-type cells with lithium increases TNF sensitivity. GSK-3β loss in these cells has a detrimental effect on the action of NF-κB, which protects against TNF-induced apoptosis [88]. Other studies have shown that GSK-3β directly promotes NF-κB stability and activation through both the degradation of p105 and activation of the p65 subunit, suggesting a likely mechanism for lithium-induced TNF hypersensitivity [89, 90] (Figure 3). The role of GSK-3β on NF-κB activation may also be mediated indirectly through inhibition of β-catenin, as cancer cells with high β-catenin levels are especially sensitive to TNF-induced death [91].
Despite the abundance of evidence implicating GSK-3β in protection from TNF-mediated apoptosis, a few conflicting reports further complicate our understanding of the pathway. A more recent study claims that GSK-3 inhibition does indeed reduce NF-κB activity but does not result in TNF-mediated apoptosis, potentially due to the activation of pro-survival genes through Wnt signaling [92]. Similarly, TNF sensitization by lithium in multiple sarcoma cell lines was found to be independent of both GSK-3β and NF-κB [93] while GSK-3β inhibition in prostate cancer and HEK cells actually increased NF-κB activity despite promoting TNF-induced apoptosis [94].
The specifics of apoptosis regulation by GSK-3β remain both ambiguous and complex, requiring further research in order to determine the mechanisms of action responsible for differential control of cell survival. In addition to variations in cell signaling and proliferation status, the effect of GSK-3β on apoptosis may depend on cellular localization. Only cytosolic GSK-3β was found to inhibit TNF-mediated apoptosis [80] while apoptosis enhances nuclear localization [95], suggesting a potential localization-based mechanism for differential apoptotic regulation. Insufficient data is available to explain the contradictory effects proposed for GSK-3β on p53-mediated apoptosis, and a more detailed study is required in order to determine the reasons for these observed differences, but differential localization of p53, MDM2, and GSK-3β may help define the regulatory role of GSK-3β in various systems.
4. Positive Regulators of GSK-3β
Several molecules are known to potentiate the downstream effects of GSK-3β (Table 3). Positive regulators of GSK-3β are often utilized for enhancing the proapoptotic effects of GSK-3β in the context of chemotherapy for cancer treatment (reviewed in [96]). These regulators typically operate through an indirect mechanism, actually serving as inhibitors for upstream negative regulators. For example, GSK-3β activity is increased upon inhibition of PI3-Kinase with wortmannin or LY294002 [97–99]. Many GSK-3β regulators act to inhibit Akt by blocking its activation or kinase activity. The kinase inhibitor staurosporine and the COX-2 inhibitor Celecoxib block the activating phosphorylation of Akt by PDK [100–104]. Additionally, curcumin dephosphorylates Akt to prevent its downstream inactivation of GSK-3β [102], as does the histone deacetylase inhibitor Trichostatin A, in a PP1-dependent manner [105]. Akt/protein kinase B signaling inhibitor-2 (API-2) appears to suppress both Akt activation and kinase activity independent of any upstream inhibitor effects [106].
Table 3.
List of known positive regulators of GSK-3β.
| Activator | Activation potency | Mode of activation | Notes |
|---|---|---|---|
| Celecoxib | IC50 = 3.5 μM | Inhibits PDK phosphorylation of Akt | COX-2 inhibitor [100]. |
| Staurosporine | IC50 = 0.22 μM | Inhibits PDK phosphorylation of Akt | General kinase inhibitor (including PKA/PKC) [101, 103, 104]. |
| Trichostatin A | Unknown | Induces Akt dephosphorylation | HDAC inhibitor acts through PP1 [105]. |
| Curcumin | Unknown | Akt dephosphorylation | Direct target not known [102]. |
| Akt/protein kinase B signaling inhibitor-2 (API-2) |
Unknown | Suppresses Akt kinase activity and activation | Does not affect upstream Akt activators [106]. |
| Wortmannin | IC50 = 5 nM | Inhibits PI3-Kinase | Indirect effect on GSK-3β [97, 98]. |
| LY294002 | IC50 = 1.4 μM | Inhibits PI3-Kinase | Likely affects ATP binding to kinase [98, 99]. |
| Rapamycin | Unknown | Potentially inhibits S6K1 | mTOR pathway can also inhibit GSK3 [107, 108]. |
| Differentiation-inducing factors (DIFs) |
Unknown | Enhances GSK-3β kinase activity and promotes nuclear localization | Reduces inhibitory phosphorylation and enhances activating phosphorylation [111, 112]. |
| Retinoids | Unknown | Reduces inhibitory phosphorylation of GSK-3β | Promotes GSK-3β-dependent cyclin D1 degradation [80, 109]. |
Alternative GSK-3β regulators have less defined and more indirect mechanisms. The mTOR inhibitor rapamycin has been shown to activate GSK-3β with some studies suggesting a potential influence of the mTOR pathway on GSK-3β regulation through phosphorylation by s6 kinase [107, 108]. Other molecules target the ability of GSK-3β to degrade cyclin D1. Vitamin A derived retinoids and multiple differentiation-inducing factors (DIFs) enhance GSK-3β activation and kinase activity [109–112] as a means for cyclin D inhibition to promote cell cycle arrest and differentiation.
5. Inhibitors of GSK-3β
While a potential therapeutic role of GSK-3β inhibitors has been suggested for some time, they have gained significant interest as a clinical tool over the past decade. GSK-3β inhibitors are currently being utilized for the treatment of various diseases including Alzheimer's disease [113, 114] and other neurodegenerative diseases [18], diabetes, inflammatory disorders [115], radiation damage, and cancer [116]. Various pharmaceutical companies have these inhibitors in clinical trials [116]. A classical example of a nonspecific GSK-3β inhibitor is lithium [21], which has been shown to inhibit GSK-3β with an IC50 of approximately 2 mM in an uncompetitive manner with respect to peptide substrate. Lithium was found to inhibit GSK-3β in a competitive manner by binding directly to magnesium-binding sites of the enzyme [117], thus providing evidence for a molecular mechanism for enzyme inactivation by lithium ions. Four distinct regions of GSK-3β have been targeted for inhibition: the Mg2+ ATP-binding active site, a separate Mg2+-binding site, the substrate-binding groove, and the scaffold-binding region [33, 118]. Several inhibitors compete with Mg2+ and/or ATP to occupy its binding site. However, the specificity of these inhibitors towards GSK-3β relative to other kinases varies significantly (Table 4). Structural studies have further elucidated molecular mechanisms for substrate selection and GSK3-β inhibition [119–125]. Beryllium was shown to compete with both ATP and Mg2+, while lithium competed only with Mg2+ [126].
Table 4.
Selected list of known GSK-3β inhibitors.
| Inhibitor | Inhibition potency | Mode of inhibition | Notes |
|---|---|---|---|
| Beryllium | IC50 = 6 mM | Mg competitor | Also inhibits cdc2 |
| Lithium | Ki =2 mM | Mg competitor | |
| Anilino maleimides (SB216763, SB415286) | Ki = 10–30 nM | ATP competitor | Does not inhibit a range of other kinases |
| Arylpyrazolopyridazines (e.g., 6-aryl pyrazole [3,4-b] pyridine 4) |
IC50 = 0.8–150 nM | ATP competitor | Also inhibits CDK2 |
| Bisindole maleimides (e.g., Ro 31-8220, GF 109203x) | IC50 = 5–170 nM | ATP competitor | Also inhibits PKC |
| Indirubins (6-bromoindirubin-3′-oxime, aka BIO) | IC50 = 5–50 nM | ATP competitor | Also inhibits CDKs |
| Paullones (alsterpaullone) | IC50 = 4–80 nM | ATP competitor | Also inhibits CDKs |
| Pseudosubstrate peptide | Ki = 0.7 mM | Substrate competitor | Specific |
The small molecule inhibitors of GSK-3 SB-216763 and SB-415286 are structurally distinct maleimides that inhibit GSK-3α/β in vitro, with Kis of 9 nM and 31 nM, respectively, in an ATP competitive manner [127]. Hymenialdisine [128] and paullones [129] also inhibit GSK-3β in an ATP competitive manner. Indirubins inhibit GSK-3β in an ATP competitive manner with a IC50 of 50–100 nM [130–132]. Small molecule inhibitors like TZDZ8 that are thiadiazolidinones inhibit GSK-3β with a IC50 of 2 μM in a noncompetitive manner [133, 134]. The other type of GSK-3β inhibitors is represented by cell-permeable, phosphorylated substrate-competitive peptides which interact with the phospho-recognition motif comprising R96, R180, and K205 to prevent substrate access to the active site. There are also GSK-3β-inhibiting peptides that contain GSK-3β interacting domains, block the interaction between Axin and GSK-3, and prevent β-catenin phosphorylation [135]. In the recent decade small molecule inhibitors of GSK-3β are emerging as a promising drug for treatments against neurodegenerative diseases, radiation damage, Alzheimer's disease, diabetes, and cancer [116].
6. Exploiting the GSK-3β Conundrum
GSK-3β signaling is a complex process influenced not only by cellular type and transformation status, but by environmental and cellular conditions. Survival signals have been mainly determined by studies involving GSK-3β inhibition, through gene silencing or pharmacologic inhibition. The resulting inhibition of apoptosis is complex, and requires further elucidation. However several studies suggest that the effects may at least in part be mediated by the effect of GSK-3β on NF-κB levels. In addition, it is clear that subcellular localization is important, as only cytosolic GSK-3β seems to be able to mediate the survival signals.
Notably, the role in promotion of apoptosis by GSK-3β has been more clearly delineated. It performs this task by both facilitating proapoptotic signals while inhibiting anti-apoptotic molecules. This signal interplay occurs mostly at the level of the mitochondria, and combined with the association with primarily nuclear GSK-3β, suggests a downstream role of GSK-3β in modulation.
So how do we exploit these paradoxical roles of GSK-3β? In healthy cells, the shift to pro-survival modes is important for cell survival under conditions of cellular stress. In these cases, the upstream signals seem to override the mitochondrial-based apoptotic machinery to allow the cells to escape potentially lethal damage. There have been attempts to exploit these pro-survival roles in neurodegenerative diseases, which are typified by high apoptosis rates. Reduction of disease-associated apoptosis by GSK-3β modulating agents can restore balance to off-kilter apoptotic machinery, resulting in decreased cellular turnover and the resultant protection of the at-risk neuronal population. In addition to diabetes, and neurodegenerative disorders, we believe that GSK-3β inhibition may play a promising role in patients receiving irradiation.
While radiation dose-escalation has been important for the treatment of multiple cranial tumors (e.g., brain metastases, primary gliomas) and benign disorders (e.g., vestibular schwannoma, meningioma), the treatment is limited by the effects of irradiation on healthy surrounding neurons. It has been demonstrated that GSK-3β inhibition can protect hippocampal neurons (in primary culture and murine pups) from irradiation-induced damage [9, 70]. Thotala et al. demonstrated improved survival of intestinal crypt cells and increased latency to murine GI-related death from irradiation [77]. This report suggested that GSK-3β inhibitors could reduce deleterious consequences of intestinal irradiation and possibly improve patient quality of life measures. It would be worthwhile to explore their utility in syngenic murine models of neural cancer, murine tumor xenografts, as well as human clinical trials of patients in the setting of re-irradiation (e.g., recurrent glioma). Reports of radiation protection have also been demonstrated with small molecular inhibitors of GSK-3β in the gastrointestinal system.
In cancer, however, the apoptotic machinery is often defective allowing cells to undergo unregulated proliferation. In this case, negative regulation of GSK-3β can serve to tip the balance in favor of apoptosis. Dickey et al. demonstrated the ability of GSK-3β inhibition to effectively enhance cell death of neuroblastoma cells in vitro and in a murine xenograft model [79]. Similar findings have been demonstrated in glioma [81, 82]. The interplay between GSK-3β regulation and other cell death stimuli is being carefully studied across a wide variety of cancer types, and there is promising data suggesting a strong role for this form of therapy in the near future. The bifunctional role of GSK-3β as a facilitator of apoptosis and a mediator of pro-survival signals has important implications in both the generation of novel therapies and the understanding of complex disease states.
The use of both positive and negative regulators of GSK-3β offers exciting treatment possibilities for a multitude of diseases. The complexity of the GSK-3β network requires careful examination, however, when considering modulating its function in a clinical setting. More studies are required to clearly understand the effects of regulating GSK-3β on the multiple signaling pathways involved in growth, development, and metabolism. The effect of GSK-3β on cell survival and apoptosis appears to be context dependent, and the required mode of action will likely depend on the specific pathway, cell type, and disease being targeted. While the vast network of GSK-3β offers a treatment option for multiple diseases, it also requires careful consideration of all the factors involved in order to prepare against potential side effects.
References
- 1.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochemical Journal. 2001;359(1):1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. Journal of Cell Science. 2003;116(7):1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jope RS, Johnson GVW. The glamour and gloom of glycogen synthase kinase-3. Trends in Biochemical Sciences. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Progress in Neurobiology. 2001;65(4):391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 5.Luo J. Glycogen synthase kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer Letters. 2009;273(2):194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pap M, Cooper GM. Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3β signaling pathway. Molecular and Cellular Biology. 2002;22(2):578–586. doi: 10.1128/MCB.22.2.578-586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez JF, Sniderhan LF, Williamson AL, Fan S, Chakraborty-Sett S, Maggirwar SB. Glycogen synthase kinase 3β-mediated apoptosis of primary cortical astrocytes involves inhibition of nuclear factor κB signaling. Molecular and Cellular Biology. 2003;23(13):4649–4662. doi: 10.1128/MCB.23.13.4649-4662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen P, Frame S. The renaissance of GSK3. Nature Reviews Molecular Cell Biology. 2001;2(10):769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 9.Yazlovitskaya EM, Edwards E, Thotala D, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Research. 2006;66(23):11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 10.Ter Haar E, Coll JT, Austen DA, Hsiao HM, Swenson L, Jain J. Structure of GSK3β reveals a primed phosphorylation mechanism. Nature Structural Biology. 2001;8(7):593–596. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- 11.Dajani R, Fraser E, Roe SM, et al. Crystal structure of glycogen synthase kinase 3β: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105(6):721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 12.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 13.Eldar-Finkelman H, Seger R, Vandenheede JR, Krebs EG. Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells. Journal of Biological Chemistry. 1995;270(3):987–990. doi: 10.1074/jbc.270.3.987. [DOI] [PubMed] [Google Scholar]
- 14.Kim L, Liu J, Kimmel AR. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99(4):399–408. doi: 10.1016/s0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- 15.Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. Journal of Neurochemistry. 2004;89(6):1313–1317. doi: 10.1111/j.1471-4159.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 16.Hernández F, Pérez M, Lucas JJ, Mata AM, Bhat R, Avila J. Glycogen synthase kinase-3 plays a crucial role in tau exon 10 splicing and intranuclear distribution of SC35: implications for Alzheimer’s disease. Journal of Biological Chemistry. 2004;279(5):3801–3806. doi: 10.1074/jbc.M311512200. [DOI] [PubMed] [Google Scholar]
- 17.Fulga TA, Elson-Schwab I, Khurana V, et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nature Cell Biology. 2007;9(2):139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- 18.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nature Reviews Drug Discovery. 2004;3(6):479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 19.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nature Genetics. 2004;36(2):131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 20.Beaulieu JM, Sotnikova TD, Yao WD, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozikowski AP, Gaisina IN, Petukhov PA, et al. Highly potent and specific GSK-3β inhibitors that block tau phosphorylation and decrease α-synuclein protein expression in a cellular model of Parkinson’s disease. ChemMedChem. 2006;1(2):256–266. doi: 10.1002/cmdc.200500039. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Lin HK, Hu YC, Xie S, Yang L, Chang C. Suppression of androgen receptor-mediated transactivation and cell growth by the glycogen synthase kinase 3β in prostate cells. Journal of Biological Chemistry. 2004;279(31):32444–32452. doi: 10.1074/jbc.M313963200. [DOI] [PubMed] [Google Scholar]
- 24.Shakoori A, Ougolkov A, Zhi WY, et al. Deregulated GSK3β activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochemical and Biophysical Research Communications. 2005;334(4):1365–1373. doi: 10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Shakoori A, Mai W, Miyashita K, et al. Inhibition of GSK-3β activity attenuates proliferation of human colon cancer cells in rodents. Cancer Science. 2007;98(9):1388–1393. doi: 10.1111/j.1349-7006.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3β participates in nuclear factor κB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Research. 2005;65(6):2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 27.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Progress in Neurobiology. 2006;79(4):173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller JR, Moon RT. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes and Development. 1996;10(20):2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 29.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287(5458):1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 30.Giles RH, Van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et Biophysica Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 31.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes and Development. 1998;12(22):3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3β facilitates staurosporine- and heat shock- induced apoptosis. Protection by lithium. Journal of Biological Chemistry. 2000;275(11):7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 33.Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cellular and Molecular Life Sciences. 2007;64(15):1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watcharasit P, Bijur GN, Zmijewski JW, et al. Direct, activating interaction between glycogen synthase kinase-3β and p53 after DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(12):7951–7955. doi: 10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loberg RD, Vesely E, Brosius FC. Enhanced glycogen synthase kinase-3β activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. Journal of Biological Chemistry. 2002;277(44):41667–41673. doi: 10.1074/jbc.M206405200. [DOI] [PubMed] [Google Scholar]
- 36.Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3β in endoplasmic reticulum stress-induced caspase-3 activation. Journal of Biological Chemistry. 2002;277(47):44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- 37.Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3β inhibitors prevent cellular polyglutamine toxicity caused by the Huntington’s disease mutation. Journal of Biological Chemistry. 2002;277(37):33791–33798. doi: 10.1074/jbc.M204861200. [DOI] [PubMed] [Google Scholar]
- 38.Cross DAE, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. Journal of Neurochemistry. 2001;77(1):94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 39.Yazlovitskaya EM, Edwards E, Thotala D, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Research. 2006;66(23):11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 40.Culbert AA, Brown MJ, Frame S, et al. GSK-3 inhibition by adenoviral FRAT1 overexpression is neuroprotective and induces Tau dephosphorylation and β-catenin stabilisation without elevation of glycogen synthase activity. FEBS Letters. 2001;507(3):288–294. doi: 10.1016/s0014-5793(01)02990-8. [DOI] [PubMed] [Google Scholar]
- 41.Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3β facilitates staurosporine- and heat shock- induced apoptosis. Protection by lithium. Journal of Biological Chemistry. 2000;275(11):7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 42.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Progress in Neurobiology. 2001;65(4):391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 43.Oren M. Decision making by p53: life, death and cancer. Cell Death and Differentiation. 2003;10(4):431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 44.Kruse JP, Gu W. Modes of p53 Regulation. Cell. 2009;137(4):609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. The Journal of Biological Chemistry. 2003;278(49):48872–48879. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beurel E, Kornprobst M, Blivet-Van Eggelpoël MJ, et al. GSK-3β inhibition by lithium confers resistance to chemotherapy-induced apoptosis through the repression of CD95 (Fas/APO-1) expression. Experimental Cell Research. 2004;300(2):354–364. doi: 10.1016/j.yexcr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Turenne GA, Price BD. Glycogen synthase kinase3 beta phosphorylates serine 33 of p53 and activates p53’s transcriptional activity. BMC Cell Biology. 2001;2 doi: 10.1186/1471-2121-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pluquet O, Qu LK, Baltzis D, Koromilas AE. Endoplasmic reticulum stress accelerates p53 degradation by the cooperative actions of Hdm2 and glycogen synthase kinase 3β . Molecular and Cellular Biology. 2005;25(21):9392–9405. doi: 10.1128/MCB.25.21.9392-9405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu L, Huang S, Baltzis D, et al. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3β . Genes and Development. 2004;18(3):261–277. doi: 10.1101/gad.1165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eom TY, Roth KA, Jope RS. Neural precursor cells are protected from apoptosis induced by trophic factor withdrawal or genotoxic stress by inhibitors of glycogen synthase kinase 3. Journal of Biological Chemistry. 2007;282(31):22856–22864. doi: 10.1074/jbc.M702973200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arboleda G, Cardenas Y, Rodriguez Y, Morales LC, Matheus L, Arboleda H. Differential regulation of AKT, MAPK and GSK3beta during C2-ceramide-induced neuronal death. Neurotoxicology. 2010;31(6):687–693. doi: 10.1016/j.neuro.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Mora A, Sabio G, Risco AM, et al. Lithium blocks the PKB and GSK3 dephosphorylation induced by ceramide through protein phosphatase-2A. Cellular Signalling. 2002;14(6):557–562. doi: 10.1016/s0898-6568(01)00282-0. [DOI] [PubMed] [Google Scholar]
- 53.Noh KT, Park YM, Cho SG, Choi EJ. GSK-3β-induced ASK1 stabilization is crucial in LPS-induced endotoxin shock. Experimental Cell Research. 2011;317(12):1663–1668. doi: 10.1016/j.yexcr.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Sengupta S, Jayaraman P, Chilton PM, Casella CR, Mitchell TC. Unrestrained glycogen synthase kinase-3β activity leads to activated T cell death and can be inhibited by natural adjuvant. Journal of Immunology. 2007;178(10):6083–6091. doi: 10.4049/jimmunol.178.10.6083. [DOI] [PubMed] [Google Scholar]
- 55.Kang YJ, Digicaylioglu M, Russo R, et al. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Annals of Neurology. 2010;68(3):342–352. doi: 10.1002/ana.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng F, Dhillon NK, Yao H, Zhu X, Williams R, Buch S. Mechanisms of platelet-derived growth factor-mediated neuroprotection—implications in HIV dementia. European Journal of Neuroscience. 2008;28(7):1255–1264. doi: 10.1111/j.1460-9568.2008.06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sui Z, Sniderhan LF, Fan S, et al. Human immunodeficiency virus-encoded Tat activates glycogen synthase kinase-3β to antagonize nuclear factor-κB survival pathway in neurons. European Journal of Neuroscience. 2006;23(10):2623–2634. doi: 10.1111/j.1460-9568.2006.04813.x. [DOI] [PubMed] [Google Scholar]
- 58.Goldbaum O, Richter-Landsberg C. Activation of PP2A-like phosphatase and modulation of tau phosphorylation accompany stress-induced apoptosis in cultured oligodendrocytes. GLIA. 2002;40(3):271–282. doi: 10.1002/glia.10119. [DOI] [PubMed] [Google Scholar]
- 59.Kihira T, Suzuki A, Kondo T, et al. Immunohistochemical expression of IGF-I and GSK in the spinal cord of Kii and Guamanian ALS patients: original Article. Neuropathology. 2009;29(5):548–558. doi: 10.1111/j.1440-1789.2009.01010.x. [DOI] [PubMed] [Google Scholar]
- 60.Koh SH, Lee YB, Kim KS, et al. Role of GSK-3β activity in motor neuronal cell death induced by G93A or A4V mutant hSOD1 gene. European Journal of Neuroscience. 2005;22(2):301–309. doi: 10.1111/j.1460-9568.2005.04191.x. [DOI] [PubMed] [Google Scholar]
- 61.Liang MH, Wendland JR, Chuang DM. Lithium inhibits Smad3/4 transactivation via increased CREB activity induced by enhanced PKA and AKT signaling. Molecular and Cellular Neuroscience. 2008;37(3):440–453. doi: 10.1016/j.mcn.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martins DF, Rosa AO, Gadotti VM, et al. The antinociceptive effects of AR-A014418, a selective inhibitor of glycogen synthase kinase-3 beta, in mice. Journal of Pain. 2011;12(3):315–322. doi: 10.1016/j.jpain.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Pérez M, Rojo AI, Wandosell F, Díaz-Nido J, Avila J. Prion peptide induces neuronal cell death through a pathway involving glycogen synthase kinase 3. Biochemical Journal. 2003;372(1):129–136. doi: 10.1042/BJ20021596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang Z, Arjunan P, Lee C, et al. Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3β phosphorylation. Journal of Experimental Medicine. 2010;207(4):867–880. doi: 10.1084/jem.20091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gall JM, Wong V, Pimental DR, et al. Hexokinase regulates Bax-mediated mitochondrial membrane injury following ischemic stress. Kidney International. 2011;79(11):1207–1216. doi: 10.1038/ki.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hotokezaka Y, van Leyen K, Lo EH, et al. AlphaNAC depletion as an initiator of ER stress-induced apoptosis in hypoxia. Cell Death & Differentiation. 2009;16(11):1505–1514. doi: 10.1038/cdd.2009.90. [DOI] [PubMed] [Google Scholar]
- 67.Li Q, Li H, Roughton K, et al. Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death and Disease. 2010;1(7, article e56) doi: 10.1038/cddis.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vasileva AK, Plotnikov EY, Kazachenko AV, Kirpatovsky VI, Zorov DB. Inhibition of GSK-3β decreases the ischemia-induced death of renal cells. Bulletin of Experimental Biology and Medicine. 2010;149(3):303–307. doi: 10.1007/s10517-010-0932-1. [DOI] [PubMed] [Google Scholar]
- 69.Kulikov R, Boehme KA, Blattner C. Glycogen synthase kinase 3-dependent phosphorylation of Mdm2 regulates p53 abundance. Molecular and Cellular Biology. 2005;25(16):7170–7180. doi: 10.1128/MCB.25.16.7170-7180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thotala DK, Hallahan DE, Yazlovitskaya EM. Inhibition of glycogen synthase kinase 3β attenuates neurocognitive dysfunction resulting from cranial irradiation. Cancer Research. 2008;68(14):5859–5868. doi: 10.1158/0008-5472.CAN-07-6327. [DOI] [PubMed] [Google Scholar]
- 71.Thotala DK, Hallahan DE, Yazlovitskaya EM. Glycogen synthase kinase 3β inhibitors protect hippocampal neurons from radiation-induced apoptosis by regulating MDM2-p53 pathway. Cell Death and Differentiation. 2011;19:387–396. doi: 10.1038/cdd.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu R, Song L, Jope RS. Lithium attenuates p53 levels in human neuroblastoma SH-SY5Y cells. NeuroReport. 1999;10(5):1123–1125. doi: 10.1097/00001756-199904060-00040. [DOI] [PubMed] [Google Scholar]
- 73.Zmijewski JW, Jope RS. Nuclear accumulation of glycogen synthase kinase-3 during replicative senescence of human fibroblasts. Aging Cell. 2004;3(5):309–317. doi: 10.1111/j.1474-9728.2004.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 75.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nature Reviews Drug Discovery. 2008;7(12):989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 76.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 77.Thotala DK, Geng L, Dickey AK, Hallahan DE, Yazlovitskaya EM. A New class of molecular targeted radioprotectors: GSK-3β inhibitors. International Journal of Radiation Oncology Biology Physics. 2010;76(2):557–565. doi: 10.1016/j.ijrobp.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three akts. Genes and Development. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 79.Dickey A, Schleicher S, Leahy K, Hu R, Hallahan D, Thotala DK. GSK-3β inhibition promotes cell death, apoptosis, and in vivo tumor growth delay in neuroblastoma Neuro-2A cell line. Journal of Neuro-Oncology. 2010;104:145–153. doi: 10.1007/s11060-010-0491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meares GP, Jope RS. Resolution of the nuclear localization mechanism of glycogen synthase kinase-3: functional effects in apoptosis. Journal of Biological Chemistry. 2007;282(23):16989–17001. doi: 10.1074/jbc.M700610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korur S, Huber RM, Sivasankaran B, et al. GSK3beta regulates differentiation and growth arrest in glioblastoma. PloS One. 2009;4(10, article e7443) doi: 10.1371/journal.pone.0007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kotliarova S, Pastorino S, Kovell LC, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-κB, and glucose regulation. Cancer Research. 2008;68(16):6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun M, Song L, Li Y, Zhou T, Jope RS. Identification of an antiapoptotic protein complex at death receptors. Cell Death and Differentiation. 2008;15(12):1887–1900. doi: 10.1038/cdd.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Venè R, Larghero P, Arena G, Sporn MB, Albini A, Tosetti F. Glycogen synthase kinase 3β regulates cell death induced by synthetic triterpenoids. Cancer Research. 2008;68(17):6987–6996. doi: 10.1158/0008-5472.CAN-07-6362. [DOI] [PubMed] [Google Scholar]
- 85.Damalas A, Ben-Ze’ev A, Simcha I, et al. Excess β-catenin promotes accumulation of transcriptionally active p53. EMBO Journal. 1999;18(11):3054–3063. doi: 10.1093/emboj/18.11.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sadot E, Geiger B, Oren M, Ben-Ze’ev A. Down-regulation of β-catenin by activated p53. Molecular and Cellular Biology. 2001;21(20):6768–6781. doi: 10.1128/MCB.21.20.6768-6781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meares GP, Mines MA, Beurel E, et al. Glycogen synthase kinase-3 regulates endoplasmic reticulum (ER) stress-induced CHOP expression in neuronal cells. Experimental Cell Research. 2011;317(11):1621–1628. doi: 10.1016/j.yexcr.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature. 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 89.Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3β regulates NF-κB1/p105 stability. Journal of Biological Chemistry. 2003;278(41):39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- 90.Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-α-induced NF-κB activation and apoptosis in hepatocytes. American Journal of Physiology. 2002;283(1):G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- 91.Deng J, Miller SA, Wang HY, et al. β-catenin interacts with and inhibits NF-κB in human colon and breast cancer. Cancer Cell. 2002;2(4):323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- 92.Gotschel F, Kern C, Lang S, et al. Inhibition of GSK3 differentially modulates NF-kappaB, CREB, AP-1 and beta-catenin signaling in hepatocytes, but fails to promote TNF-alpha-induced apoptosis. Experimental Cell Research. 2008;314(6):1351–1366. doi: 10.1016/j.yexcr.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 93.Schotte P, Van Loo G, Carpentier I, Vandenabeele P, Beyaert R. Lithium sensitizes tumor cells in an NFκB-independent way to caspase activation and apoptosis induced by tumor necrosis factor (TNF): evidence for a role of the TNF receptor-associated death domain protein. Journal of Biological Chemistry. 2001;276(28):25939–25945. doi: 10.1074/jbc.M104014200. [DOI] [PubMed] [Google Scholar]
- 94.Liao X, Zhang L, Thrasher JB, Du J, Li B. Glycogen synthase kinase-3beta suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Molecular Cancer Therapeutics. 2003;2(11):1215–1222. [PubMed] [Google Scholar]
- 95.Bijur GN, Jope RS. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3β . Journal of Biological Chemistry. 2001;276(40):37436–37442. doi: 10.1074/jbc.M105725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi-Yanaga F, Sasaguri T. GSK-3β regulates cyclin D1 expression: a new target for chemotherapy. Cellular Signalling. 2008;20(4):581–589. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 97.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochemical Journal. 1993;296(2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3- kinase/Akt cell survival pathway. Journal of Biological Chemistry. 1998;273(32):19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 99.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)- 8-phenyl-4H-1-benzopyran-4-one (LY294002) Journal of Biological Chemistry. 1994;269(7):5241–5248. [PubMed] [Google Scholar]
- 100.Arico S, Pattingre S, Bauvy C, et al. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. Journal of Biological Chemistry. 2002;277(31):27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 101.Hill MM, Andjelkovic M, Brazil DP, Ferrari S, Fabbro D, Hemmings BA. Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a staurosporine-insensitive kinase. Journal of Biological Chemistry. 2001;276(28):25643–25646. doi: 10.1074/jbc.C100174200. [DOI] [PubMed] [Google Scholar]
- 102.Hussain AR, Al-Rasheed M, Manogaran PS, et al. Curcumin induces apoptosis via inhibition of PI3′-kinase/AKT pathway in acute T cell leukemias. Apoptosis. 2006;11(2):245–254. doi: 10.1007/s10495-006-3392-3. [DOI] [PubMed] [Google Scholar]
- 103.Meggio F, Donella Deana A, Ruzzene M, et al. Different susceptibility of protein kinases to staurosporine inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. European Journal of Biochemistry. 1995;234(1):317–322. doi: 10.1111/j.1432-1033.1995.317_c.x. [DOI] [PubMed] [Google Scholar]
- 104.Noël A, Barrier L, Rinaldi F, Hubert C, Fauconneau B, Ingrand S. Lithium chloride and staurosporine potentiate the accumulation of phosphorylated glycogen synthase kinase 3β/Tyr216, resulting in glycogen synthase kinase 3β activation in SH-SY5Y human neuroblastoma cell lines. Journal of Neuroscience Research. 2011;89(5):755–763. doi: 10.1002/jnr.22587. [DOI] [PubMed] [Google Scholar]
- 105.Alao JP, Stavropoulou AV, Lam EWF, Coombes RC. Role of glycogen synthase kinase 3 beta (GSK3β) in mediating the cytotoxic effects of the histone deacetylase inhibitor trichostatin A (TSA) in MCF-7 breast cancer cells. Molecular Cancer. 2006;5, article 40 doi: 10.1186/1476-4598-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang L, Dan HC, Sun M, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Research. 2004;64(13):4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 107.Dong J, Peng J, Zhang H, et al. Role of glycogen synthase kinase 3β in rapamycin-mediated cell cycle regulation and chemosensitivity. Cancer Research. 2005;65(5):1961–1972. doi: 10.1158/0008-5472.CAN-04-2501. [DOI] [PubMed] [Google Scholar]
- 108.Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Molecular Cell. 2006;24(2):185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benkoussa M, Brand C, Delmotte MH, Formstecher P, Lefebvre P. Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter. Molecular and Cellular Biology. 2002;22(13):4522–4534. doi: 10.1128/MCB.22.13.4522-4534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma Y, Feng Q, Sekula D, Diehl JA, Freemantle SJ, Dmitrovsky E. Retinoid targeting of different D-type cyclins through distinct chemopreventive mechanisms. Cancer Research. 2005;65(14):6476–6483. doi: 10.1158/0008-5472.CAN-05-0370. [DOI] [PubMed] [Google Scholar]
- 111.Mori J, Takahashi-Yanaga F, Miwa Y, et al. Differentiation-inducing factor-1 induces cyclin D1 degradation through the phosphorylation of Thr286 in squamous cell carcinoma. Experimental Cell Research. 2005;310(2):426–433. doi: 10.1016/j.yexcr.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 112.Takahashi-Yanaga F, Taba Y, Miwa Y, et al. Dictyostelium differentiation-inducing factor-3 activates glycogen synthase kinase-3β and degrades cyclin D1 in mammalian cells. Journal of Biological Chemistry. 2003;278(11):9663–9670. doi: 10.1074/jbc.M205768200. [DOI] [PubMed] [Google Scholar]
- 113.Medina M, Avila J. Glycogen synthase kinase-3 (GSK-3) inhibitors for the treatment of Alzheimer’s disease. Current Pharmaceutical Design. 2010;16(25):2790–2798. doi: 10.2174/138161210793176581. [DOI] [PubMed] [Google Scholar]
- 114.Avila J, Hernández F. GSK-3 inhibitors for Alzheimer’s disease. Expert Review of Neurotherapeutics. 2007;7(11):1527–1533. doi: 10.1586/14737175.7.11.1527. [DOI] [PubMed] [Google Scholar]
- 115.Klamer G, Song E, Ko KH, O’Brien TA, Dolnikov A. Using small molecule GSK3β inhibitors to treat inflammation. Current Medicinal Chemistry. 2010;17(26):2873–2881. doi: 10.2174/092986710792065090. [DOI] [PubMed] [Google Scholar]
- 116.Phukan S, Babu VS, Kannoji A, Hariharan R, Balaji VN. GSK3β: role in therapeutic landscape and development of modulators. British Journal of Pharmacology. 2010;160(1):1–19. doi: 10.1111/j.1476-5381.2010.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ryves WJ, Dajani R, Pearl L, Harwood AJ. Glycogen synthase kinase-3 inhibition by lithium and beryllium suggests the presence of two magnesium binding sites. Biochemical and Biophysical Research Communications. 2002;290(3):967–972. doi: 10.1006/bbrc.2001.6305. [DOI] [PubMed] [Google Scholar]
- 118.Martinez A, Castro A, Dorronsoro I, Alonso M. Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Medicinal Research Reviews. 2002;22(4):373–384. doi: 10.1002/med.10011. [DOI] [PubMed] [Google Scholar]
- 119.Aoki M, Yokota T, Sugiura I, et al. Structural insight into nucleotide recognition in tau-protein kinase I/glycogen synthase kinase 3β . Acta Crystallographica D. 2004;60(3):439–446. doi: 10.1107/S090744490302938X. [DOI] [PubMed] [Google Scholar]
- 120.Bax B, Carter PS, Lewis C, et al. The structure of phosphorylated GSK-3β complexed with a peptide, FRATtide, that inhibits β-catenin phosphorylation. Structure. 2001;9(12):1143–1152. doi: 10.1016/s0969-2126(01)00679-7. [DOI] [PubMed] [Google Scholar]
- 121.Bertrand JA, Thieffine S, Vulpetti A, et al. Structural characterization of the GSK-3β active site using selective and non-selective ATP-mimetic inhibitors. Journal of Molecular Biology. 2003;333(2):393–407. doi: 10.1016/j.jmb.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 122.Bhat R, Xue Y, Berg S, et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. The Journal of Biological Chemistry. 2003;278(46):45937–45945. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- 123.Dajani R, Fraser E, Roe SM, et al. Structural basis for recruitment of glycogen synthase kinase 3β to the axin-APC scaffold complex. EMBO Journal. 2003;22(3):494–501. doi: 10.1093/emboj/cdg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin D, Lee SC, Heo YS, et al. Design and synthesis of 7-hydroxy-1H-benzoimidazole derivatives as novel inhibitors of glycogen synthase kinase-3β . Bioorganic and Medicinal Chemistry Letters. 2007;17(20):5686–5689. doi: 10.1016/j.bmcl.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 125.Zhang HC, Bonaga LV, Ye H, Derian CK, Damiano BP, Maryanoff BE. Novel bis(indolyl)maleimide pyridinophanes that are potent, selective inhibitors of glycogen synthase kinase-3. Bioorganic & Medicinal Chemistry Letters . 2007;17(10):2863–2868. doi: 10.1016/j.bmcl.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 126.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends in Pharmacological Sciences. 2003;24(9):441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 127.Coghlan MP, Culbert AA, Cross DAE, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chemistry and Biology. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 128.Meijer L, Thunnissen AMWH, White AW, et al. Inhibition of cyclin-dependent kinases, GSK-3β and CK1 by hymenialdisine, a marine sponge constituent. Chemistry and Biology. 2000;7(1):51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 129.Zaharevitz DW, Gussio R, Leost M, et al. Discovery and initial characterization of the paullones, a novel class of smallmolecule inhibitors of cyclin-dependent kinases. Cancer Research. 1999;59(11):2566–2569. [PubMed] [Google Scholar]
- 130.Hoessel R, Leclerc S, Endicott JA, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nature Cell Biology. 1999;1(1):60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 131.Leclerc S, Garnier M, Hoessel R, et al. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? Journal of Biological Chemistry. 2001;276(1):251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 132.Meijer L, Skaltsounis AL, Magiatis P, et al. GSK-3-selective inhibitors derived from tyrian purple indirubins. Chemistry and Biology. 2003;10(12):1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 133.Martinez A, Alonso M, Castro A, Pérez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 β (GSK-3β) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. Journal of Medicinal Chemistry. 2002;45(6):1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 134.Coghlan MP, Culbert AA, Cross DAE, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chemistry and Biology. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 135.Thomas GM, Frame S, Goedert M, Nathke I, Polakis P, Cohen P. A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of Axin and β-catenin. FEBS Letters. 1999;458(2):247–251. doi: 10.1016/s0014-5793(99)01161-8. [DOI] [PubMed] [Google Scholar]


