Abstract
Glioblastoma multiforme (GBM) is most common primary brain tumor in adults. GBM is very aggressive due to its poor cellular differentiation and invasiveness, which makes complete surgical resection virtually impossible. Therefore, GBM’s invasive nature as well as its intrinsic resistance to current treatment modalities makes it a unique therapeutic challenge. Extensive examination of human GBM specimens has uncovered that these tumors overexpress a variety of receptors that are virtually absent in the surrounding non-neoplastic brain. Human GBMs overexpress receptors for cytokines, growth factors, ephrins, urokinase-type plasminogen activator (uPA), and transferrin, which can be targeted with high specificity by linking their ligands with highly cytotoxic molecules, such as Diptheria toxin and Pseudomonas exotoxin A. We review the preclinical development and clinical translation of targeted toxins for GBM. In view of the clinical experience, we conclude that although these are very promising therapeutic modalities for GBM patients, efforts should be focused on improving the delivery systems utilized in order to achieve better distribution of the immuno-toxins in the tumor/resection cavity. Delivery of targeted toxins using viral vectors would also benefit enormously from improved strategies for local delivery.
Keywords: IL-13, IL-13Rα2, TGF-α, EGFR, EGFRvIII, transferrin receptor, pseudomonas exotoxin, diphtheria toxin, gene therapy, targeted toxins
INTRODUCTION
Glioblastoma multiforme (GBM) is highly malignant due to its poor cellular differentiation, invasiveness, and surgical inaccessibility to complete resection. GBM is characterized by the presence of necrosis, microvascular proliferation, nuclear atypia, and invasion into the surrounding non-neoplastic brain 1. GBM’s infiltrative nature and intrinsic resistance to current treatment modalities make it a unique therapeutic challenge 2. However, extensive scrutiny of GBM samples from human patients has uncovered that these tumors overexpress several receptors that distinguish them from the surrounding normal brain parenchyma. Human GBMs overexpress receptors, which are virtually absent in the normal brain such as those for cytokines 3–9, growth factors 10–14, ephrins 9, 15–17, urokinase-type plasminogen activator (uPA) 18, transferrin 19, 20. Thus, specific targeting of cytotoxic molecules towards tumor cells has been attempted by linking them with ligands for these GBM specific receptors (Figure 1).
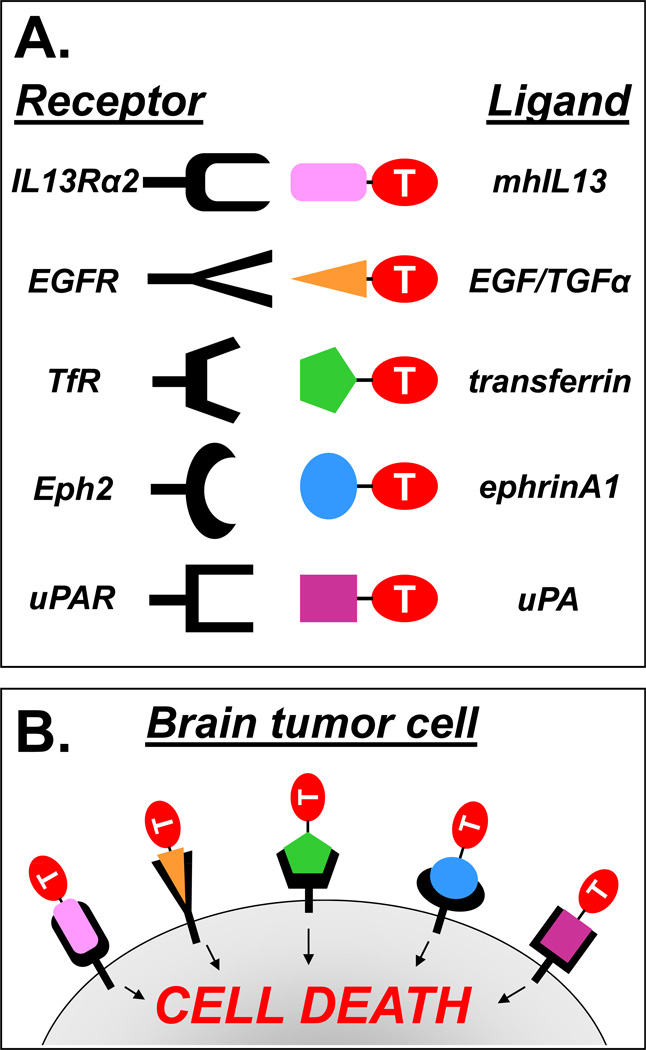
Figure 1. Illustration depicting the use of various targeted approaches under development for the treatment of GBM.
A) The receptors (left) and their ligands (right) under development for targeted toxin therapies are shown. Various toxins (T) are linked to specific ligands in order to elicit the binding and internalization of the targeted toxins to receptors that are overexpressed on GBM cells. The rationale underlying the use of targeted toxins is shown in (B).
Receptors for IL-13 have been detected in 50–75% of human GBM specimens 3, 9, 11, 21. In normal tissues, receptors for IL-13 consist of the IL13α1R chain which requires heterodimerization with the IL4Rα chain in order to bind IL-13 with high affinity 4, 22, 23. IL-13 and IL-4 compete for the shared physiological signaling receptor IL13/IL4R present in normal cells 4, 22, 23. GBM cells overexpress a distinct monomeric receptor for IL-13, which consists of the IL13Rα2 chain 3, 24–26. IL-13 binds to GBM-associated IL-13Rα2 with high affinity, while IL-4 does not interact with this receptor 3, 24–26. Although the heterodimeric receptor complex composed of IL-13α1 receptor and IL-4 receptor (IL13/IL4R) is widely expressed among normal tissues 27, GBM-associated IL13Rα2 seems to be restricted mainly to the testes among normal peripheral tissues, and virtually absent from the CNS 3.
Overexpression of EphA2, a receptor tyrosine kinase for ephrinA1, has been observed in most of human GBM specimens 16, 17 but is undetectable in normal brain 17. EphA2 is an attractive target due to the fact that it is not only expressed on GBM cells, but also in the tumor microvasculature 9. In addition, expression of EphA2 in GBM has been shown to be associated with a more aggressive phenotype and a poorer clinical outcome 16, 17.
Amplification of the epidermal growth factor receptor (EGFR) gene is the most common genetic alteration found in human GBM 28. This results in overexpression of EGFR protein, which has been detected in at least half of human GBM specimens 11, 29, 30. Between 50–60% of EGFR+ GBMs also express a mutated form of the receptor, EGFRvIII, which is a truncated and constitutively active form of EGFR that is not present in normal tissues 31. The association of EGFR overexpression and clinical outcome for GBM patients remains controversial 29, 30.
Receptors for transferring, TfR1 and TfR2, have been detected in the majority of GBM patients 11, 32, 33. TfRs are mainly restricted to the luminal surface of the brain capillaries with minimal dispersion throughout the normal brain tissue 34–36, thus making TfRs attractive candidates for targeted toxins. The binding of the ligand transferrin (Tf) to its receptor TfR mediates the transport of iron into actively dividing cells. Each Tf molecule has two lobes, each of which serves as an iron-binding site; thus, each Tf molecule is capable of binding and transporting two ferric ions (Fe3+) into a cell. TfR is only able to bind Tf when both lobes of the transferrin molecule are bound to ferric ions (holo-Tf). After binding of holo-Tf to TfR, the holo-Tf/TfR complex undergoes receptor-mediated endocytosis. Upon entry of the holo-Tf/TfR complex into the cell, two Fe3+ ions are released from holo-Tf, thus re-forming the Tf ligand in its iron-free state (apo-Tf) while remaining bound to the TfR. The apo-Tf/TfR complex is then recycled back to the cell surface where the iron-free apo-Tf is released from the TfR back into the extracellular space. The unbound apo-Tf can then bind two more ferric irons and repeat the endocytic cycle 37. The endocytic capacity of TfR has been exploited to introduce cytotoxic toxins within GBM cells.
Overexpression of receptors for urokinase-type plasminogen activator (uPAR) has also been observed in human GBM and is associated with poorer prognosis due to its role in invasion 18, 38–40. These receptors are virtually confined to GBM cells and that their presence seems to be associated with a more aggressive tumor phenotype, targeting of highly cytotoxic compounds towards tumor cells has been attempted using their natural ligands. Considering that uPAR is found in tumor microvasculature 41, this receptor is also an attractive target to impact tumor angiogenesis 42.
Targeting of IL-13Rα2
A number of recombinant proteins consisting of catalytic toxins fused to IL-13 have been developed to deliver highly toxic agents to IL13α2R+ GBM cells 4, 43, 44. Pseudomonas exotoxin A [PE] is a cytotoxic bacterial protein which encompasses three functional domains. Domain I binds the α2-macroglobulin receptor, which is ubiquitously expressed in normal tissues; the exotoxin-α2-macroglobulin receptor complex undergoes internalization by endocytosis 45. Domain II is a site of proteolytic cleavage that activates PE and is required for catalyzing the translocation of the catalytic domain III into the cytosol. Once in the cytosol, Domain III directs the processed fragment of the toxin to the endoplasmic reticulum where it inactivates the elongation factor 2 through ADP ribosylation, inhibiting protein synthesis and leading to cell death 45. The mutant exotoxin, PE38QQR 46, does not bind to the ubiquitous α2-macroglobulin receptor due to the deletion of domain I 46, hence it can be linked to various ligands in order to promote its internalization into target tumor cells. To target the PE toxin to human glioma cells, a fusion protein was constructed that links the N-terminal domain of PE38QQR to native hIL-13 (hIL-13-PE, Cintredekin Besudotox) 4. The sensitivity of GBM cells to hIL-13-PE correlates positively with the density of IL-13Rα2 47 and it has been demonstrated that this toxin exerts an anti-tumor effect in preclinical models of human GBM 47–50. Systemic administration of the hIL-13-PE is limited by dose 48 due to toxic side effects. Preclinical 4, 7, 48, 51, 52 and clinical trials 53 have shown that local administration can be used to successfully deliver chimeric toxins to brain tumors. However, since the toxin remains active for only up to six hours at the site of injection, intratumoral administration requires multiple injections to be effective 51, 54. Convection enhanced delivery (CED) of the hIL-13-PE has been tested in a Phase III clinical trial for GBM 55 and is associated with dose-related neurological side effects in most of the enrolled patients 56 (Table 1)
Table 1.
Summary of clinical trials using convection enhanced delivery (CED) of targeted toxin approaches for the treatment of brain tumors
| Receptor target |
Ligand | Toxin | Clinical Stage |
# of patients |
Main findings | Publication |
|---|---|---|---|---|---|---|
| IL-13Rα2 | IL-13 | PE38 | Phase I | 46 | Interim analysis of three Phase I trials. | Kunwar et al., 2003 |
| IL-13Rα2 | IL-13 | PE38 | Phase I | 22 | MTD was established as 0.5 µg/ml administered via CED. | Vogelbaum et al., 2007 |
| IL-13Rα2 | IL-13 | PE38 | Phase I | 51 | Final analysis of three Phase I trials: MTD was established as 0.5 µg/ml administered via CED. Infusion duration of up to 6 days was well tolerated. Postoperative catheter placement is critical for optimal drug distribution. | Kunwar et al., 2007 |
| IL-13Rα2 | IL-13 | PE38 | Phase III | 296 | Efficacy of drugs delivered by CED may be hampered by ineffective delivery. | Sampson et al., 2010 |
| IL-13Rα2 | IL-13 | PE38 | Phase III | 296 | No survival difference between IL13-PE38QQR when compared to Gliadel wafers | Kunwar et al., 2010 |
| IL-4R | IL-4 | PE38 | Phase I | 9 | No systemic toxicity observed. Necrosis was observed in 6 of 9 patients. | Rand et al., 2000 |
| IL-4R | IL-4 | PE38 | Phase I/II | 31 | No drug-related systemic toxicity; treatment-related adverse effects were limited to the central nervous system. MTD was defined as 6 µg/ml × 40ml. | Weber et al., 2003 |
| IL-4R | IL-4 | PE38 | Phase I/II | 31 | Case study of long-term survivor. | Rainov et al., 2005 |
| EGFR | TGFα | PE38 | Phase I | 20 | Progress Report: 3 of 15 patients, with residual disease at the time of therapy, demonstrated radiographic responses and one patient with a complete response that survived longer than 83 weeks | Sampson et al., 2003 |
| EGFR | TGFα | PE38 | Phase I | 20 | Case Study of long-term survivor. | Sampson et al., 2005 |
| EGFR | TGFα | PE-38 | Phase I | 20 | Final Results: MTD was not reached. Dose escalation was stopped at 100 ng/ml due to inconsistent drug delivery. | Sampson et al., 2008 |
| Transferrin-R | Transferrin | DT | Phase I | 18 | No symptomatic systemic toxicity occurred. MTD was defined as 0.67 µg/ml × 40ml. | Laske et al., 1997 |
| Transferrin-R | Transferrin | DT | Phase II | 31 | 35% of the patients displayed complete or partial responses, and only 38% of the patients had progressive disease after treatment. Toxicities associated with Tf-CRM107 were progressive cerebral edema and seizures | Weaver et al., 2003 |
Initially it was thought that binding sites for human IL-13 were exclusively localized in GBM cells and not present in the non-neoplastic brain 57. However, although IL-13Rα2 seems to indeed be confined to GBM cells 9, 21, 26, 58, several reports show that normal brain cells express IL4αR. Expression of physiological IL4αR chain was observed in human non-neoplastic astrocytes in vitro 58 and in vivo in reactive astrocytes surrounding human astrocytoma specimens 59. These findings suggest that the specificity of the hIL-13-PEQQR may be lower than hitherto anticipated. In fact, clinical trials in GBM patients showed that intracranial administration of hIL-13-PE38QQR led to dose limiting toxicities in some patients, including neurological symptoms secondary to necrotic and inflammatory processes as well as irreversible hemiparesis and the death of one patient due to neurologic decline possibly related to Cintredekin Besudotox 56. Grade III and IV imaging changes were observed in tumor infiltrated and normal brain parenchyma that were indicative of tissue damage 60. Brain tissue damage was regarded as a result of nonspecific internalization of hIL-13-PE38QQR by normal brain cells 56. In fact, we have recently demonstrated that a single injection of hIL-13-PE38QQR into the naïve mouse brain leads to acute neurological deterioration and severe neuropathological changes, even at low doses 50.
To optimize the targeting of the toxin to the GBM-associated IL-13Rα2, the IL-13 gene has been engineered to generate a mutant form of IL-13 (mhIL-13, IL-13.E13K) 61. It has been shown that IL13.E13K fused to PE (mhIL-13-PE) binds to GBM-associated IL13α2R with 50-fold higher affinity compared to native hIL-13 61, 62. Importantly, unlike its native counterpart, mhIL13-PE does not interact with the physiological IL13/IL4R 61, 62, hence, decreasing the capacity of the chimeric toxin to bind to normal cells. Although this second-generation cytotoxin exerts a stronger anti-tumor effect than first-generation hIL-13-PE in intracranial human GBM models, its administration into naïve mouse brain leads to dose-dependent neurotoxicity 50.
In a recent publication from our lab, we showed the development of a novel third-generation IL-13-based cytotoxin 50. We developed an adenoviral vector (Ad, Fig. 2) encoding mhIL13-PE to provide long-term high local expression of the targeted toxin, leading to an effective cytotoxic response in IL-13Rα2-expressing GBM cells without adverse side effects to surrounding normal brain tissue. The expression of mhIL-13 fused to PE toxin from an Ad vector allows direct and continued targeting of mhIL-13-PE to GBM cells in situ. We demonstrated that a single intratumoral injection of the therapeutic vector in intracranial human GBM xenografts and syngeneic GL26 tumors implanted in immune-competent mice leads to tumor regression and long-term survival in 50–70% of the animals (Fig. 3) 50. To further increase the safety of this therapeutic vector, we also encoded a mutated IL-4 (mIL4, IL-4.Y124D). Since mIL-4 is known to bind and block the IL13R/IL4R present in normal cells without interacting with IL-13Rα2 61, 62, we hypothesized that mIL4 would block any potential binding of the mhIL-13-PE to normal cells, without effecting the binding of the chimeric toxin to malignant cells. The expression of these transgenes is under the tight control of the regulatable bidirectional TRE promoter, allowing the inhibition of transgene expression by withdrawal of the inducer if adverse side effects were to occur. The TetON system consists of rtTA2S-M2 (TetON), which triggers expression of mhIL-13-PE and mIL-4 in the presence of Dox, and the Tet-repressor tTSkid, which inhibits transgene expression in the absence of Dox; both systems function to inhibit potential leakiness of this system 63, 64. This approach has several advantages over traditional protein formulations of IL-13 cytotoxins (Fig. 4): i) once synthesized by infected cells, mhIL13-PE is released 50 and exerts a powerful bystander effect, inducing apoptosis of GBM cells expressing the IL-13α2R located within the diffusion range of the toxin and amplifying the therapeutic efficiency of our approach; ii) this approach is highly specific and exhibits negligible toxicity towards normal brain tissue 50 since mhIL-13-PE specifically binds to GBM cells expressing IL-13Rα2, hence sparing normal brain cells. Additionally, the co-expression of mIL4 blocks any putative negligible binding of the toxin to normal cells.
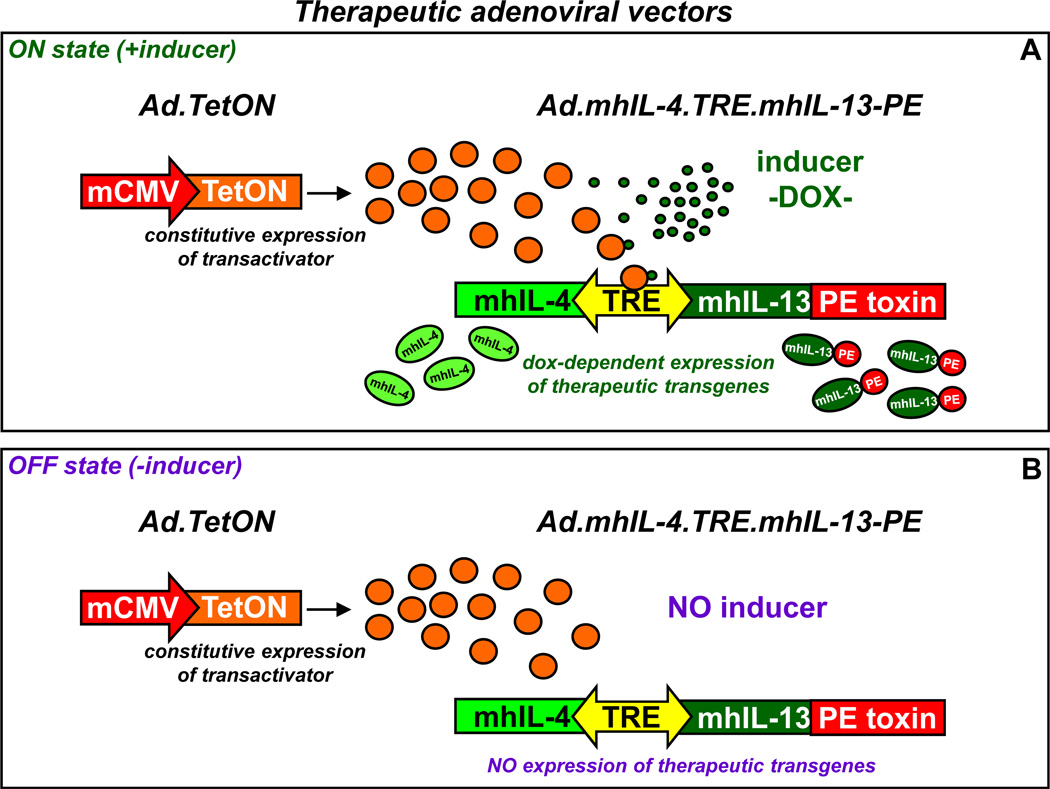
Figure 2. Regulated transgene expression from the therapeutic adenoviral vectors.
Ad-mediated regulated therapeutic cytotoxin expression. Ad.mhIL-4.TRE.mhIL-13-PE expresses mhIL-4 and mhIL-13-PE under the control of the bidirectional TRE promoter, which is activated by rtTA2sM2 in the presence of Dox (ON state, A). In the absence of the inducer (OFF state, B) the transactivator is unable to induce transgene expression.
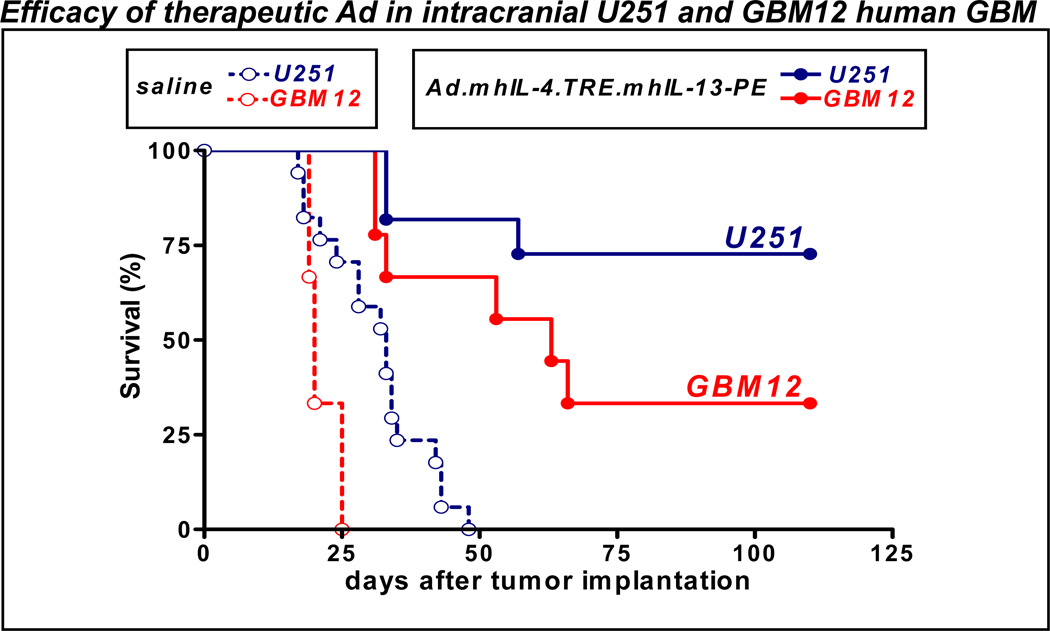
Figure 3. Intratumoral administration of Ad.mhIL-4.TRE.mhIL-13 mediates tumor regression and long term survival in orthotopic models of GBM.
Nude and Rag1−/− mice were implanted in the brain with human U251 glioma cells and human primary GBM12 cells, respectively. Five days later, they were treated with either a single intratumoral injection of control vector Ad.mhIL-4.TRE.mhIL-13 or saline. Animals were fed Dox-chow to activate transgene expression.
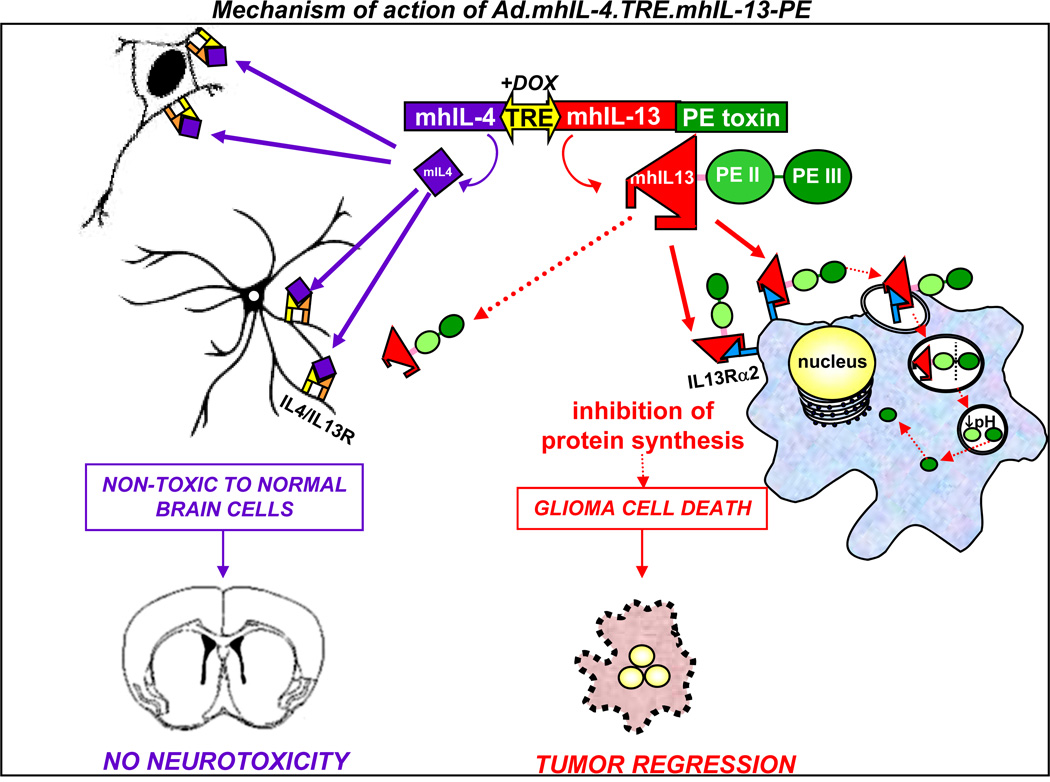
Figure 4. Gene therapy-mediated delivery of mhIL-13-PE leads to anti-tumor efficacy and long term survival in the absence of neurological toxicity.
The diagram depicts the mechanism of action of the targeted gene therapeutic strategy. The targeting of IL-13α2 receptor overexpressed in glioma cells has been approached by constructing an Ad vector encoding the truncated form of PE toxin fused to a mutated form of human IL-13 (mhIL-13), which has higher affinity for the glioma-associated IL-13α2 receptor and negligible binding to the physiological receptor, IL13/IL4R. Binding of mhIL-13-PE to IL13Rα2 promotes its internalization into glioma cells. Domain II (PEII) mediates the translocation of the toxin into the endosomes by endocytosis. Once in the endosomes, furin mediates proteolytic cleavage that activates the catalytic Domain III (PEIII). Due to the low pH of the endosome, the processed fragment of the toxin is translocated to the cytosol and inhibits protein synthesis, leading to glioma cell death. The absence of IL-13Rα2 protects normal cells from mhIL-13-PE mediated cell death. The safety of our approach has been further enhanced by co-expressing a mutated form of IL-4 (mhIL-4) encoded in the therapeutic bi-directional Ad which acts as an antagonist of the physiological receptor comprising the IL-13α1R and IL-4αR chains. Secreted IL-4 would inhibit the already negligible binding of mhIL-13-PE to normal brain cells, without affecting the binding of the mhIL-13-PE to GBM cells.
Previous reports aiming at controlling the expression of ADP-ribosylating toxins consisted of plasmids expressing native or attenuated Diphtheria toxin (DT) 65, 66. Plasmids expressing DT under the control of the first generation Tet-OFF system showed superior control of transgene expression in vitro when compared to lacR-IPTG system 65. While the first generation Tet system fails to completely inhibit transgene expression in the “OFF” state 67, the third generation Tet system used by us constitutes a non leaky inducible system ideal for delivering such toxic genes. Another approach to increase the specificity and reduce the toxicity of targeted toxins is to express them under the control of a cell-type specific` promoter. Native DT expressed under the control of a prostate specific antigen (PSA) promoter and delivered using an Ad vector in a mouse model of prostate cancer led to ~80% long term survival 68. However, transgene expression is much higher when driven by ubiquitous promoters than when using cell-type specific promoters 69, 70. The use of strong promoters elicits high transgene expression levels using low doses of Ads, which minimizes the risk of neurotoxicity due to high viral vector loads 71, 72. Considering that the specificity of our approach relies on the targeting of mIL-13-PE to IL-13Rα2, which is only expressed in GBM cells and absent in the normal brain, we expressed the components of the Tet-ON system under the control of the potent murine CMV promoter 50. We have previously demonstrated that this promoter elicits high levels of transduction in vitro in human GBM cells and in vivo in mouse and dog brain 63, 73, 74
Since mIL-13-PE kills GBM cells that express IL-13Rα2, treatment with Ad.mIL-4.TRE.mIL-13-PE could promote the selection of IL-13Rα2-negative GBM cells, which could lead to the development of a mIL-13-PE-resistant tumor. Thus, combination with other chemotherapeutic, proapoptotic agents or immunotherapeutic approaches could be attempted 75. Additionally, patients that do not display overexpression of IL-13Rα2 may benefit from PE toxin fused to ligands that bind other receptors that are overexpressed in GBM. For example, the receptor tyrosine kinase Eph2R 9, 15 is also overexpressed in human GBM specimens, but virtually absent in the non-neoplastic brain 9, 17. Targeting of this receptor has been attempted by fusing the ligand of Eph2R, ephrinA1, to PE38QQR, which exhibits a potent and specific cytotoxic effect against GBM cells 76.
Targeting the epidermal growth factor receptor (EGFR)
Two EGFR ligands, TGF-α and EGF, have been fused to toxic proteins and assessed both in pre-clinical animal models of GBM, and in human patients in Phase I clinical trials. In these studies, TGF-α was fused to the mutated variant of pseudomonas exotoxin PE-38 77, 78. Efficacy of TGF-α-PE-38 fusion protein (TP-38) was assessed in an intracranial brain tumor model consisting of epidermoid carcinoma A431 cells mixed with TP-38 and implanted into the caudate nuclei of athymic mice. Mice receiving tumor cells mixed with either 0.03 µg or 0.1 µg of the fusion protein displayed 100% and 90% survival compared to mice injected with cells alone, which exhibited a median survival of 19 days. Mice treated with higher doses of TP-38 died before the control mice, most likely due to toxicity of the drug 77. Toxicity of TP-38 was then assessed in both athymic rats and Rhesus macaques to determine the maximum tolerated dose (MTD). Naïve athymic rats were injected with 20 µL in the caudate nucleus with increasing doses of TP-38. Animals treated with high dose of TP-38 exhibited evidence of demyelination and necrosis and the MTD was determined to be 0.66 77. To assess toxicity studies in Rhesus macaques, 200 µL of TP-38 was infused into the brain. A neurological assessment was performed 7 days later followed by a histopathological analysis of the brain. Neurotoxicity was only observed at the highest dose tested (6 µg), and thus the MTD was determined to be 6 µg 77. Based on these data and extrapolation from brain mass, body mass, and body surface area, 1 µg was selected as the starting “low” dose for a human Phase I clinical trial 77.
A Phase I clinical trial of TP-38 was undertaken at Duke University and University of California San Francisco with the primary objective to determine the MTD of TP-38 in twenty adult human patients with recurrent brain tumors following infusion of TP-38 into the brain 77, 79–81. The dose escalation study commenced at a total dose of 1 µg, at a concentration of 25 ng/mL, and a rate of 0.4 mL/hour, for a total of 40 mL. Doses were escalated to 2 µg and 4 µg. The authors concluded that most toxicities encountered were solely neurological and most likely unrelated to TP-38, rather instead a consequence of infusion volume, recurrent tumor, or stereotactic catheter placement 79. The MTD of TP-38 was never reached in this study, however, two dose-limiting toxicities (DLT) were observed and were of neurologic nature 79. The dose escalation of TP-38 was stopped at 4 µg due to inconsistent drug delivery. The secondary objective of this study was to assess efficacy of TP-38. The median survival after TP-38 was 28 weeks. Two patients demonstrated radiographic responses, including one patient who demonstrated a nearly complete response and remained alive >83 weeks post-therapy. The sponsors of the study describe that the efficacy of this approach was limited due to ineffective transfusion of TP-38 in many patients; only 3 catheters produced intraparenchymal infusate distribution 79.
EGF has also been fused to the diphtheria toxin to target EGFR in brain tumors. Investigators used a His-Ala linker to fuse the catalytic and translocation domains of diphtheria toxin (DAB389) to human EGF with promising results obtained in vitro in a battery of brain tumor cell lines 7, 82. In vitro, efficacy of DAB389EGF was strongly correlated with EGFR density on the GBM target cell lines. One report of the efficacy and safety of DAB389EGF has been published to date. In this dose escalation study, athymic mice were injected (s.c.) with U87MG human glioma cells in the flank 10. When a large peripheral tumor mass developed, animals were treated with intratumoral injections of either 1, 3, 5, or 10 µg of DAB389EGF, given every other day for three to six doses. The MTD of DAB389EGF was demonstrated to be 3 µg given every other day. Animals receiving high doses exhibited clinical symptoms of weight loss, diminished activity, and dehydration. These clinical symptoms correlated with altered blood chemistry including abnormal blood urea nitrogen, creatinine, aspartate transaminase, and alanine transaminase. Histopathological analysis of the liver and kidney at the high doses revealed renal tubular necrosis 10. At the MTD, tumor regression was observed in all animals, while 25% of the animals (4 out of 16 mice) exhibited a tumor relapse within one month. Relapsed tumors were found to retain their EGF receptor, and responded to a second round of intratumoral DAB389EGF.
Another approach uses an antibody-toxin approach to target EGFR overexpressing tumor cells. One example of this approach consists of variable domains of the antagonistic monoclonal antibody 14E1, which is specific for human full-length EGFR, fused to a truncated form of the pseudomonas exotoxin A (ETA). Importantly, scFv(14E1)-ETA specifically recognizes wildtype human EGF receptor and its constituently active mutant EGFRvIII 83–85. Systemic delivery of scFv(14E1)-ETA in mice was found to completely suppress or reduce pulmonary metastases of intravenously injected renal carcinoma cells overexpressing EGFR or EGFRvIII 83. The cytotoxic effects of scFv(14E1)-ETA and TP-38 were tested on neuroblastoma cells, which displayed elevated levels of EGFR, both in chemosensitive and chemoresistant cell lines. Both scFv(14E1)-ETA and TP-38 resulted in decreased cell viability in cell lines that were insensitive to cetuximab or EGFR tyrosine kinase inhibitors 86 currently in use in clinical trials. Furthermore, it was demonstrated that cisplatin treatment resulted in an increase in EGFR expression in neuroblastoma cells and a concomitant increase in cytotoxicity of both scFv(14E1)-ETA and TP-38. Cisplatin-resistant cell lines also displayed an increased sensitivity to both targeted toxin approaches 86. These highly encouraging data demonstrate that the therapeutic efficacy of scFv(14E1)-ETA ought to be further explored in orthotopic models of neuroblastoma, and also glioblastoma multiforme.
Targeting the transferrin receptor (TfR)
The transferrin receptor (TfR) is another receptor that is overexpressed on GBM cells 32 and has been exploited for its use to specifically target toxins to GBM. To take advantage of TfR endocytic pathway, researchers have fused human transferrin to an mutated diphtheria toxin (CRM107), joined together by a stable, non-reducible thioether bond 87. The CRM107 diphtheria variant has a single point mutation in its subunit B that prevents the toxin from binding to the cell surface without effecting its cytotoxic effects, i.e., inhibiting protein synthesis 88. In vitro, Tf-CRM107 was 1,000 to 100,000 more toxic than the unconjugated CRM107 89. To assess efficacy and safety in vivo, subcutaneous tumors comprised of U251 cells in the flanks of nude mice were treated with escalating doses of Tf-CRM107. Each mouse was treated with one of three escalating doses every 2 days. The identical dose was delivered for a total of four times in each tumor bearing animal. Tumor regression was observed at all three doses 53. Toxicity studies revealed that when injected into the brain parenchyma, the dose of Tf-CRM107 required to elicit significant toxicity was 4–5 orders of magnitude greater than the therapeutic dose used to treat subcutaneous U251 tumors 87.
A dose escalation Phase I clinical trial was undertaken to assess the toxicity and determine the MTD of Tf-CRM107 in patients with progressive malignant brain tumors (primary or metastatic) who had previously failed conventional therapies 53, 90. In total 18 patients, 10 of which were diagnosed with glioblastoma multiforme, underwent treatment involving the sterotactic placement of multiple catheters followed by infusion of the drug starting at a rate of 0.5 µL/min, eventually increasing to a maximum rate of 4–10 µL/min. The concentrations of drug delivered started at 0.1 µg/mL and escalated until the MTD was achieved. Total infusion volumes of 20, 40, 60, 80, and 120 mL were tested, with the optimal infusion volume that could be safely administered defined at 40mL. Patients were re-treated with Tf-CRM107 at every 4–6 week intervals until there was evidence of tumor resolution by MRI, or until toxicities were noted. Treatment with Tf-CRM107 resulted in a ≥50% decrease in tumor volume in 9 out of 15 patients, and tumor response appeared to be dose dependent. Two patients showed complete response, one of which was tumor free for 23 months after a single infusion of Tf-CRM107. Intratumoral infusions were well tolerated with no treatment related deaths of life threatening toxicity. Localized toxicity, including peritumoral focal brain injury, was observed in patients receiving the higher doses (≥1.0 µg/mL), but there was no evidence of Tf-CRM107-induced tissue damage outside of the CNS. This Phase I clinical trial established the MTD at 0.67 µg/mL delivered in 40 mL.
Using this MTD, a Phase II clinical trial was then begun using CED infusion of Tf-CRM107 from two catheters. The primary objectives of this study were to evaluate the efficacy of Tf-CRM107 in patients with refractory or progressive GBM or anaplastic astrocytoma and to further evaluate the safety of Tf-CRM107. All patients received 0.67 µg/mL of Tf-CRM107 over a period of 4–5 days until the entire 40mL was delivered and then received additional treatments 4–10 weeks later. 44 patients at 9 study sites were treated, of which 31 of the patients received the second administration of Tf-CRM107. In total, 35% of the patients displayed complete or partial responses, and only 38% of the patients had progressive disease after treatment. Toxicities associated with Tf-CRM107 were progressive cerebral edema and seizures 90.
A recent publication described the development of the next generation of transferrin-diphtheria toxin ligand conjugate and the assessment of its therapeutic efficacy 91. Yoon et al. tested the hypothesis that sub-optimal efficacy of Tf-CRM107 was due to the rapid cycling of transferrin through the cell that resulting in a very limited time to deliver the diphtheria toxin. Given the physiological cycling of native human holo-Tf (iron loaded) to apo -Tf (devoid of Fe3+ ions), they speculated that up to 30 cycles of transferrin trafficking might be required for efficacious delivery of transferrin bound toxins into the cell. To overcome this limitation, Yoon et al. used mathematical modeling of Tf/TfR trafficking to identify that decreasing the Fe3+ release rate following receptor mediated endocytosis could induce the cell to traffic holo-Tf towards the cell surface, effectively “recycling” the transferrin-diphtheria ligand conjugate and increasing the probability of efficacious delivery of the toxin to the cytosol. Yoon et al. had previously developed recombinant mutant variants of Tf that exhibited reduced iron release kinetics 92. These mutant Tf-diphtheria toxin conjugates displayed higher efficiency of drug delivery compared to wildtype Tf in both HeLa cells 92 and also the GBM cell lines U87 and U251 91. When these mutant Tf-diphtheria toxin conjugates were used to treat nude mice bearing flank tumors U87 tumors overexpressing EGFRvIII, they exhibited increased tumor regression compared to animals treated with Tf-CRM107 91. These promising data demonstrate the renewed clinical promise of using transferrin to deliver toxins for the treatment of GBM.
Targeting of Eph2
The endogenous ligand for EphA2 receptor tyrosine kinase, ephrin A1, has been fused to PE38QQR for the treatment of GBM 9, 76. EphrinA1-PE38QQR exerts a potent cytotoxic effect on human GBM cells depending on their level of EphA2 expression 9. The cytotoxic effect of ephrinA1-PE38QQR appears specific for EphA2+ tumor cells since it is not toxic to normal human endothelial cells and EphA2− tumor cells 76.
Although EphA2 and IL-13Rα2 are overexpressed in a fraction of GBM patients 9, when combined they are expressed in virtually all patients with GBM 9. Thus, it has been proposed that a combinatorial approach using IL-13- and ephrin1-based cytotoxins would improve the outcome of patients with GBM 9, 76.
Targeting of uPAR
Tallying with the idea of targeting more than one receptor overexpressed in GBM to achieve therapeutic efficacy in as many GBM patients as possible, bispecific toxins have been developed that target uPAR and EGFR or IL-13Rα2 42, 93. These bispecific toxins improved efficacy and reduced toxicity than the monovalent toxins.
A bispecific toxin consisting of EGF and a urokinase fused to truncated pseudomonas exotoxin PE38 was generated to target EGFR in GBM cells, and uPAR in the tumor microvasculature 42. This toxin, EGFATFKDEL, has the advantage of being less immunogenic since immunodominant B-cell epitopes of the PE38 molecule were mutated 42. In preclinical studies, EGFATFKDEL showed superior efficacy when compared to toxins that target EGFR or uPAR alone 42.
A recombinant diptheria toxin-uPA fusion protein (DTAT) was developed that contains the translocation and catalytic portions of diphtheria toxin (responsible for cell entry and killing, respectively), fused to the non-internalizing amino terminal fragment portion of human plasminogen activator 93. The DTAT fusion protein targets malignant glioma cells and the endothelial cells of the neovasculature that express the urokinase-type plasminogen activator receptor (uPAR). In the same study, a second protein was engineered (DTAT13) that targets uPAR and IL13Rα2 expressing GBM cells. In preclinical models of GBM, DTAT13 exhibited similar efficacy as DTAT but with reduced toxicity 93. Authors proposed that the bi-specific DTAT13 might target a broader range of GBM patients and that the bi-specificity reduces the amount of toxin required for therapeutic efficacy than if the two agents were administered separately 93.
CONCLUSIONS
The use of targeted toxins for glioma therapeutics is the focus of intense research, related to both the development of novel improved cytotoxins, with enhanced efficacy and lower “off target” toxicities, the implementation of improved in vivo delivery systems including devices and biologicals (i.e., viral vectors, plasmids), and the discovery of novel glioma specific receptors. Several of these approaches have moved from preclinical GBM models to phase I, II and III clinical trials. The results from the preclinical models have been very encouraging 47–50; in spite of this, the clinical experience has not been as successful 94. For example, results from the PRECISE clinical trial have not reported a significant improvement in the median survival of GBM patients and several adverse events were observed 56. One of the strongest reasons which could explain the lack of more encouraging efficacy results from the PRECISE phase III trial could be the lack of consistency in the delivery of the immunotoxin Cintredekin Besudotox 55, 56, 94–96. In order to overcome the short half life of the protein Cintredekin Besudotox, clinical trials used convection enhanced delivery (CED) to administer Cintredekin Besudotox intracranially in GBM patients, who received 96h infusions through 1–3 catheters placed before and/or after tumor resection into the brain tumor mass or the brain parenchyma surrounding the tumor cavity, respectively 95. Although CED produces widespread drug dissemination in some patients, this technique is complex and leads to varied, discrepant results when comparing different centers 95, 96. The anatomy of the target area as well as the accuracy of catheter positioning had strong influence on the distribution of the protein, resulting in heterogeneous and limited distribution of the drug 95, 96. In fact, the efficacy of Cintredekin Besudotox in GBM patients was strongly dependent on the accuracy of catheter placement 55, 56. In addition, preliminary information from a recent Phase III clinical trial indicated that CED of Cintredekin Besudotox did not significantly improve the median survival of GBM patients when compared to patients treated with Gliadel wafers 97. In summary, although this is a very exciting field which could lead to novel improved treatments for GBM patients, substantial efforts should be devoted to improving the delivery approaches utilized in order to enable distribution of the immuno-toxins safely and accurately into the tumor/resection cavity. Also, delivery of targeted immuno-toxins using viral vectors would benefit enormously from improved devices for local delivery.
ACKNOWLEDGEMENTS
This work is supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grant 1R21-NSO54143; 1UO1 NS052465.01. The brain tumor program in our Institute is funded by NIH/NINDS Grants 1RO1-NS 057711; to M.G.C.; NIH/NINDS Grants 1 RO1 NS 054193; RO1 NS 42893 to P.R.L. The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics to PRL and MGC, respectively, The Drown Foundation; The Linda Tallen & David Paul Kane Foundation Annual Fellowship and the Board of Governors at CSMC. M.C was supported by NIH/NINDS 1F32 NS058156.01
REFERENCES
- 1.Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE, McNiel EA, Ohlfest JR, Freese AB, Moore PF, Lerner J, Lowenstein PR, Castro MG. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85(2):133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16(6):1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 3.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6(5):440–449. [PMC free article] [PubMed] [Google Scholar]
- 4.Debinski W, Obiri NI, Pastan I, Puri RK. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995;270(28):16775–16780. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- 5.Debinski W, Thompson JP. Retargeting interleukin 13 for radioimmunodetection and radioimmunotherapy of human high-grade gliomas. Clin Cancer Res. 1999;5(10 Suppl):3143s–3147s. [PubMed] [Google Scholar]
- 6.Joshi BH, Leland P, Asher A, Prayson RA, Varricchio F, Puri RK. In situ expression of interleukin-4 (IL-4) receptors in human brain tumors and cytotoxicity of a recombinant IL-4 cytotoxin in primary glioblastoma cell cultures. Cancer Res. 2001;61(22):8058–8061. [PubMed] [Google Scholar]
- 7.Liu TF, Willingham MC, Tatter SB, Cohen KA, Lowe AC, Thorburn A, Frankel AE. Diphtheria toxin-epidermal growth factor fusion protein and Pseudomonas exotoxin-interleukin 13 fusion protein exert synergistic toxicity against human glioblastoma multiforme cells. Bioconjug Chem. 2003;14(6):1107–1114. doi: 10.1021/bc034111+. [DOI] [PubMed] [Google Scholar]
- 8.Puri RK, Leland P, Kreitman RJ, Pastan I. Human neurological cancer cells express interleukin-4 (IL-4) receptors which are targets for the toxic effects of IL4-Pseudomonas exotoxin chimeric protein. Int J Cancer. 1994;58(4):574–581. doi: 10.1002/ijc.2910580421. [DOI] [PubMed] [Google Scholar]
- 9.Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14(1):199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 10.Liu TF, Hall PD, Cohen KA, Willingham MC, Cai J, Thorburn A, Frankel AE. Interstitial diphtheria toxin-epidermal growth factor fusion protein therapy produces regressions of subcutaneous human glioblastoma multiforme tumors in athymic nude mice. Clin Cancer Res. 2005;11(1):329–334. [PubMed] [Google Scholar]
- 11.Liu TF, Tatter SB, Willingham MC, Yang M, Hu JJ, Frankel AE. Growth factor receptor expression varies among high-grade gliomas and normal brain: epidermal growth factor receptor has excellent properties for interstitial fusion protein therapy. Mol Cancer Ther. 2003;2(8):783–787. [PubMed] [Google Scholar]
- 12.Phillips PC, Levow C, Catterall M, Colvin OM, Pastan I, Brem H. Transforming growth factor-alpha-Pseudomonas exotoxin fusion protein (TGF-alpha-PE38) treatment of subcutaneous and intracranial human glioma and medulloblastoma xenografts in athymic mice. Cancer Res. 1994;54(4):1008–1015. [PubMed] [Google Scholar]
- 13.Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 12(9):675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzoleni S, Politi LS, Pala M, Cominelli M, Franzin A, Sergi Sergi L, Falini A, De Palma M, Bulfone A, Poliani PL, Galli R. Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer Res. 70(19):7500–7513. doi: 10.1158/0008-5472.CAN-10-2353. [DOI] [PubMed] [Google Scholar]
- 15.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 16.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6(12):1795–1806. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori T, Abe T, Wakabayashi Y, Hikawa T, Matsuo K, Yamada Y, Kuwano M, Hori S. Up-regulation of urokinase-type plasminogen activator and its receptor correlates with enhanced invasion activity of human glioma cells mediated by transforming growth factor-alpha or basic fibroblast growth factor. J Neurooncol. 2000;46(2):115–123. doi: 10.1023/a:1006339717748. [DOI] [PubMed] [Google Scholar]
- 19.Hall WA, Godal A, Juell S, Fodstad O. In vitro efficacy of transferrin-toxin conjugates against glioblastoma multiforme. J Neurosurg. 1992;76(5):838–844. doi: 10.3171/jns.1992.76.5.0838. [DOI] [PubMed] [Google Scholar]
- 20.Martell LA, Agrawal A, Ross DA, Muraszko KM. Efficacy of transferrin receptor-targeted immunotoxins in brain tumor cell lines and pediatric brain tumors. Cancer Res. 1993;53(6):1348–1353. [PubMed] [Google Scholar]
- 21.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67(17):7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 22.Obiri NI, Leland P, Murata T, Debinski W, Puri RK. The IL-13 receptor structure differs on various cell types and may share more than one component with IL-4 receptor. J Immunol. 1997;158(2):756–764. [PubMed] [Google Scholar]
- 23.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111(4):677–690. doi: 10.1067/mai.2003.1333. quiz 691. [DOI] [PubMed] [Google Scholar]
- 24.Debinski W, Miner R, Leland P, Obiri NI, Puri RK. Receptor for interleukin (IL) 13 does not interact with IL4 but receptor for IL4 interacts with IL13 on human glioma cells. J Biol Chem. 1996;271(37):22428–22433. doi: 10.1074/jbc.271.37.22428. [DOI] [PubMed] [Google Scholar]
- 25.Debinski W, Slagle B, Gibo DM, Powers SK, Gillespie GY. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J Neurooncol. 2000;48(2):103–111. doi: 10.1023/a:1006446426611. [DOI] [PubMed] [Google Scholar]
- 26.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5(5):985–990. [PubMed] [Google Scholar]
- 27.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4(5):388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51(8):2164–2172. [PubMed] [Google Scholar]
- 29.Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11(4):1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 30.Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- 31.Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009;16(6):748–754. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Recht L, Torres CO, Smith TW, Raso V, Griffin TW. Transferrin receptor in normal and neoplastic brain tissue: implications for brain-tumor immunotherapy. J Neurosurg. 1990;72(6):941–945. doi: 10.3171/jns.1990.72.6.0941. [DOI] [PubMed] [Google Scholar]
- 33.Calzolari A, Larocca LM, Deaglio S, Finisguerra V, Boe A, Raggi C, Ricci-Vitani L, Pierconti F, Malavasi F, De Maria R, Testa U, Pallini R. Transferrin receptor 2 is frequently and highly expressed in glioblastomas. Transl Oncol. 3(2):123–134. doi: 10.1593/tlo.09274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill JM, Ruff MR, Weber RJ, Pert CB. Transferrin receptors in rat brain: neuropeptide-like pattern and relationship to iron distribution. Proc Natl Acad Sci U S A. 1985;82(13):4553–4557. doi: 10.1073/pnas.82.13.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connor JR, Fine RE. The distribution of transferrin immunoreactivity in the rat central nervous system. Brain Res. 1986;368(2):319–328. doi: 10.1016/0006-8993(86)90576-7. [DOI] [PubMed] [Google Scholar]
- 36.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 37.Lin LN, Mason AB, Woodworth RC, Brandts JF. Calorimetric studies of the binding of ferric ions to human serum transferrin. Biochemistry. 1993;32(36):9398–9406. doi: 10.1021/bi00087a019. [DOI] [PubMed] [Google Scholar]
- 38.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3(7):489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Fei Z, Bu X, Zhen H, Zhang Z, Gu J, Chen Y. Expression and significance of urokinase type plasminogen activator gene in human brain gliomas. J Surg Oncol. 2000;74(2):90–94. doi: 10.1002/1096-9098(200006)74:2<90::AID-JSO2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa Y, Kinoh H, Iwadate Y, Onimaru M, Ueda Y, Harada Y, Saito S, Furuya A, Saegusa T, Morodomi Y, Hasegawa M, Saito S, Aoki I, Saeki N, Yonemitsu Y. Urokinase-targeted fusion by oncolytic Sendai virus eradicates orthotopic glioblastomas by pronounced synergy with interferon-beta gene. Mol Ther. 18(10):1778–1786. doi: 10.1038/mt.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prager GW, Breuss JM, Steurer S, Mihaly J, Binder BR. Vascular endothelial growth factor (VEGF) induces rapid prourokinase (pro-uPA) activation on the surface of endothelial cells. Blood. 2004;103(3):955–962. doi: 10.1182/blood-2003-07-2214. [DOI] [PubMed] [Google Scholar]
- 42.Tsai AK, Oh S, Chen H, Shu Y, Ohlfest JR, Vallera DA. A novel bispecific ligand-directed toxin designed to simultaneously target EGFR on human glioblastoma cells and uPAR on tumor neovasculature. J Neurooncol. doi: 10.1007/s11060-010-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Hall WA, Jin N, Todhunter DA, Panoskaltsis-Mortari A, Vallera DA. Targeting glioblastoma multiforme with an IL-13/diphtheria toxin fusion protein in vitro and in vivo in nude mice. Protein Eng. 2002;15(5):419–427. doi: 10.1093/protein/15.5.419. [DOI] [PubMed] [Google Scholar]
- 44.Todhunter DA, Hall WA, Rustamzadeh E, Shu Y, Doumbia SO, Vallera DA. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng Des Sel. 2004;17(2):157–164. doi: 10.1093/protein/gzh023. [DOI] [PubMed] [Google Scholar]
- 45.Pastan I, Chaudhary V, FitzGerald DJ. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- 46.Debinski W, Pastan I. An immunotoxin with increased activity and homogeneity produced by reducing the number of lysine residues in recombinant Pseudomonas exotoxin. Bioconjug Chem. 1994;5(1):40–46. doi: 10.1021/bc00025a006. [DOI] [PubMed] [Google Scholar]
- 47.Puri RK, Leland P, Obiri NI, Husain SR, Kreitman RJ, Haas GP, Pastan I, Debinski W. Targeting of interleukin-13 receptor on human renal cell carcinoma cells by a recombinant chimeric protein composed of interleukin-13 and a truncated form of Pseudomonas exotoxin A (PE38QQR) Blood. 1996;87(10):4333–4339. [PubMed] [Google Scholar]
- 48.Husain SR, Joshi BH, Puri RK. Interleukin-13 receptor as a unique target for anti-glioblastoma therapy. Int J Cancer. 2001;92(2):168–175. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1182>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 49.Husain SR, Obiri NI, Gill P, Zheng T, Pastan I, Debinski W, Puri RK. Receptor for interleukin 13 on AIDS-associated Kaposi's sarcoma cells serves as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin Cancer Res. 1997;3(2):151–156. [PubMed] [Google Scholar]
- 50.Candolfi M, Xiong W, Yagiz K, Liu C, Muhammad AK, Puntel M, Foulad D, Zadmehr A, Ahlzadeh GE, Kroeger KM, Tesarfreund M, Lee S, Debinski W, Sareen D, Svendsen CN, Rodriguez R, Lowenstein PR, Castro MG. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1008261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawakami K, Kawakami M, Kioi M, Husain SR, Puri RK. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J Neurosurg. 2004;101(6):1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- 52.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1(11):1253–1258. [PubMed] [Google Scholar]
- 53.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3(12):1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 54.Mintz A, Gibo DM, Madhankumar AB, Debinski W. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor alpha2-expressing glioma. J Neurooncol. 2003;64(1–2):117–123. doi: 10.1007/BF02700026. [DOI] [PubMed] [Google Scholar]
- 55.Sampson JH, Archer G, Pedain C, Wembacher-Schroder E, Westphal M, Kunwar S, Vogelbaum MA, Coan A, Herndon JE, Raghavan R, Brady ML, Reardon DA, Friedman AH, Friedman HS, Rodriguez-Ponce MI, Chang SM, Mittermeyer S, Croteau D, Puri RK. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 113(2):301–309. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 56.Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, Sampson JH, Ram Z, Gutin PH, Gibbons RD, Aldape KD, Croteau DJ, Sherman JW, Puri RK. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami M, Kawakami K, Takahashi S, Abe M, Puri RK. Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer. 2004;101(5):1036–1042. doi: 10.1002/cncr.20470. [DOI] [PubMed] [Google Scholar]
- 58.Liu H, Jacobs BS, Liu J, Prayson RA, Estes ML, Barnett GH, Barna BP. Interleukin-13 sensitivity and receptor phenotypes of human glial cell lines: non-neoplastic glia and low-grade astrocytoma differ from malignant glioma. Cancer Immunol Immunother. 2000;49(6):319–324. doi: 10.1007/s002620000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H, Prayson RA, Estes ML, Drazba JA, Barnett GH, Bingaman W, Liu J, Jacobs BS, Barna BP. In vivo expression of the interleukin 4 receptor alpha by astrocytes in epilepsy cerebral cortex. Cytokine. 2000;12(11):1656–1661. doi: 10.1006/cyto.2000.0773. [DOI] [PubMed] [Google Scholar]
- 60.Parney IF, Kunwar S, McDermott M, Berger M, Prados M, Cha S, Croteau D, Puri RK, Chang SM. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg. 2005;102(2):267–275. doi: 10.3171/jns.2005.102.2.0267. [DOI] [PubMed] [Google Scholar]
- 61.Debinski W, Gibo DM, Obiri NI, Kealiher A, Puri RK. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16(5):449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 62.Madhankumar AB, Mintz A, Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia. 2004;6(1):15–22. doi: 10.1016/s1476-5586(04)80049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiong W, Candolfi M, Kroeger KM, Puntel M, Mondkar S, Larocque D, Liu C, Curtin JF, Palmer D, Ng P, Lowenstein PR, Castro MG. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol Ther. 2008;16(2):343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong W, Goverdhana S, Sciascia SA, Candolfi M, Zirger JM, Barcia C, Curtin JF, King GD, Jaita G, Liu C, Kroeger K, Agadjanian H, Medina-Kauwe L, Palmer D, Ng P, Lowenstein PR, Castro MG. Regulatable Gutless Adenovirus Vectors Sustain Inducible Transgene Expression in the Brain in the Presence of an Immune Response against Adenoviruses. J Virol. 2006;80(1):27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paulus W, Baur I, Oberer DM, Breakefield XO, Reeves SA. Regulated expression of the diphtheria toxin A gene in human glioma cells using prokaryotic transcriptional control elements. J Neurosurg. 1997;87(1):89–95. doi: 10.3171/jns.1997.87.1.0089. [DOI] [PubMed] [Google Scholar]
- 66.Keyvani K, Baur I, Paulus W. Tetracycline-controlled expression but not toxicity of an attenuated diphtheria toxin mutant. Life Sci. 1999;64(19):1719–1724. doi: 10.1016/s0024-3205(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 67.Curtin JF, Candolfi M, Xiong W, Lowenstein PR, Castro MG. Turning the gene tap off; implications of regulating gene expression for cancer therapeutics. Mol Cancer Ther. 2008;7(3):439–448. doi: 10.1158/1535-7163.MCT-07-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, McCadden J, Ferrer F, Kruszewski M, Carducci M, Simons J, Rodriguez R. Prostate-specific expression of the diphtheria toxin A chain (DT-A): studies of inducibility and specificity of expression of prostate-specific antigen promoter-driven DT-A adenoviral-mediated gene transfer. Cancer Res. 2002;62(9):2576–2582. [PubMed] [Google Scholar]
- 69.Morelli AE, Larregina AT, Smith-Arica J, Dewey RA, Southgate TD, Ambar B, Fontana A, Castro MG, Lowenstein PR. Neuronal and glial cell type-specific promoters within adenovirus recombinants restrict the expression of the apoptosis-inducing molecule Fas ligand to predetermined brain cell types, and abolish peripheral liver toxicity. J Gen Virol. 1999;80(Pt 3):571–583. doi: 10.1099/0022-1317-80-3-571. [DOI] [PubMed] [Google Scholar]
- 70.Williams JC, Stone D, Smith-Arica JR, Morris ID, Lowenstein PR, Castro MG. Regulated, adenovirus-mediated delivery of tyrosine hydroxylase suppresses growth of estrogen-induced pituitary prolactinomas. Mol Ther. 2001;4(6):593–602. doi: 10.1006/mthe.2001.0499. [DOI] [PubMed] [Google Scholar]
- 71.Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ, Southgate TD, Klatzmann D, Lassmann H, Castro MG, Lowenstein PR. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5(11):1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 72.Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001;3(1):36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- 73.Candolfi M, Curtin JF, Xiong WD, Kroeger KM, Liu C, Rentsendorj A, Agadjanian H, Medina-Kauwe L, Palmer D, Ng P, Lowenstein PR, Castro MG. Effective High-Capacity Gutless Adenoviral Vectors Mediate Transgene Expression in Human Glioma Cells. Mol Ther. 2006;14(3):371–381. doi: 10.1016/j.ymthe.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Candolfi M, Pluhar GE, Kroeger K, Puntel M, Curtin J, Barcia C, Muhammad AK, Xiong W, Liu C, Mondkar S, Kuoy W, Kang T, McNeil EA, Freese AB, Ohlfest JR, Moore P, Palmer D, Ng P, Young JD, Lowenstein PR, Castro MG. Optimization of adenoviral vector-mediated transgene expression in the canine brain in vivo, and in canine glioma cells in vitro. Neuro Oncol. 2007;9(3):245–258. doi: 10.1215/15228517-2007-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Candolfi M, Kroeger KM, Muhammad AK, Yagiz K, Farrokhi C, Pechnick RN, Lowenstein PR, Castro MG. Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety. Curr Gene Ther. 2009;9(5):409–421. doi: 10.2174/156652309789753301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6(12 Pt 1):3208–3218. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- 77.Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, Friedman HS, Herndon JE, 2nd, Kunwar S, Marcus S, McLendon RE, Paolino A, Penne K, Provenzale J, Quinn J, Reardon DA, Rich J, Stenzel T, Tourt-Uhlig S, Wikstrand C, Wong T, Williams R, Yuan F, Zalutsky MR, Pastan I. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65(1):27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 78.Karpel-Massler G, Schmidt U, Unterberg A, Halatsch ME. Therapeutic inhibition of the epidermal growth factor receptor in high-grade gliomas: where do we stand? Mol Cancer Res. 2009;7(7):1000–1112. doi: 10.1158/1541-7786.MCR-08-0479. [DOI] [PubMed] [Google Scholar]
- 79.Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, Friedman HS, Greer K, Herndon JE, 2nd, Kunwar S, McLendon RE, Paolino A, Petry NA, Provenzale JM, Reardon DA, Wong TZ, Zalutsky MR, Pastan I, Bigner DD. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20(5):267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sampson JH, Reardon DA, Friedman AH, Friedman HS, Coleman RE, McLendon RE, Pastan I, Bigner DD. Sustained radiographic and clinical response in patient with bifrontal recurrent glioblastoma multiforme with intracerebral infusion of the recombinant targeted toxin TP-38: case study. Neuro Oncol. 2005;7(1):90–96. doi: 10.1215/S1152851703000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu TF, Cohen KA, Willingham MC, Tatter SB, Puri RK, Frankel AE. Combination fusion protein therapy of refractory brain tumors: demonstration of efficacy in cell culture. J Neurooncol. 2003;65(1):77–85. doi: 10.1023/a:1026286214901. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt M, Maurer-Gebhard M, Groner B, Kohler G, Brochmann-Santos G, Wels W. Suppression of metastasis formation by a recombinant single chain antibody-toxin targeted to full-length and oncogenic variant EGF receptors. Oncogene. 1999;18(9):1711–1721. doi: 10.1038/sj.onc.1202489. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt M, Reiser P, Hills D, Gullick WJ, Wels W. Expression of an oncogenic mutant EGF receptor markedly increases the sensitivity of cells to an EGF-receptor-specific antibody-toxin. Int J Cancer. 1998;75(6):878–884. doi: 10.1002/(sici)1097-0215(19980316)75:6<878::aid-ijc10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt M, Vakalopoulou E, Schneider DW, Wels W. Construction and functional characterization of scFv(14E1)-ETA - a novel, highly potent antibody-toxin specific for the EGF receptor. Br J Cancer. 1997;75(11):1575–1584. doi: 10.1038/bjc.1997.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michaelis M, Bliss J, Arnold SC, Hinsch N, Rothweiler F, Deubzer HE, Witt O, Langer K, Doerr HW, Wels WS, Cinatl J., Jr. Cisplatin-resistant neuroblastoma cells express enhanced levels of epidermal growth factor receptor (EGFR) and are sensitive to treatment with EGFR-specific toxins. Clin Cancer Res. 2008;14(20):6531–6537. doi: 10.1158/1078-0432.CCR-08-0821. [DOI] [PubMed] [Google Scholar]
- 87.Johnson VG, Wrobel C, Wilson D, Zovickian J, Greenfield L, Oldfield EH, Youle R. Improved tumor-specific immunotoxins in the treatment of CNS and leptomeningeal neoplasia. J Neurosurg. 1989;70(2):240–248. doi: 10.3171/jns.1989.70.2.0240. [DOI] [PubMed] [Google Scholar]
- 88.Greenfield L, Johnson VG, Youle RJ. Mutations in diphtheria toxin separate binding from entry and amplify immunotoxin selectivity. Science. 1987;238(4826):536–539. doi: 10.1126/science.3498987. [DOI] [PubMed] [Google Scholar]
- 89.Johnson VG, Wilson D, Greenfield L, Youle RJ. The role of the diphtheria toxin receptor in cytosol translocation. J Biol Chem. 1988;263(3):1295–1300. [PubMed] [Google Scholar]
- 90.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65(1):3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 91.Yoon DJ, Kwan BH, Chao FC, Nicolaides TP, Phillips JJ, Lam GY, Mason AB, Weiss WA, Kamei DT. Intratumoral therapy of glioblastoma multiforme using genetically engineered transferrin for drug delivery. Cancer Res. 2010;70(11):4520–4527. doi: 10.1158/0008-5472.CAN-09-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoon DJ, Chu DS, Ng CW, Pham EA, Mason AB, Hudson DM, Smith VC, MacGillivray RT, Kamei DT. Genetically engineering transferrin to improve its in vitro ability to deliver cytotoxins. J Control Release. 2009;133(3):178–184. doi: 10.1016/j.jconrel.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hall WA, Vallera DA. Efficacy of antiangiogenic targeted toxins against glioblastoma multiforme. Neurosurg Focus. 2006;20(4):E23. doi: 10.3171/foc.2006.20.4.15. [DOI] [PubMed] [Google Scholar]
- 94.Japsen B. NeoPharm drug fails test: cancer therapy bust sends stock into dive. Chicago Tribune. 2006 Sect. Business 1. [Google Scholar]
- 95.Sampson JH, Brady ML, Petry NA, Croteau D, Friedman AH, Friedman HS, Wong T, Bigner DD, Pastan I, Puri RK, Pedain C. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. Neurosurgery. 2007;60(2 Suppl 1) doi: 10.1227/01.NEU.0000249256.09289.5F. ONS89–98; discussion ONS98–9. [DOI] [PubMed] [Google Scholar]
- 96.Sampson JH, Raghavan R, Provenzale JM, Croteau D, Reardon DA, Coleman RE, Rodriguez Ponce I, Pastan I, Puri RK, Pedain C. Induction of hyperintense signal on T2-weighted MR images correlates with infusion distribution from intracerebral convection-enhanced delivery of a tumor-targeted cytotoxin. AJR Am J Roentgenol. 2007;188(3):703–709. doi: 10.2214/AJR.06.0428. [DOI] [PubMed] [Google Scholar]
- 97.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, Croteau D, Pedain C, Leland P, Husain SR, Joshi BH, Puri RK. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]