Abstract
Objective. The aim of this study was to compare retinal nerve fiber layer thickness (RNFLT) between spectral-domain (SD-) and time-domain optical coherence tomography (TD-OCT) in MS patients and healthy controls (HC). Furthermore, RNFLT between MS eyes with and without optic neuritis (ON) and HC should be explored. Finally, the relationship between RNFLT, disease duration, EDSS, and disease modifying therapy (DMT) should be established. Design. Prospective, cross-sectional study. Participants. 28 MS patients and 35 HC. Methods. Both groups underwent TD- and SD-OCT measurements. RFNLT was correlated between the two machines and between MS eyes with and without ON and HC. Furthermore, RNFLT was correlated to disease duration, EDSS and DMT. Results. A strong correlation (Pearson's r = 0.921, P < 0.001), but a statistically significant difference of 2 μm (P < 0.001), was found between the two devices. RNFLT was significantly different between MS eyes with history of ON (mean RFNLT (SD) 72.21 μm (15.83 μm)), MS eyes without history of ON 93.03 μm (14.25 μm), and HC 99.07 μm (7.23 μm) (P < 0.001). Conclusions. The measurements between different generation of OCT machines are not interchangeable, which should be taken into account if comparing results between different machines and switching OCT machine in longitudinal studies.
1. Introduction
Optical coherence tomography (OCT) is a noninvasive technique for high-resolution, cross-sectional tomographic imaging of retinal tissue using backscattered light. OCT imaging is very similar to ultrasound B-Scan imaging but uses infrared-light instead of ultrasound waves. Two-dimensional, cross-sectional images are obtained from multiple axial scans (A-Scans) at different transverse locations [1].
Until recently, third-generation time-domain OCT (TD-OCT) using Stratus OCT (Carl Zeiss Meditec AG, Jena, Germany) has been widely used to acquire images at a rate of 400 axial scans per second with an axial resolution of 10 μm [2]. The recently introduced fourth-generation spectral-domain OCT (SD-OCT) has improved depth resolution by a factor of three (axial resolution up to 3.8 μm) and allows a significantly higher acquisition speed (40'000 axial scans per second) resulting in improved image quality and minimized motion artefacts [3]. Furthermore, software improvements allow reconstruction of a three-dimensional image of the retina.
Heidelberg Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) uses an integrated eye tracking system (IETS) to compensate for eye movement artefact during data acquisition. IETS also allows an automatic re-centration, which can be used for more reliable follow-up scans. Heidelberg noise reduction technology helps producing significantly improved images by adjusting data and reducing noise using mean values from several scans [4]. Heidelberg Spectralis OCT needs to be validated for accuracy, reproducibility, and comparability to previous models before it can be reliably used for clinical and research purposes.
Recent studies have shown differences between SD-OCT machines (Cirrus SD-OCT, Carl Zeiss Meditec AG, Jena, Germany [5–10], RTVue-100, Optovue Inc., Fremont, CA, USA [11], and Spectralis, Heidelberg Engineering, Heidelberg, Germany, [12, 13]) and TD-OCT in healthy controls and glaucoma patients. These studies showed better reproducibility compared to TD-OCT and significant differences in RNFLT measurements between the two generations of machines.
So far only few studies have examined the role of SD-OCT in multiple sclerosis (MS) patients [14, 15]. Therefore, our specific study aims are (1) to compare retinal nerve fiber layer thickness (RNFLT) measurements between the validated third-generation Stratus OCT and the new fourth-generation Heidelberg Spectralis OCT in MS patients and healthy controls, (2) to compare RNFLT between MS eyes affected by optic neuritis (ON eyes) to eyes without a history of ON (NON eyes) and control eyes, and finally (3) to determine the relationship between RNFLT, disease duration, expanded disability status scale (EDSS), and disease-modifying therapy (DMT) in MS patients and refraction in both groups.
2. Materials and Methods
2.1. Study Design and Patient Population
In a prospective, cross-sectional study, subjects with MS and controls were identified from the UBC Hospital MS Clinic with the aid of advertisement and pamphlets. All MS subjects had confirmed diagnosis of MS made by a neurologist with specific experience in managing MS patients, and based on the modified McDonald criteria [16].
2.2. Inclusion and Exclusion Criteria
Patients with a recent history of optic neuritis (ON) (<6 months), history of ocular diseases (age-related macular degeneration, diabetic retinopathy, uveitis, and glaucoma), and history of other diseases that could mimic MS or affect OCT testing (neuromyelitis optica, parkinson's disease, and Alzheimer disease) and subjects with difficulties maintaining fixation were not included.
2.3. Outcome Measures
2.3.1. Clinical Data
Clinical history information such as disease duration from time of disease onset, previous history of optic neuritis, and other neurological information like EDSS score was obtained by history and from hospital charts after patient recruitment. Myopia was defined as spherical equivalent of <−0.50 diopters, emmetropia between −0.5 and +0.5 diopters, and hyperopia as >+0.5 diopters measured by SD-OCT.
2.3.2. Optical Coherence Tomography
OCT was performed in a random order by an experienced person that was masked to clinical data, using TD-OCT and SD-OCT within one-hour period with no pupil dilation (half of subjects had TD-OCT prior to SD-OCT and vice versa).
TD-OCT (Stratus OCT 3000, Software Version 4.0.7; Carl Zeiss Meditec, Jena, Germany): the standard Fast RNFL acquisition protocol was used. Three scans, each composed of 256 A scans, were automatically acquired consecutively using a circle scan with a standardized diameter of 3.4 mm by the same experienced operator. Several scans were taken and the best-centered scan with a quality score of ≥6 was chosen for analysis (as suggested by the manufacturer). An automated computer algorithm delineated the anterior and posterior margins of the RNFL.
SD-OCT (Heidelberg Spectralis OCT, Software Version 5.1.2, Heidelberg Engineering, Heidelberg, Germany): The RNFL protocol in high-resolution mode (axial resolution 3.8 μm, 19'000 scans per second) was used. Sixteen consecutive circular B-scans (each composed of 1536 A scans) with a diameter of 3.4 mm were automatically averaged to reduce speckle noise. The online tracking system compensated for eye movements. Several scans were taken by the same experienced operator and the best centered with a quality of at least 24 (which is about the equivalent of 6 in Stratus OCT) was chosen for analysis. The included software algorithm delineated the anterior and posterior margins of the RNFL.
2.4. Statistical Analysis
Microsoft Office 2007 and SPSS Version 16.0 for Windows were used to do statistical analysis. Descriptive, mean comparison (t-test and one-way ANOVA) and correlation analysis (Pearson's) were used to compare OCT measures between different groups: SD-OCT versus TD-OCT RNFLT measurements; MS eyes versus control eyes; myopic versus emmetropic and hyperopic eyes. P values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Patient Demographics and Clinical Characteristics
The study recruitment took place between August 2009 and February 2010. Twenty-eight MS patients (age mean: 38.88 yrs; SD: 11.65 yrs, mean disease duration: 83.12 months, SD: 83.76; 25 with relapsing-remitting and 3 with secondary progressive MS; EDSS range between 1.5 and 6.5, mean: 2.8, SD: 1.6) and 35 healthy controls (age mean: 43.46 yrs; SD: 9.08 yrs) participated in this study (Table 1). Fourteen (out of 27) patients used DMT for MS. Sixteen (out of 27) patients had an EDSS score of less than 3.0. All subjects were examined by SD-OCT and TD-OCT machines. Two subjects were excluded in the control group due to (1) software failure to delineate RNFL correctly and (2) OCT artefacts due to high myopia. One patient in the MS group was excluded due to inability to measure exact refraction after refractive surgery. Out of the remaining 120 eyes 32 eyes were myopic (refraction range between −8.25 and −0.75 diopters), 74 eyes were emmetropic (refraction = 0 diopters), and 14 eyes were hyperopic (refraction range between +1 and +6 diopters). Fourteen (out of 54) MS eyes were previously affected by a single optic neuritis event.
Table 1.
Descriptive statistics of MS and control group.
| Control group | MS group | |
|---|---|---|
| N | 35 | 28 |
| Mean age in years (±SD) | 38.88 (11.65) | 43.46 (9.08) |
| Gender | 15 female 20 male |
23 female 5 male |
| Number excluded | 2 | 1 |
| Average RNFL SD-OCT (micrometer ± SD) | 98.59 (6.74) | 88.80 (17.39) |
| Average RNFL TD-OCT (micrometer ± SD) | 100.67 (8.88) | 90.91 (18.09) |
| Number of eyes with optic neuritis | n/a | 14 (26%) |
| Number of patients with SPMS | n/a | 3 (11%) |
| Mean EDSS (SD) | n/a | 2.8 (1.6) |
| Mean disease duration in months (SD) | n/a | 83.12 (83.67) |
| On disease-modifying therapy | n/a | 52% (14/27) |
3.2. Comparing Time-Domain and Spectral-Domain OCT
SD-OCT and TD-OCT RNFLT values were strongly correlated in all quadrants with correlation coefficient ranging from 0.808 (P < 0.01) in inferior quadrant to 0.878 (P < 0.01) in temporal quadrant. The overall RNFLT was also strongly correlated (correlation coefficient = 0.921; P < 0.001) between the two machines (Figure 1). However, RNFLT values showed minor but statistically significant differences between the two machines (P < 0.001) (Table 2).
Figure 1.
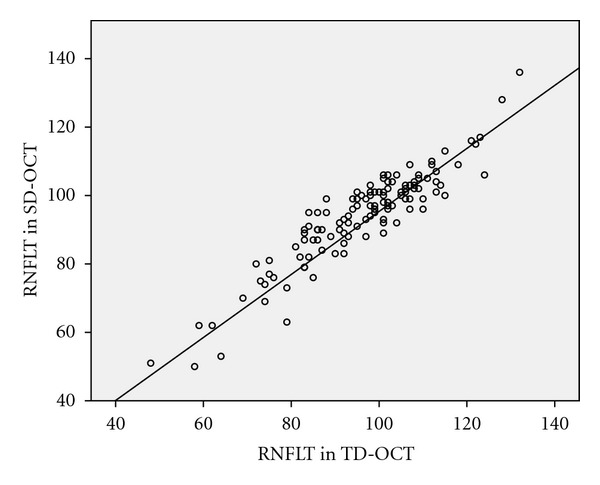
Correlation between average RNFLT in μm in SD-OCT (y-axis) and TD-OCT (x-axis).
Table 2.
Differences in RNFLT between SD- and TD-OCT in MS group and control group (SD-OCT minus TD-OCT).
| Control group | MS group | |||
|---|---|---|---|---|
| Mean difference in μm (95% CI) | Pearson's Corr r | Mean difference in μm (95% CI) | Pearson's Corr r | |
| Average | −2.40 (−3.70 to −1.09) | 0.83 (P < 0.001) | −1.69 (−3.44 to 0.06) | 0.93 (P < 0.001) |
| Superior | 3.75 (0.99 to 6.51) | 0.77 (P < 0.001) | 5.54 (2.20 to 8.87) | 0.90 (P < 0.001) |
| Temporal | 0.04 (−1.41 to 1.50) | 0.88 (P < 0.001) | −0.54 (−3.02 to 1.94) | 0.87 (P < 0.001) |
| Inferior | −6.56 (−8.28 to −4.84) | 0.85 (P < 0.001) | −6.81 (−9.51 to −4.11) | 0.91 (P < 0.001) |
| Nasal | −6.93 (−9.47 to −4.38) | 0.80 (P < 0.001) | −3.37 (−7.13 to 0.40) | 0.81 (P < 0.001) |
3.3. Comparing RNFLT Measurements between MS and Control Eyes
Overall, MS patients had significantly lower RNFLT measured by both SD-OCT and TD-OCT (Table 3). Moreover, RNFLT was significantly different between MS eyes with history of optic neuritis (mean RFNL (SD) 72.21 μm (15.83 μm)), MS eyes without history of optic neuritis 93.03 μm (14.25 μm), and healthy controls 99.07 μm (7.23 μm) (P < 0.001) measured by SD-OCT (Figure 2).
Table 3.
Overview RNFLT between SD- and TD-OCT in MS and control groups, separated by quadrants.
| SD-OCT μm mean (SD) | TD-OCT μm mean (SD) | |||
|---|---|---|---|---|
| Control | MS | Control | MS | |
| Average | 98.59 (6.79) | 88.80 (17.55) | 100.67 (8.96) | 90.9107 (18.26) |
| Superior | 121.27 (16.22) | 107.25 (26.95) | 117.48 (16.87) | 101.88 (27.04) |
| Temporal | 71.17 (10.88) | 65.02 (15.92) | 70.86 (12.88)* | 65.82 (17.57)* |
| Inferior | 127.16 (13.10) | 114.80 (21.64) | 133.23 (13.59) | 120.36 (27.08) |
| Nasal | 74.55 (11.21) | 68.07 (21.53) | 81.031 (15.75) | 72.20 (24.34) |
*All measures are significantly different between MS patients and control except for temporal quadrant measured by TD-OCT.
Figure 2.
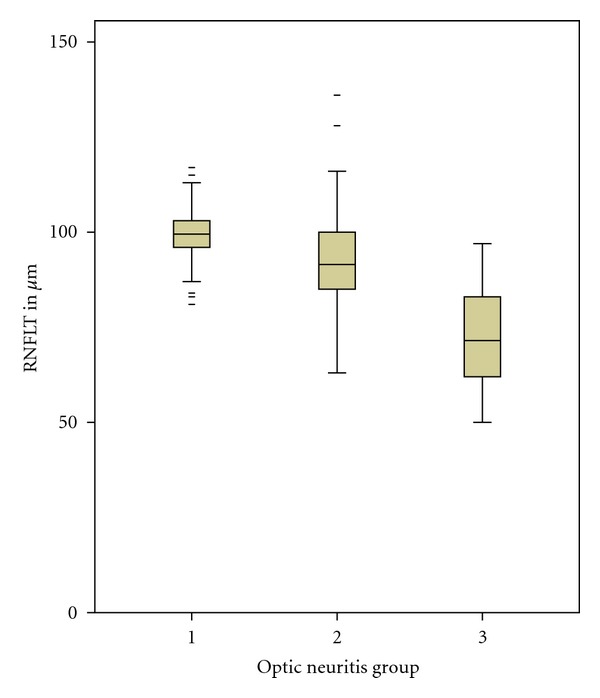
Boxplot of differences between RNFLT in control group (left box, group 1) and groups of MS eyes without optic neuritis (center box, group 2) and MS eyes with optic neuritis (left box, group 2) measured by SD-OCT.
3.4. Correlation between Retinal Nerve Fiber Layer Thickness, Disease Duration, EDSS, DMT, and Refraction
When all MS eyes were considered, duration of the disease since onset of first symptoms weakly correlated with superior (r = −0.28; P = 0.048) and temporal (r = −0.33; P = 0.02) quadrant RNFLT. There was no significant correlation between disease duration and mean RNFLT values (r = −0.13; P = 0.35), or RNFLT values in the inferior (r = −0.14; P = 0.31) or nasal (r = −0.19; P = 0.17) quadrants. When only MS eyes without a history of ON were considered, a moderate correlation (r = −0.44; P = 0.01) was found between mean RNFLT and disease duration, with a significant correlation in the superior and inferior quadrants (r = −0.51; P = 0.001, and r = −0.38; P = 0.02, resp.) and no significant correlation in the temporal and nasal quadrants (P = 0.91 and P = 0.08, resp.).
When EDSS was correlated to mean RNFLT in all MS eyes, a weak correlation was found (r = − 0.3; P = 0.05). This was significant in the superior quadrant only (r = −0.33; P = 0.02), nonsignificant in the other quadrants (P = 0.46 for temporal quadrant, P = 0.07 for inferior quadrant, and P = 0.39 for nasal quadrant). When only MS eyes without a history of ON were considered, a moderate correlation was found between EDSS and mean RNFLT (r = −0.35; P = 0.03). This was also significant in the superior (r = −0.38; P = 0.02) and inferior quadrants (r = −0.33; P = 0.05), but not in the temporal (P = 0.31) and nasal quadrants (P = 0.43).
The minimal difference between RFNLT in patients with DMT (86.07 μm) and without DMT (90.82 μm) was not statistically different (P = 0.327).
Myopic and emmetropic eyes showed significantly different RFNLT measurements in both SD-OCT (mean RNFLT (SD) 87.75 μm (12.52 μm) versus 96.46 μm (14.61 μm)) and TD-OCT (89.59 μm (13.65 μm) versus 99.19 μm (15.11 μm)). There was a significant correlation between refraction in diopters and RNFLT (r = 0.4; P = 0.005).
4. Discussion
The main aim of this study was to compare RNFLT measurements between SD-OCT and TD-OCT in MS patients and healthy controls. Our results show strong correlations (Pearson's r = 0.921) between the measurements of the Heidelberg Spectralis SD-OCT and TD-OCT. RNFLT values were significantly lower in SD-OCT (mean difference 2 μm). These results are similar to those of Watson et al. [14], who found Spectralis SD-OCT to measure 3 μm lower than TD-OCT in a study of 50 MS eyes, and Seibold et al. [12], who found the same results in a series of 80 healthy eyes. On the other hand, Arthur et al. [13] compared Spectralis SD-OCT with TD-OCT in 30 healthy eyes and found Spectralis to measure 6 μm lower, a larger difference than our results. Studies using other SD-OCT devices showed similar discrepancies with Cirrus SD-OCT measuring lower RNFLT and RTVue measuring higher RNFLT than TD-OCT (Table 4).
Table 4.
Overview published studies comparing RNFLT in SD-OCT versus TD-OCT.
| Author | SD-OCT used | Study population (eyes) | Results |
|---|---|---|---|
| Chang et al. [5] | Cirrus | 54 glaucoma 50 controls |
Cirrus is equivalent to Stratus for detecting glaucoma |
| Knight et al. [6] | Cirrus | 101 glaucoma 29 controls |
Cirrus 7 μm lower then Stratus in both groups |
| Leung et al. [7] | Cirrus | 83 glaucoma 97 controls |
Cirrus 12 μm lower for control, 6 μm lower in glaucoma |
| Sung et al. [8] | Cirrus | 103 glaucoma 60 controls |
Cirrus 13 μm lower for control, 14 μm lower in glaucoma |
| Vizzeri et al. [9] | Cirrus | 78 glaucoma 32 controls |
Cirrus 8 μm lower for control, 6 μm lower in glaucoma |
| Kim et al. [10] | Cirrus | 27 controls | Cirrus 10 μm lower |
| Gonzalez-Garcia et al. [11] | RTVue-100 | 76 glaucoma 60 controls |
RTVue 2 μm higher |
| Seibold et al. [12] | Cirrus, Spectralis, RTVue-100 | 80 controls | Spectralis 3 μm lower, Cirrus 12 μm lower, RTVue 3 μm higher, |
| Arthur et al. [13] | Spectralis | 30 controls | Spectralis 6 μm lower |
| Watson et al. [14] | 3D OCT-1000, Cirrus, RTVue-100, Spectralis | 50 MS | Spectralis 3 μm lower, Cirrus 8 μm lower, RTVue 3 μm higher, 3D OCT-1000 2 μm higher |
| Bock et al. [15] | Cirrus | 110 MS | Cirrus 8 μm lower |
The discrepancy between different devices may be explained by a difference in calibration due to a higher resolution and improved software algorithm in more recent models [6]. This has been addressed in evaluation of SD-OCT in macular thickness [17, 18]. The phenomenon of thickness-dependent interdevice differences was not observed in our data [15]. The minimal difference observed in our study is lower than the axial resolution of the SD-OCT, hence clinically not significant. However, results from these machines cannot be interchangeably interpreted in a population study and ongoing longitudinal studies switching generation of OCT should take these differences into consideration.
There has been increasing interest in RNFLT measurements in MS patients in order to determine whether OCT can be used as a surrogate marker for follow-up examinations. Therefore, a large amount of cross-sectional data has been previously published. Many studies have shown differences between RNFLT in MS eyes with optic neuritis, MS eyes without optic neuritis, and healthy controls, for example, [15, 19–27]. All these studies were using the older TD-OCT technology. We were able to reproduce these differences using the newer generation of OCT machine. Spectral-domain OCT has several advantages over the older TD-OCT technology: there is no pupil dilation needed, the speed of the machines is higher, reducing the possibilities of motion artefacts, and the lack of the previously used bright flashlight makes the examination much more comfortable for the patient. Furthermore, the higher resolution and the improved software algorithm allowing automatic re-centration for follow-up exams help in improving accuracy and reproducibility for follow-up exam in longitudinal studies. Up to date, no longitudinal study in an MS cohort has been published using SD-OCT technology. Two longitudinal studies using TD-OCT have not been able to show any change in RNFLT in a two-year follow-up period [27, 28]. Only Talman et al. [21] could detect significant RNFLT changes in a 4.5-year study of 299 patients using TD-OCT (loss of 2.9 μm at 2 to 3 years and 6.1 μm at 3 to 4.5 years; P < 0.001). This pattern was observed in both eyes with and without history of ON. Proportions of eyes with RNFL loss greater than test-retest variability (≥6.6 μm) increased from 11% at baseline to 44% at final visit (3–4.5 years) (P < 0.001). The progressive axonal loss of approximately 2 μm per year could only be detected over a relatively long period of time. This is most likely due to the relatively low resolution of the TD-OCT machine. The new generation SD-OCT is more sensitive to smaller changes and may be more reliable detecting RNFL changes over shorter time periods. Longitudinal studies using SD-OCT technology will be needed to establish if OCT measurements can be used as a surrogate marker in MS and be used to monitor disease progression and disease-modifying therapy.
We were interested in contribution of disease duration, EDSS, refraction, and status of DMT on RNFLT measurements. Our results showed no significant correlation for disease duration and EDSS when all MS eyes were compared but moderate correlation when only eyes without a history of ON were considered. This may be due to the fact that ON causes a 18–22% loss of RNFLT and the small progressive loss of RNFLT is not evident at this time anymore [29]. The difference between patients with and without DMT was statistically not significant, but our sample size was too small for a final conclusion.
We also compared RNFLT measurements between refraction range groups (described in methods). We showed a relatively large difference between myopic and nonmyopic eyes using both devices. Thinner RNFLT measurements in myopes may be explained by increased scan diameter due to the telecentric optics of the OCT in increased myopia and myopic tilted discs resulting in elevated and decreased RNFLT at different sites. Furthermore, the centration is very difficult even on a frozen fundus image due to the asymmetry of the disc.
RNFLT values were significantly lower in myopic eyes as the diameter of the scan increases with higher myopic refraction. Rauscher et al. [30] have reported an average decrease of RNFL of 3 μm per diopter of myopia. A possible explanation is the telecentric system, which keeps the angle of the OCT beam constant at 12 degrees. In our measurements, the scan diameter increased to 3.8 mm in −5 diopters and to 4.2 mm in −10 diopters. This results in thinning of about 10 μm in −5 diopters and about 20 μm in −10 diopters [31]. This was not a major issue with the older TD-OCT as the axial resolution is only 10 μm but gets more importance with the SD-OCT devices with higher resolution up to 3.8 μm. This must be taken into consideration designing future studies. Higher myopic refraction should be excluded or properly matched between groups. Furthermore, normative databases are needed to be refraction adjusted.
The main aim of the study was not to characterize RNFLT in MS population. Therefore, the MS population involved was randomly selected and examiner was not blinded to subjects' diagnosis and history of optic neuritis. Furthermore, the groups were not gender- or age-matched and both eyes of each subject were included. However, this was not a major issue in comparing RNFLT in the same subject between two different machines and did not affect the results of our main study aim.
Conflict of Interests
None of the authors had any conflict of interests.
Acknowledgments
A. P. Lange was funded by the Swiss National Science Foundation and the UBC Hospital NMO Research Program.
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Investigative Ophthalmology and Visual Science. 2004;45(6):1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung CKS, Ye C, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography. A study on diagnostic agreement with Heidelberg retinal tomograph. Ophthalmology. 2010;117(2):267–274. doi: 10.1016/j.ophtha.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto A, Hangai M, Yoshimura N. Spectral-domain optical coherence tomography with multiple B-scan averaging for enhanced imaging of retinal diseases. Ophthalmology. 2008;115(6):1071–1078. doi: 10.1016/j.ophtha.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Chang RT, Knight OJ, Feuer WJ, Budenz DL. Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology. 2009;116(12):2294–2299. doi: 10.1016/j.ophtha.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Knight OJ, Chang RT, Feuer WJ, Budenz DL. Comparison of retinal nerve fiber layer measurements using time domain and spectral domain optical coherent tomography. Ophthalmology. 2009;116(7):1271–1277. doi: 10.1016/j.ophtha.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung CKS, Cheung CYL, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116(7):1257–1263. doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Sung KR, Kim DY, Park SB, Kook MS. Comparison of retinal nerve fiber layer thickness measured by Cirrus HD and Stratus optical coherence tomography. Ophthalmology. 2009;116(7):1264–1270. doi: 10.1016/j.ophtha.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 9.Vizzeri G, Weinreb RN, Gonzalez-Garcia AO, et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. British Journal of Ophthalmology. 2009;93(6):775–781. doi: 10.1136/bjo.2008.150698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JS, Ishikawa H, Sung KR, et al. Retinal nerve fibre layer thickness measurement reproducibility improved with spectral domain optical coherence tomography. British Journal of Ophthalmology. 2009;93(8):1057–1063. doi: 10.1136/bjo.2009.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Garcia AO, Vizzeri G, Bowd C, Medeiros FA, Zangwill LM, Weinreb RN. Reproducibility of RTVue retinal nerve fiber layer thickness and optic disc measurements and agreement with Stratus optical coherence tomography measurements. American Journal of Ophthalmology. 2009;147(6):1067–1074. doi: 10.1016/j.ajo.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seibold LK, Mandava N, Kahook MY. Comparison of retinal nerve fiber layer thickness in normal eyes using time-domain and spectral-domain optical coherence tomography. American Journal of Ophthalmology. 2010;150(6):807–814. doi: 10.1016/j.ajo.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Arthur SN, Smith SD, Wright MM, et al. Reproducibility and agreement in evaluating retinal nerve fibre layer thickness between Stratus and Spectralis OCT. Eye. 2011;25(2):192–200. doi: 10.1038/eye.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson GM, Keltner JL, Chin EK, Harvey D, Nguyen A, Park SS. Comparison of retinal nerve fiber layer and central macular thickness measurements among five different optical coherence tomography instruments in patients with multiple sclerosis and optic neuritis. Journal of Neuro-Ophthalmology. 2011;31(2):110–116. doi: 10.1097/WNO.0b013e3181facbbd. [DOI] [PubMed] [Google Scholar]
- 15.Bock M, Brandt AU, Dörr J, et al. Time domain and spectral domain optical coherence tomography in multiple sclerosis: a comparative cross-sectional study. Multiple Sclerosis. 2010;16(7):893–896. doi: 10.1177/1352458510365156. [DOI] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 Revisions to the "McDonald Criteria". Annals of Neurology. 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 17.Wolf-Schnurrbusch UEK, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Investigative Ophthalmology and Visual Science. 2009;50(7):3432–3437. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 18.Giani A, Cigada M, Choudhry N, et al. Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. American Journal of Ophthalmology. 2010;150(6):815–824. doi: 10.1016/j.ajo.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69(22):2085–2092. doi: 10.1212/01.wnl.0000294876.49861.dc. [DOI] [PubMed] [Google Scholar]
- 20.Zaveri MS, Conger A, Salter A, et al. Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis. Archives of Neurology. 2008;65(7):924–928. doi: 10.1001/archneur.65.7.924. [DOI] [PubMed] [Google Scholar]
- 21.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Annals of Neurology. 2010;67(6):749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73(4):302–308. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Annals of Neurology. 2005;58(3):383–391. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- 24.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113(2):324–332. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 25.Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Annals of Neurology. 2006;59(6):963–969. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 26.Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Multiple Sclerosis. 2008;14(7):893–905. doi: 10.1177/1352458508091367. [DOI] [PubMed] [Google Scholar]
- 27.Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. Journal of the Neurological Sciences. 2009;281(1-2):74–79. doi: 10.1016/j.jns.2009.02.354. [DOI] [PubMed] [Google Scholar]
- 28.Henderson APD, Trip SA, Schlottmann PG, et al. A preliminary longitudinal study of the retinal nerve fiber layer in progressive multiple sclerosis. Journal of Neurology. 2010;257(7):1083–1091. doi: 10.1007/s00415-010-5467-x. [DOI] [PubMed] [Google Scholar]
- 29.Henderson APD, Trip SA, Schlottmann PG, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131(1):277–287. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]
- 30.Rauscher FM, Sekhon N, Feuer WJ, Budenz DL. Myopia affects retinal nerve fiber layer measurements as determined by optical coherence tomography. Journal of Glaucoma. 2009;18(7):501–505. doi: 10.1097/IJG.0b013e318193c2be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skaf M, Bernardes AB, Cardillo JA, et al. Retinal nerve fibre layer thickness profile in normal eyes using third-generation optical coherence tomography. Eye. 2006;20(4):431–439. doi: 10.1038/sj.eye.6701896. [DOI] [PubMed] [Google Scholar]


