Abstract
Background
Fluoxetine, a selective serotonin reuptake inhibitor, has recently been found to possess anti-inflammatory properties. The present study investigated the effects of fluoxetine on inflammatory tissue destruction in a rat model of ligature-induced periodontitis (PD).
Methods
Male Wistar rats were randomly assigned into three groups (n=10 animals/group): 1) Control rats (without ligature); 2) rats with ligature + placebo (saline; oral gavage); 3) rats with ligature + fluoxetine (20 mg/kg/day in saline; oral gavage). Histological analyses were performed on the furcation region and mesial of mandibular first molars of rats sacrificed at 15 days after ligature-induced PD. Reverse transcriptase-polymerase chain reaction (RT-PCR) and zymography were performed to analyze the mRNA expression of interleukin (IL)-1β, cyclooxygenase (COX)-2, matrix metalloproteinase (MMP)-9 and inducible nitric oxide synthase (iNOS), and the MMP-9 activity, respectively, in gingival tissues samples.
Results
Compared to the ligature + placebo group, alveolar bone loss was reduced in the fluoxetine group (P < 0.05), and the integrity of collagen fibers in the gingival tissue was maintained. Moreover, in gingival tissue sampled 3 days after ligature attachment, fluoxetine administration reduced IL-1β and COX-2 mRNA expression. Fluoxetine down-regulated MMP-9 activity, without affecting MMP-9 mRNA expression induced by ligature, compared to the ligature + placebo group (P < 0.05). These data suggested that fluoxetine suppressed proinflammatory responses, as well as proteolytic enzyme activity, induced by ligature.
Conclusions
In the present study, fluoxetine suppressed the inflammatory response and protected against periodontal bone resorption and destruction of collagen fibers, suggesting that fluoxetine can constitute a promising therapeutic approach for periodontal diseases.
Keywords: Fluoxetine, inflammation, periodontitis, bone resorption, collagen
Introduction
Although the etiology of periodontal disease (PD) is related to putatively virulent microorganisms in the biofilm, it has also been demonstrated that significant tissue destruction derives from immune/inflammatory responses of a PD-susceptible host to microbial challenge.1,2 The persistent presence of these bacteria in the gingival crevice disrupts homeostatic balance between periodontal bacteria and host cells, which results in the synthesis and release of proinflammatory cytokines, proteolytic enzymes and chemical inflammatory mediators by a variety of resident and infiltrating host cells in the periodontium.1,3
A better understanding of these host mechanisms have led to the search for new therapeutic agents aimed at modulation of host response to bacteria through the inhibition of immune/inflammatory mediators. Some pharmacological agents, such as inhibitors of cyclooxygenase (COX)-2, matrix metalloproteinases (MMPs) and nitric oxide (NO), have been reported to reduce inflammatory parameters and bone resorption in vivo.4–7 Therefore, these pharmacological agents could offer a valuable adjunct strategy to periodontal treatment when the use of conventional therapies alone fails to control the progression of PD.
Fluoxetine is a selective serotonin reuptake inhibitor used as an antidepressant drug.8 However, recent studies have shown additional analgesic, anti-inflammatory and immunomodulatory effects of fluoxetine.9–11 Most notably, several reports have revealed that fluoxetine possesses anti-inflammatory properties. For example, studies using animal models have shown that fluoxetine can decrease proinflammatory cytokine levels in inflammatory diseases, including rheumatoid arthritis.11–13 Fluoxetine was also found to reduce prostaglandin-E2 (PGE2) levels in subcutaneous exudates and paw edema in carrageenan-induced inflammation.10,14 Moreover, fluoxetine can reduce both PGE2 and NO production by human synovial cells.9 More recently, we demonstrated that fluoxetine reduced the production of important cytokines/chemokines by dendritic cells (DCs), as well as the expression of a co-stimulatory molecule that is essential to T cell activation.15 It was shown that fluoxetine suppressed the ability of DCs to present bacterial antigens to T cells and thus downregulated the resulting T-cell proliferation, suggesting a possible modulation of the immunological synapse by fluoxetine in the context of PD.15
Although fluoxetine can reduce the production of important inflammatory mediators, no studies have thus far evaluated its impact on PD. Given its anti-inflammatory effect, along with its safety and high medical prescription rates in clinical practice, the present study investigated whether fluoxetine would affect the development of PD. To test this hypothesis, we assessed the effects of systemically administered fluoxetine on inflammatory host responses, bone resorption and collagen destruction using a rat model of ligature-induced periodontitis.
Material and Methods
Animals
Male Wistar rats (60-day-old) obtained from CEMIB (Multidisciplinary Center for Biological Research, University of Campinas, SP, Brazil) were housed in plastic cages with food and water given ad libitum and maintained in a specific pathogen-free facility at the Piracicaba Dental School. The protocol used for this rat experiment was approved by the Ethical Committee on Animal Research (Protocol #1499–1) at the University of Campinas. The behavior and physical appearance of the animals were monitored daily, and their weight was assessed at the beginning and end of each experimental period.
Induction of periodontal disease (PD) and treatment
To induce PD, rats were first anesthetized with an intramuscular injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). A cotton ligature was placed in a subgingival position around the cervix of both sides of mandibular first molars in each animal.16 In order to immobilize the ligature, two knots were made at the mesial aspect of the first molars.
After the ligature placement, animals were randomly assigned to three experimental groups (n=10 animals/group): 1) Control rats (without ligature); 2) rats with ligature + placebo (saline); 3) rats with ligature + fluoxetine (20 mg/kg/day11 in saline). Fluoxetine hydrochloride# was dissolved in saline solution (vehicle). All treatments (saline or fluoxetine) were given orally (gavages) 1 hour before the attachment of ligature and daily during experimental periods.
Rats were euthanized under general anesthesia at 3 or 15 days after the attachment of the ligature, respectively, according to the protocols established by Rodini et al.17 and Holzhausen et al.5 At Day 3, gingival tissue samples of the same size were collected from the mandibular first molars regions, immediately frozen, and kept at −80°C until processing for reverse transcriptase- polymerase chain reaction (RT-PCR) analysis and protein assays. Mandibular alveolar bone specimens of rats collected at Day 15 were submitted to histological analysis.
Histological analysis
The alveolar bone specimens were immediately fixed with 10% neutral buffered formalin and then decalcified with 10% EDTA aqueous solution for 60 days. The decalcified specimens were dehydrated and embedded in paraffin. Serial sections obtained in a mesiodistal direction (5 μm thickness) were stained with hematoxylin and eosin (H&E) for measurement of bone loss or reacted with picrosirius red for the evaluation of collagen content.
Measurement of periodontal bone loss
The images of five semi-serial sections stained with H&E were digitized at a magnification of x50. The influence of fluoxetine on periodontal bone loss was histometrically assessed by measuring the area (mm2) of bone resorption in the furcation region, according to a method previously reported18. Evaluation was performed by a single examiner (L.S.B.-A.) blind to the treatment assignment using an image analysis system**. Alveolar bone specimens from control group (no ligature) were also measured to compare the results from both ligature groups.
Collagen assessment in the connective tissue
To evaluate the effects of fluoxetine on the inflammatory change of collagen fibers in the connective tissue, three equidistant sections were obtained from the region corresponding to the mesial of first molars and then stained with picrosirius red19. The images of the sections were obtained by polarization microscopy and digitized at x400 magnification. First, the images of collagen fibers that displayed red hue were selected using an image processing software††. The selected images were then transferred to another image software‡‡, where they were binarized, and the percentage of area filled by collagen fibers was calculated. The sections from control group were also evaluated.
RNA isolation
Total RNA was isolated from gingival tissues using a specific reagent§§ following the manufacturer’s instructions. The isolated RNA was resuspended in ultrapure DNase/RNase free water and stored at −80°C. The RNA concentration and quality were determined using a spectrophotometer¶¶. DNAse## was used to eliminate DNA contamination.
Detection of interleukin (IL)-1β, cyclooxygenase (COX)-2, matrix metalloproteinase (MMP)-9 and inducible nitric oxide synthase (iNOS) mRNA using RT-PCR
Complementary DNA (cDNA) was synthetized from total RNA (0.5 μg) using reverse transcriptase and random primers***. Subsequently, resultant cDNA was amplified by PCR with Taq DNA polymerase†††. The primer sequences used for amplification of target mRNA genes were: IL-1β, sense 5′-TCCATGAGCTTTGTACAAGG-3′, antisense 5′-GGTGCTGATGTACCAGTTGG-3′, 237bp; COX-2, sense 5′-TGATGACTGCCCAACTCCCATG-3′, antisense 5′-AATGTTGAAGGTGTCCGGCAGC-3′, 702 bp; MMP-9, sense 5′-GGATTACCTGTACCGCTATGGTTA-3′, antisense 5′-TTGGATCCAATAGGTGATGTTATG-3′, 241bp;iNOS, sense 5′-ACAACAGGAACCTACCAGCTCA-3′, antisense 5′-GATGTTGTAGCGCTGTGTGTCA-3′, 651 bp. Amplification of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (sense 5′-ACCACAGTCCATGCCATCAC-3′, antisense 5′-TCCACCACCCTGTTGCTGTA-3′, 450 bp) was used as an internal control.
PCR conditions were 30–35 cycles at 94°C for 30 s; 55–60°C for 30 s and 72°C for 1 min. The size of the PCR products was determined by comparison with the 100 bp ladder‡‡‡. The agarose gels containing the amplified products were scanned and analyzed by ImageJ, which provided numeric values that allowed a semi-quantitative comparison between target genes and the internal control gene.
Preparation of gingival tissue homogenates
The gingival tissues collected from mandibular first molars were homogenized in a lysis buffer [25 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 10% glycerol, 1% Triton X-100, 0.1% SDS, 1% NP-40,§§§] using a tissue homogenizer¶¶¶. After the centrifugation of homogenized samples, the supernatants were removed and stored at −80°C for further analyses. Measurement of total protein in the supernatants was performed using a protein assay kit###, following the manufacturer’s instructions.20
Gelatinase activity of MMP-9 using zymography
The measurement of MMP-9 activity was performed using gelatin zymography.21 Aliquots of gingival tissue homogenates containing the same protein amount were run under non-reducing conditions without heat denaturation onto 10% sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) co-polymerized with 1.6 mg/mL of gelatin**** as substrate. After SDS-PAGE, the gels were washed twice in 2% Triton X-100 for 30 min each, and then the gels were incubated overnight in activation buffer (50 mM Tris-HCl, pH 7.4, 5 mM CaCl2) for 16 h at 37°C. The MMP-9 gelatinolytic activity was detected after staining the gels with Coomassie blue††††. A gelatinase zymography standard‡‡‡‡ was used in all gels. The intensities of the bands in the photographed gels were analyzed to determine the gelatinase activity using image analysis software§§§§.
Statistical analysis
Data from assays are presented as means ± standard deviation (SD). The results were subjected to one-way analysis of variance (ANOVA), and statistical differences among the three groups were analyzed using the Student’s t-test at a significance level of 5%. Data were analyzed using statistical software¶¶¶¶.
Results
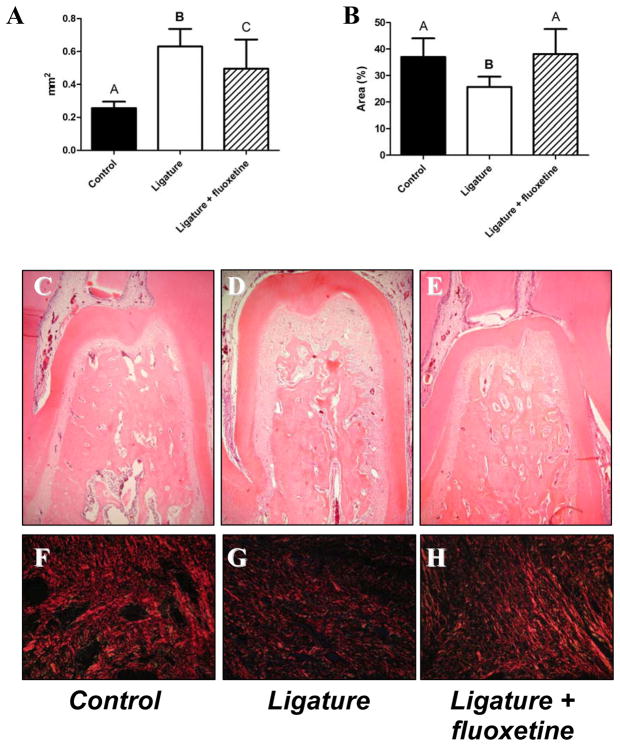
The effects of fluoxetine on ligature-induced bone resorption in rats were evaluated. According to histometric analysis (Fig. 1A), the experimental group treated with fluoxetine showed significantly lower levels of ligature-induced bone loss in the furcation region when compared to that of ligature-alone group (P < 0.05, Fig. 1). Histo-morphological images of the furcation region show that the pronounced bone loss caused by ligature (Fig. 1D) compared to control (Fig. 1C) was abolished by treatment with fluoxetine (Fig. 1E). Furthermore, the group treated with fluoxetine maintained the amount of collagen comparable to that of control group (P > 0.05, Fig. 1B). In contrast, the ligature-alone group showed a significantly lower amount of collagen than either control or the fluoxetine-treated group (P < 0.05, Fig. 1B). The configuration of collagen fibers determined by fluorescent immuno-histochemistry showed less picrosirius red staining pattern in the ligature-alone group compared to either control or the fluoxetine-treated group (Fig. 1, F: control, G: ligature-alone group, and H: ligature + fluoxetine).
Figure 1. Effects of fluoxetine treatment on alveolar bone loss and collagen content in a rat model of ligature-induced periodontitis.
Data represent the mean ± standard deviation (SD) of 10 rats for each group. A) Measurement of bone loss (mm2) in the furcation region of first molars of control (rats having no ligature; healthy sites), ligature (saline-treated rats with ligature), and ligature + fluoxetine (fluoxetine-treated rats, 20 mg/kg/day) groups after 15 days of periodontal disease induction. B) Quantitative analysis of red-stained collagen fibers (% area) in the connective tissue immediately above the bone crest in the mesial of the mandibular first molars of control, ligature-alone and ligature + fluoxetine groups after 15 days of periodontal disease induction. Fluoxetine reduced alveolar bone loss as compared to ligature-alone group (P < 0.05) and maintained collagen fiber levels similar to control group (P > 0.05). Different letters on the top of each bar indicate statistical differences (Figure 1A: A vs. B, P < 0.01; A vs. C, P < 0.05; B vs. C, P < 0.01; Figure 1B: A vs. B, P < 0.01, ANOVA followed by Student’s t test). C, D, and E are the images of histochemical staining at the furcation region of control, ligature, and ligature + fluoxetine groups, respectively (hematoxylin and eosin staining, magnification of x50). F, G, and H are fluorescent images of the collagen fibers stained with picrosirius red in the connective tissue immediately above the bone crest in the mesial of mandibular first molars of control, ligature, and ligature + fluoxetine groups, respectively (picrosirius red stain, magnification of x400).
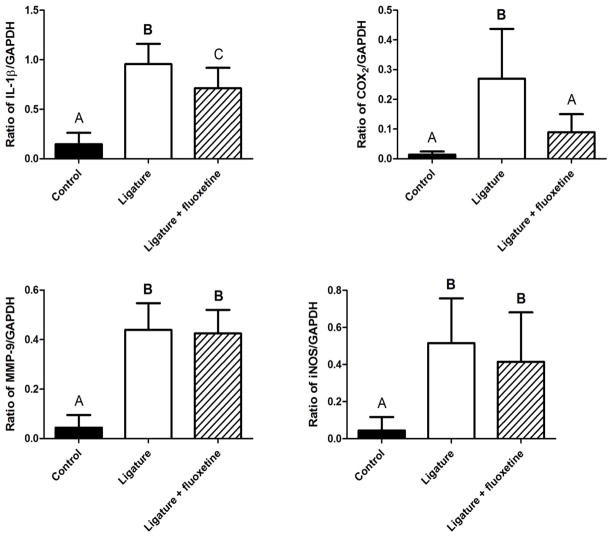
RT-PCR analyses showed that IL-1β, COX-2, MMP-9 and iNOS mRNA expressions were elevated in the ligature-alone group compared to control (Fig. 2). However, fluoxetine treatment significantly reduced the expressions of IL-1β and COX-2 mRNA induced in the gingival tissues compared to the ligature-alone group (P < 0.05). At the same time, no differences were found for MMP-9 and iNOS mRNA expressions induced by the attachment of ligature when fluoxetine was compared to ligature-alone group (P > 0.05).
Figure 2. Effects of fluoxetine treatment on expressions of IL-1β, COX-2, MMP-9 and iNOS mRNA levels monitored in gingival tissues.
mRNA expression levels (ratio of PCR products for target gene/GAPDH measured in agarose gel) of respective genes, IL-1β, COX-2, MMP-9 and iNOS in the gingival tissues were calculated. The column and bar indicate mean ± SD. Data were collected from the samples isolated from the gingival tissues of 1) control, 2) ligature-alone and 3) ligature + fluoxetine on Day-3. Different letters on the top of each bar indicate statistical differences (IL-1β: A vs. B, P < 0.01; A vs. C, P < 0.05; B vs. C, P < 0.01. COX-2: A vs. B, P < 0.01; MMP-9: A vs. B, P < 0.01; iNOS: A vs. B, P < 0.01. ANOVA followed by Student’s t test).
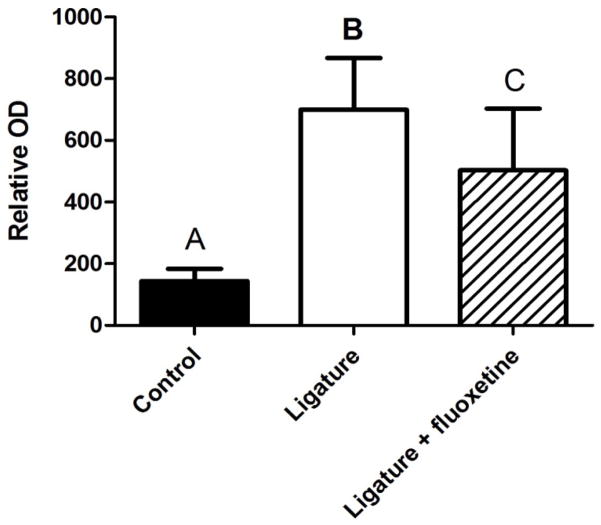
The gelatin gel zymography results showed that MMP-9 activity in gingival tissue was remarkably up-regulated by ligature attachment, and that fluoxetine suppressed such MMP-9 activity in the ligature-induced periodontal disease (Fig. 3).
Figure 3.
Effects of fluoxetine treatment on gelatinase activity of equal amounts of protein contained in the gingival tissue homogenates of control, ligature-alone and fluoxetine + ligature groups were subjected to gelatinase activity assay, as shown in the Material and Methods section. Different letters on the top of each bar indicate statistical differences (A vs. B, P < 0.01; A vs. C, P < 0.05; B vs. C, P < 0.01. ANOVA followed by Student’s t test).
Discussion
The present study showed that oral treatment with fluoxetine positively modulated important key periodontal inflammatory mediators in rats submitted to a ligature-induced PD model, which mimics the PD process very well.5,18,22 Furthermore, fluoxetine reduced alveolar bone resorption and had a positive effect on collagen breakdown in gingival tissues. Altogether, these findings not only confirm the potential anti-inflammatory capacity of fluoxetine, but are also the first to demonstrate the potency of fluoxetine on PD.
The modulation of the local inflammatory host responses by fluoxetine observed in this investigation [i.e., reduction of IL-1β and COX-2 expression, total protein concentration (data not shown) and MMP-9 activity in gingival tissues] seems to be responsible for its positive influence on bone resorption. Among all these inflammatory indicators, we can speculate that most fluoxetine inhibitory effects can be attributed to the reduction of IL-1β expression. Noting that IL-1β levels significantly decrease after successful periodontal therapy,23 it is still one of the most powerful pro-inflammatory factors.24 In fact, multifunctional IL-1β orchestrates a cascade of destructive events in periodontal tissues, triggering the production of an array of inflammatory mediators/enzymes, including PGE2 and MMP-9,25 both of which were also affected by fluoxetine in the present study.
PGE2 has been extensively related to inflammation and bone resorption, and the biosynthesis of PGE2 in the context of inflammation is regulated by COX-2.26 In this context, inhibitors of both PGE2 production and COX-2 expression can markedly reduce alveolar bone resorption in vivo.6 The effects of fluoxetine on PGE2 have only scarcely been reported;9,14,27 therefore, studies such as the present work are still necessary to strengthen reporting about this specific anti-inflammatory activity of fluoxetine. In this investigation, COX-2 mRNA expression was reduced in the gingival tissues after drug treatment, suggesting that fluoxetine possibly down-modulated the PGE2 generation by suppressing the production of COX-2 protein in the inflamed sites, thus contributing to the reduction of bone loss observed here.
In the present study, enhanced expression of MMP-9 mRNA after ligature attachment was not suppressed by fluoxetine; however, fluoxetine presented a positive influence on collagen from the gingival connective tissue. These results encouraged the authors of this study to perform additional analysis aimed at clarifying whether fluoxetine could affect the gelatinolytic activity of MMP-9 (gelatinase B, type IV collagenase). Because significant MMP-9 activity is found in progressive diseased sites, a measurable level of influence on this activity is relevant.28 MMP-9 can efficiently degrade collagen fibers in concert with MMP-1 and MMP-3, which results in tissue destruction during inflammation.29 We found that fluoxetine could down-modulate MMP-9 enzymatic activity without affecting expression of MMP-9 mRNA induced by ligature-mediated inflammation. Fluoxetine effects on MMPs activity/expression have been poorly investigated. Benekareddy et al.30 showed that fluoxetine regulates MMP-2/MMP-9 in the adult rat hippocampus. Recently, it was shown that fluoxetine inhibited monocrotaline-induced extracellular matrix remodeling of pulmonary artery and inflammation of lungs, effects that were in part related to its inhibition of MMPs and cytokine productions, including IL-1β.31 Altogether, this information and the results of the present study suggest that the reduction of MMP-9 activity observed here was partially responsible for the maintenance of collagen content in the gingival tissues of rats treated with fluoxetine. Thus, in the context of PD, our finding is of considerable therapeutic interest. Because the only drug approved by the Food and Drug Administration (U.S.F.D.A.) for periodontal disease therapy is a collagenase (and/or MMP) inhibitor, doxycycline, we anticipate that fluoxetine could be clinically used for the suppression of connective tissue destruction occurring in the context of PD.
Fluoxetine has shown inhibitory effects on NO produced by synovial cells from patients with rheumatoid arthritis. 9 Considering the importance of NO in the pathogenesis of periodontal disease3 and the fact that NO inhibitors have been shown to reduce periodontal bone resorption7, the present study also evaluated the effects of fluoxetine on gingival iNOS expression. Our investigation, however, failed to demonstrate any effect of fluoxetine on iNOS, suggesting that the bone resorption reduction observed here was probably unrelated to NO. Nonetheless, since the effects of fluoxetine on iNOS/NO are not well known and since existing reports have shown contradictory results,32,33 we suggest that more studies are needed to evaluate whether fluoxetine can influence NO in the context of PD.
Some mechanisms have been proposed to explain the anti-inflammatory properties of fluoxetine.14,27,34 Diamond et al.35 suggested that the effects of fluoxetine might be mediated by cyclic adenosine monophosphate. Moreover, fluoxetine has been reported to reduce the transcription activity of nuclear factor (NF)-κB,11,27 which can, to some extent, explain the anti-inflammatory effect observed in the present study. Therefore, our findings and those from previous studies led us to consider a relevant suppression effect of fluoxetine on the inflammation induced in the rat model of ligature-induced periodontal disease.
As for its therapeutic value: like all pharmacologic treatments, some tolerance issues have arisen from the use of fluoxetine, including headache, gastrointestinal disorders (i.e. nausea, diarrhea), insomnia, somnolence and weight loss.8,36,37 Nonetheless, fluoxetine is considered very safe and well tolerated by patients over short- and long-term periods, since side effects are transitory and dissipate quickly if the appropriate dosage is used.8,36,37 In the present study, we observed greater weight loss in the ligature + fluoxetine group in comparison to the other groups (data not shown), which could be explained by the side effects, such as gastrointestinal disorders. Importantly, fluoxetine does not change psychological variables in mentally healthy individuals38 and its psychotropic effects only manifest in the context of psychiatric target symptoms38,39, suggesting that it can be safely used as an adjunct to the conventional periodontal treatment in mentally healthy patients with periodontitis. Moreover, minimal adverse effects are reported by healthy patients using fluoxetine daily for several weeks.38
While the present study’s findings have merit with respect to periodontal therapy, the present investigation can also be useful in research aimed at new therapeutic strategies to treat other peripheral or central nervous disorders,40 important inflammatory conditions, and autoimmune disorders, such as rheumatoid arthritis.
In conclusion, our results suggest that fluoxetine may be considered a promising new therapeutic approach to treat PD. To better understand the mechanisms by which fluoxetine modulates host response, further studies must be conducted to test its effects on immune cells in the pathogenesis of PD. We believe that further clinical trials should be conducted to clarify the benefits of fluoxetine (and other selective serotonin reuptake inhibitors) as an adjunct regimen in periodontal therapy.
Acknowledgments
The authors thank the following Brazilian Government Agencies for fellowships to L.S.B.-A.: Fundação de Amparo à Pesquisa do Estado de São Paulo, São Paulo, SP (FAPESP #2008/00566–6); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasília, DF (PDEE/CAPES-BEX #4073/08–8). This study was supported in part by NIH grants DE-018499 and DE-019917 from the National Institute of Dental and Craniofacial Research, Bethesda, MD, USA.
Footnotes
The authors have no conflict of interest to declare.
Sigma-Aldrich, St Louis, MO, USA
Image-Pro® Media Cybernetics, Silver Spring, MD, USA
AdobePhotoshop, version 7.0.1, Adobe Systems Incorporated, San Jose, CA, USA
ImageJ, version 1.31p, National Institutes of Health, Bethesda, MD, USA
TRIzol® reagent, Invitrogen, Carlsbad, CA, USA
Nanodrop Spectrophotometer, model ND-3300, NanoDrop Technologies, Wilmington, DE
DNAse I, Invitrogen, Carlsbad, CA, USA
SuperScript sythesis system, Invitrogen, Carlsbad, CA, USA
Invitrogen, Carlsbad, CA, USA
Invitrogen, Carlsbad, CA, USA
Sigma-Aldrich, St Louis, MO, USA
Marconi Piracicaba, model MA-102, Piracicaba, SP, Brazil
Pierce® BCA Protein Assay Kit, Thermo Scientific, Rockford, IL, USA
Sigma-Aldrich, St Louis, MO, USA
Coomassie™ Brilliant Blue G-250, USB Corporation, Cleveland, OH, USA
CHEMICON International, Inc., Temecula, CA, USA
ImageJ, National Institutes of Health, Bethesda, MD, USA
BioEstat 5.0, Sociedade Civil Mamirauá, Belém, PA, Brazil
References
- 1.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 2.Han X, Kawai T, Taubman MA. Interference with immune-cell-mediated bone resorption in periodontal disease. Periodontol 2000. 2007;45:76–94. doi: 10.1111/j.1600-0757.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 3.Lappin DF, Kjeldsen M, Sander L, et al. Inducible nitric oxide synthase expression in periodontitis. J Periodontal Res. 2000;35:369–373. doi: 10.1034/j.1600-0765.2000.035006369.x. [DOI] [PubMed] [Google Scholar]
- 4.Golub LM, Lee HM, Ryan ME, et al. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 5.Holzhausen M, Rossa C, Júnior, Marcantonio E, Júnior, et al. Effect of selective cyclooxygenase-2 inhibition on the development of ligature-induced periodontitis in rats. J Periodontol. 2002;73:1030–1036. doi: 10.1902/jop.2002.73.9.1030. [DOI] [PubMed] [Google Scholar]
- 6.Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- 7.Leitão RF, Ribeiro RA, Chaves HV, et al. Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76:956–963. doi: 10.1902/jop.2005.76.6.956. [DOI] [PubMed] [Google Scholar]
- 8.Calil HM. Fluoxetine: a suitable long-term treatment. J Clin Psychiatry. 2001;62:24–29. [PubMed] [Google Scholar]
- 9.Yaron I, Shirazi I, Judovich R, et al. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum. 1999;42:2561–2568. doi: 10.1002/1529-0131(199912)42:12<2561::AID-ANR8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Salam OM, Nofal SM, El-Shenawy SM. Evaluation of the anti-inflammatory and anti-nociceptive effects of different antidepressants in the rat. Pharmacol Res. 2003;48:157–165. doi: 10.1016/s1043-6618(03)00106-3. [DOI] [PubMed] [Google Scholar]
- 11.Roumestan C, Michel A, Bichon F, et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guemei AA, El Din NM, Baraka AM, et al. Do desipramine [10,11-dihydro-5-[3-(methylamino) propyl]-5H-dibenz[b,f]azepine monohydrochloride] and fluoxetine [N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]-propan-1-amine] ameliorate the extent of colonic damage induced by acetic acid in rats? J Pharmacol Exp Ther. 2008;327:846–850. doi: 10.1124/jpet.108.141259. [DOI] [PubMed] [Google Scholar]
- 13.Sacre S, Medghalchi M, Gregory B, et al. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62:683–693. doi: 10.1002/art.27304. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi M, Rossoni G, Sacerdote P. Effects of chlomipramine and fluoxetine on subcutaneous carrageenin-induced inflammation in the rat. Inflamm Res. 1995;44:466–469. doi: 10.1007/BF01837911. [DOI] [PubMed] [Google Scholar]
- 15.Branco-de-Almeida LS, Kajiya M, Cardoso CR, et al. Selective serotonin reuptake inhibitors attenuate the antigen presentation from dendritic cells to effector T lymphocytes. FEMS Immunol Med Microbiol. 2011;62(3):283–294. doi: 10.1111/j.1574-695X.2011.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irie K, Tomofuji T, Tamaki N, et al. Effects of ethanol consumption on periodontal inflammation in rats. J Dent Res. 2008;87:456–460. doi: 10.1177/154405910808700511. [DOI] [PubMed] [Google Scholar]
- 17.Rodini CO, Batista AC, Dionísio TJ, et al. Morphologic evaluation and expression of matrix metalloproteinases-2 and 9 and nitric oxide during experimental periodontal disease in rat. J Mol Histol. 2008;39:275–282. doi: 10.1007/s10735-008-9163-4. [DOI] [PubMed] [Google Scholar]
- 18.Nociti FH, Jr, Nogueira-Filho GR, Primo MT, et al. The influence of nicotine on the bone loss rate in ligature-induced periodontitis. A histometric study in rats. J Periodontol. 2000;71:1460–1464. doi: 10.1902/jop.2000.71.9.1460. [DOI] [PubMed] [Google Scholar]
- 19.Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22:97–104. [Google Scholar]
- 20.de Brito Penna Forte LF, Cortelli SC, Cortelli JR, et al. Psychological stress has no association with salivary levels of beta-defensin 2 and beta-defensin 3. Oral Pathol Med. 2010 Nov;39(10):765–769. doi: 10.1111/j.1600-0714.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- 21.Marques MR, dos Santos MC, da Silva AF, et al. Parathyroid hormone administration may modulate periodontal tissue levels of interleukin-6, matrix metalloproteinase-2 and matrix metalloproteinase-9 in experimental periodontitis. J Periodontal Res. 2009;44:744–750. doi: 10.1111/j.1600-0765.2008.01186.x. [DOI] [PubMed] [Google Scholar]
- 22.Graves DT, Fine D, Teng YT, et al. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou LT, Liu CM, Rossomando EF. Crevicular interleukin-1 beta in moderate and severe periodontitis patients and the effect of phase I periodontal treatment. J Clin Periodontol. 1995;22:162–167. doi: 10.1111/j.1600-051x.1995.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 24.McCauley LK, Nohutcu RM. Mediators of periodontal osseous destruction and remodeling: principles and implications for diagnosis and therapy. J Periodontol. 2002;73:1377–1391. doi: 10.1902/jop.2002.73.11.1377. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz Z, Goultschin J, Dean DD, et al. Mechanisms of alveolar bone destruction in periodontitis. Periodontol 2000. 1997;14:158–172. doi: 10.1111/j.1600-0757.1997.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 26.Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432–444. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y, Lim CM, Kim SW, et al. Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Res. 2009;1281:108–116. doi: 10.1016/j.brainres.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 28.Teng YT, Sodek J, McCulloch CA. Gingival crevicular fluid gelatinase and its relationship to periodontal disease in human subjects. J Periodontal Res. 1992;27:544–552. doi: 10.1111/j.1600-0765.1992.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 29.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 30.Benekareddy M, Mehrotra P, Kulkarni VA, et al. Antidepressant treatments regulate matrix metalloproteinases-2 and -9 (MMP-2/MMP-9) and tissue inhibitors of the metalloproteinases (TIMPS 1–4) in the adult rat hippocampus. Synapse. 2008;62(8):590–600. doi: 10.1002/syn.20529. [DOI] [PubMed] [Google Scholar]
- 31.Li XQ, Wang HM, Yang CG, et al. Fluoxetine inhibited extracellular matrix of pulmonary artery and inflammation of lungs in monocrotaline-treated rats. Acta Pharmacol Sin. 2011 Feb;32(2):217–222. doi: 10.1038/aps.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ha E, Jung KH, Choe BK, et al. Fluoxetine increases the nitric oxide production via nuclear factor kappa B-mediated pathway in BV2 murine microglial cells. Neurosci Lett. 2006;397:185–189. doi: 10.1016/j.neulet.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Roman A, Rogóz Z, Kubera M, et al. Concomitant administration of fluoxetine and amantadine modulates the activity of peritoneal macrophages of rats subjected to a forced swimming test. Pharmacol Rep. 2009;61:1069–1077. doi: 10.1016/s1734-1140(09)70169-0. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi M, Sacerdote P, Panerai AE. Fluoxetine reduces inflammatory edema in the rat: involvement of the pituitary adrenal axis. Eur J Pharmacol. 1994;263:81–84. doi: 10.1016/0014-2999(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 35.Diamond M, Kelly JP, Connor TJ. Antidepressants suppress production of the Th1 cytokine interferon-gamma, independent of monoamine transporter blockade. Eur Neuropsychopharmacol. 2006;16:481–490. doi: 10.1016/j.euroneuro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Zajecka J, Amsterdam JD, Quitkin FM, et al. Changes in adverse events reported by patients during 6 months of fluoxetine therapy. J Clin Psychiatry. 1999;60:389–394. doi: 10.4088/jcp.v60n0608. [DOI] [PubMed] [Google Scholar]
- 37.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta- analysis. J Clin Psychiatry. 2010;71:1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 38.Gelfin Y, Gorfine M, Lerer B. Effect of clinical doses of fluoxetine on psychological variables in healthy volunteers. Am J Psychiatry. 1998;155(2):290–2. doi: 10.1176/ajp.155.2.290. [DOI] [PubMed] [Google Scholar]
- 39.Bonne O, Krausz Y, Aharon Y, et al. Clinical doses of fluoxetine and cerebral blood flow in healthy volunteers. Psychopharmacology (Berl) 1999 Mar;143(1):24–8. doi: 10.1007/s002130050915. [DOI] [PubMed] [Google Scholar]
- 40.Hartung HP, Kieseier BC. The role of matrix metalloproteinases in autoimmune damage to the central and peripheral nervous system. J Neuroimmunol. 2000;107:140–147. doi: 10.1016/s0165-5728(00)00225-3. [DOI] [PubMed] [Google Scholar]