Abstract
The uterine expression of the chemokine IL8 increases dramatically with the onset of labour both at term and preterm. The IL8 promoter contains binding sites for the transcription factors nuclear factor-kappa B (NFκB), activator protein-1 (AP-1), and CCAAT/enhancer-binding protein (CEBP). In this study we investigated the roles of these transcription factors in IL1B regulation of the IL8 gene in human myometrium. Using chromatin immune precipitation (ChIP) assay, we showed that each of NFκB, CEBP, and AP-1 binds to the IL8 promoter upon IL1B stimulation. To examine the relative importance of each site in IL8 gene expression, site-directed mutagenesis of each of these sites was performed. We found that the NFκB site was essential for basal and IL1B-stimulated gene expression. Mutation of the AP-1 site reduced both basal and IL1B-stimulated expression but to a lesser extent. Mutation of the CEBP site had no effect upon basal expression but eliminated the IL1B response. Small interfering RNA (siRNA) silencing of NFκB abolished the IL8 response to IL1B significantly; siRNA against AP-1 reduced it to a lesser extent whilst knockdown of CEBP enhanced the response. Our data confirms a central and essential role for NFκB in regulation of IL8 in human myometrium.
1. Introduction
The onset of labour is associated with changes in gene expression in the myometrium consistent with activation of inflammatory mediators and leukocyte chemotaxis [1, 2]. The uterine expression of the chemokine IL8 increases dramatically with the onset of term and preterm labour [3, 4] and is thought to promote cervical remodelling [5, 6] and myometrial contractility by promoting neutrophil infiltration and activation [4, 7]. Myometrial IL8 expression is increased by stretch and the inflammatory cytokines in a MAPK- and NFκB-dependent manner [8, 9]. Consistent with these observations, IL8 expression has been shown to be critically dependent on a region in its promoter, spanning nucleotides −1 to −133, which contains binding sites for the transcription factors AP-1, CEBP, and NFκB [10–13]. The binding sites are in close proximity to each other and to the coding region of the gene, forming a transcriptional enhanceosome (Figure 1(a)). Many researchers have investigated the role and interaction of these transcription factors in regulating the IL8 gene and have shown different mechanisms of regulation depending on the cell type [13–21]. Studies in reproductive tissues show that NFκB is important in IL1B-driven IL8 expression in amnion and myometrium [14], AP-1 is involved in the stretch-induced expression of IL8, OXTR, and COX2 in human myometrial and amnion cells [9, 22, 23], and CEBP has been shown to bind to two different regions of the IL8 promoter and interact with NFκB to regulate the IL8 expression [24].
Figure 1.
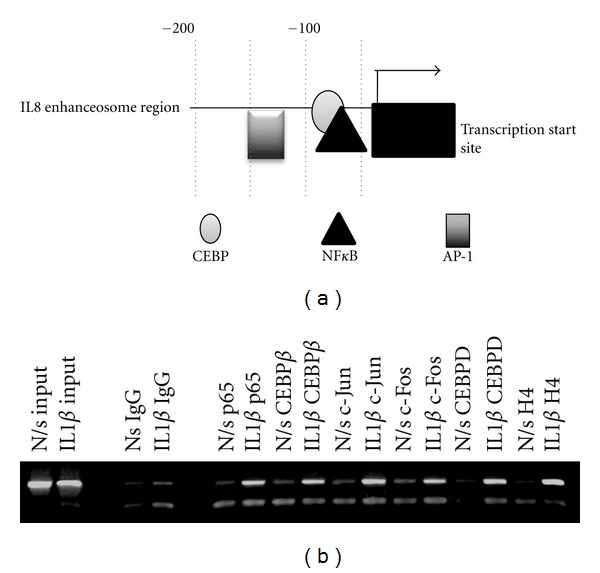
(a) Schematic of the IL8 promoter enhanceosome region. (b) ChIP analysis demonstrates the in vivo binding of NFκBp65, CEBPβ and d, c-Jun, c-Fos, and H4 to the transcriptional enhanceosome of the IL8 promoter. ChIP assay using antibodies against NFκBp65 and CEBPβ and -d, c-Jun, and c-Fos was performed in nonstimulated (N/s) and IL1B stimulated conditions. The immunoprecipitates were subjected to PCR analysis using primer pairs spanning the IL8 promoter transcriptional enhanceosome. The first two lanes are “input” lanes where no immunoprecipitation was performed prior to PCR. The second two lanes contained IgG antibody for immunoprecipitation (negative controls).
The marked inflammatory infiltration of the myometrium and cervix associated with human labour is driven by increased expression of IL8 and other chemokines. The transcription factors, NFκB, CEBP, and AP-1, have all been implicated in IL8 expression in various tissues/cell types, but which actually regulate myometrial IL8 expression remains unclear. In this study, we have used IL1B stimulation to mimic labour in order to define the relative importance of NFκB, CEBP, and AP-1 in myometrial IL8 expression.
2. Materials and Methods
2.1. Myometrial Cell Culture
Myometrial cells were extracted from biopsies taken at the time of elective caesarean section at term with the Ethics Committee approval and patient consent. None of the patients was in labour or had received uterotonics or tocolytics. Tissue was minced and digested for 45 min in DMEM with 1 mg/mL collagenase type IA and IX (Sigma-Aldrich Corp., St. Louis, MO, USA). Cells were centrifuged at 400 ×g for 10 min and grown in DMEM with 10% fetal calf serum, L-glutamine, and penicillin-streptomycin (37°C and 5% CO2). Cells were serum starved for 16 h before treatment with 1 ng/mL IL1B (R&D Systems, Inc., Minneapolis, MN, USA). Cells were used at passage three for all experiments.
2.2. Western Blotting
To obtain whole cell lysates, cells were lysed for 20 min on ice in radioimmunoprecipitation assay buffer (1% NP-40, 1% Triton, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10 mM Tris, PH 8.0, and 2 mM NaF). Protein concentrations were determined using detergent-compatible protein assay reagents (Bio-Rad Laboratories). Protein samples (50 μg) were denatured by boiling for 5 min and run on a 10% SDS-PAGE for 60 min at 140 V, followed by a transfer to a Hybond ECL nitrocellulose membrane (Amersham Biosciensces). The membrane was blocked in 5% milk protein solution over night or for 1 hour, washed and hybridized with the primary antibody for 1 hour at room temperature in a fresh blocking buffer (1 × PBS, 1% milk protein and 0.1% Tween-20) containing antibodies for RELA and CEBPβ (Santa Cruz Biochemicals, SC-8008 and SC-7962, resp.) or beta actin (Abcam, Ab6276). This process was repeated with the secondary antibody. The antibodies were used at 1 : 1000 dilution. Immunoreactivity was then visualised using a chemiluminescent substrate for HRP (ECL plus; Amersham Biosciensces).
2.3. Site-Directed Mutagenesis
The promoter region of the IL8 gene (−135/+46 bp) was amplified by polymerase chain reaction, and the fragment was ligated into the luciferase reporter plasmid pGL2-Basic (Promega, Southampton, UK) to give the wild-type construct. The mutation of individual base pairs in plasmid DNA was achieved using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, UK) according to manufacturer's instructions. The mutagenic oligonucleotide primers were designed individually and are shown in Table 1.
Table 1.
Primers used in site-directed mutagenesis.
| Construct | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| IL8 mAP-1 | 5′-GATCTGAAGTGTGATATCTCAGGTTTGCCCTGAGGG-3′ | 5′-CCCTCAGGGCAAACCTGAGATATCACACTTCAGATC-3′ |
| IL8 mκB | 5′-GGGCCATCAGTTGCAAATCGTTAACTTTCCTCTGAC-3′ | 5′-GTCAGAGGAAAGTTAACGATTTGCAACTGATGGCCC-3′ |
| IL8 mCEBP | 5′-GGGCCATCAGCTACGAGTCGTGGAATTTCCTCTGAC-3′ | 5′-GTCAGAGGAAATTCCACGACTCGTAGCTGATGGCCC-3′ |
2.4. Transient Transfection
Myocytes were grown in 24-well plates to 80% confluence. Transient transfections were performed using FuGene 6 transfection reagent (Roche, Indianapolis, IN, USA). Cytomegalovirus-Renilla vector (1/10th of reporter) was used to control for transfection efficiency and cell number. Luciferase reporter vectors containing either the wild-type IL8 promoter or the mNFκB (mutation of NFκB) or the mCEBP (mutation of CEBP) or the mAP-1 (mutation of AP-1) were transfected at 0.2 μg/well. Cells were cultured for a total of 48 h (including 24 h of IL1B stimulation for half the experiment) followed by harvesting and analysis with a dual firefly/Renilla luciferase assay (Luclite, Packard Bell, and Coelenterazine CN Biosciences). Transfections were performed in triplicates. Luciferase/Renilla activity was measured 48 hours after transfection.
2.5. Chromatin Immunoprecipitation (ChIP)
DNA-protein complexes were crosslinked in situ with 1% formaldehyde. The cells were lysed, and chromatin was sheared into 200 to 1000 bp fragments by sonication. Antibodies recognizing the C-terminal of RELA, CEBPβ (Santa Cruz Biochemicals, SC-8008 and SC-7962 resp.), and acetyl-histone H4 (Upstate cell signalling solutions, 06-866) were used (1 : 1000 dilution) for immunoprecipitation, and the chromatin fragments containing the crosslinked protein were purified by immunoabsorption and elution from protein A/G beads. The crosslinks were reversed, and the DNA was purified using a QIAquick nucleotide removal kit (QIAGEN Inc., Valencia, CA, USA). The DNA region crosslinked to the protein was determined by PCR analysis. PCR was performed using AmpliTaq Gold DNA polymerase (Applied Biosystems Ltd.). Pre-PCR cycle was 10 min at 95°C followed by 35 cycles of 95°C for 1 min, 56–60°C for 1 min, and 72°C for 1 min followed by final extension at 72°C for 10 min. The primers are shown in Table 2.
Table 2.
IL8 ChIP primer covering bps −177 to +22.
| Forward primer sequence | Reverse primer sequence |
|---|---|
| 5′-GAAAACTTTCGTCATACTCCG-3′ | 3′-GAAAGTTTGTGCCTTATGGAG-5′ |
2.6. siRNA Transfection
ON-TARGETplus SMART pool human NFκBp65, CEBPβ, CEBPδ, c-Fos, and c-Jun siRNAs (Dharmacon) were used. SiGLO (Dharmacon) was used as a positive control, giving a high transfection efficiency of approximately 90%, and ON-TARGETplus nontargeting pool (Dharmacon) was used as a negative control. The siRNAs were transfected using DharmaFECT 2 (Dharmacon) transfection reagent at a final concentration of 100 nM according to manufacturer's instructions. Briefly, cells were grown to 80% confluence. Two separate tubes were used for siRNA preparation. Tube one contained the siRNA diluted in phenol-red-free DMEM, and tube two contained the transfection reagent diluted in phenol-red-free DMEM. Each tube was incubated at room temperature for 5 min before mixing the contents of the two tubes. The mixture was incubated at room temperature for an additional 20 min. Prewarmed DMEM containing 10% FBS and 2 mM L-glutamine, but no antibiotics were then added to the mixture. The culture media was removed from the cells, and the cells were fed with the siRNA mixture. The media was changed to DMEM containing 10% FBS, 2 mM L-glutamine, and 100 U/mL penicillin-streptomycin 24 hours after the transfection. The siRNA transfections were performed at different time courses (12, 24, 48, and 72 h and 5, 6, and 7 days). The changes were of the same pattern at all time points, but maximal response was observed at 5 days. The cell viability was monitored closely before harvesting the cells for appropriate analysis.
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
At the end of incubation assays, 1 mL of medium was collected and immediately frozen at −80°C for analysis by IL8 ELISA kit (Biosource, KHC0081). The IL8 ELISA had a sensitivity of 5 pg/mL; the inter- and intra assay variations were 7.8% and 5.3%, respectively. ELISA data were normalised to cellular protein. Protein concentrations were determined using detergent-compatible protein assay reagents (Bio-Rad Laboratories).
2.8. Statistical Analysis
Data are expressed as mean ± SEM. Statistical analysis was performed by ANOVA with the level of significance defined as P < 0.05.
3. Results
3.1. IL1B Stimulates Binding of NFκB, CEBP, and AP-1 to the IL8 Promoter
ChIP studies showed that IL1B induces the binding of NFκBp65, CEBP and AP-1 to the endogenous IL8 promoter. IL1B stimulated the recruitment of NFκBp65, CEBPβ, and -δ, cFos, and cJun to their respective cis-elements in the transcriptional enhanceosome at 6 hrs (Figure 1(b)). This was associated with concomitant acetylation of H4 at that region of the promoter.
3.2. Site-Directed Mutagenesis Reveals an Essential Role for NFκB in the Regulation of IL8 in Human Myometrium
A sequence extending from −135 to +46 which contains the transcription enhanceosome was used as a wild-type control. Site-directed mutagenesis was performed to make three further constructs with mutations for each of the NFκB, CEBP, or AP-1 sites individually (Figure 2). Basal luciferase activity following transient transfection of the wild-type construct was significantly increased by 2-fold upon IL1B stimulation. In primary human myometrial cells, mutation of the NFκB site reduced the unstimulated reporter activity to 10% of that of the wild type and obliterated the response to IL1B. Mutation of the CEBP site had no effect on basal promoter activity but did abolish the response to IL1B. Mutation of the AP-1 site decreased basal IL8 promoter activity although a response to IL1B stimulation was retained (Figure 2).
Figure 2.
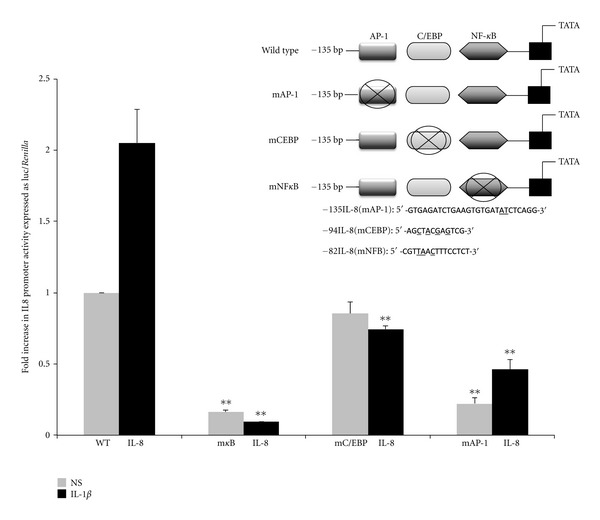
Representation of the IL8 promoter region and the mutations used in this study, as indicated by the underlined nucleotides. mAP-1: mutation of AP-1. mNFκB: mutation of NFκB and mCEBP: mutation of CEBP binding site. Functional effect of site-directed mutagenesis of transcription factor binding sites in the IL8 promoter in primary human myometrial cells. Data are presented as mean ± SEM (n = 4). An Anova test was performed with a Dunnett's multiple comparison post-test. **indicates a significant difference of P less than 0.01 compared to WT promoter.
3.3. Small Interfering RNA (siRNA) Knockdown of NFκB, CEBP, or AP-1 Demonstrates a Central Role for NFκB in the Expression of IL8
Knockdown mediated by siRNA against NFκBp65, CEBPβ, c-Jun, and c-Fos was performed with protein expression studies showing highly-specific and better than 95% gene silencing (Figure 3(a)). The effect of siRNA knockdowns against NFκBp65, CEBPβ, c-Jun, and c-Fos was measured on IL8 protein production and its response to IL1B stimulation (for 24 h) using ELISA.
Figure 3.
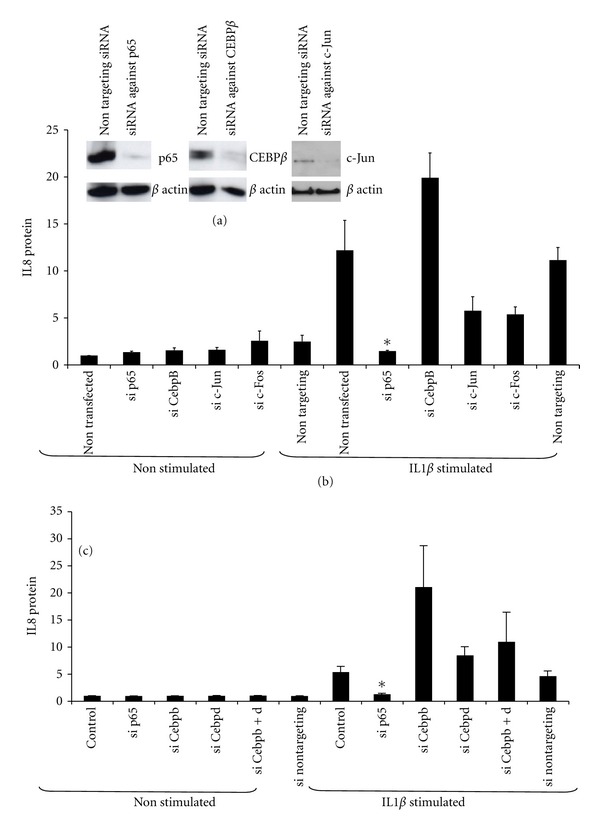
(a) siRNA knockdown of p65 (a), CEBPβ (b), and c-Jun (c). (b) The effects of siRNA against NFκBp65, CEBPβ, c-Jun, and c-Fos on IL8 protein production in a nonstimulated and IL1B-stimulated state. Data is normalised to the nontargeting control. Data are presented as mean ± SEM (n = 4). An ANOVA was performed with a Dunnett's multiple comparison post-test. *Indicates a significant difference of P less than 0.05. (c) The effects of siRNA against NFκBp65, CEBPβ, and CEBPD on IL8 protein production in a nonstimulated and IL1B-stimulated state. Data is normalised to the non-targeting control. Data are presented as mean ± SEM (n = 4). An ANOVA was performed with a Dunnett's multiple comparison post-test. *Indicates a significant difference of P less than 0.05.
Silencing of each individual transcription factor had no effect on basal IL8 protein release into the culture medium, measured by ELISA. Knock-down NFκBp65 abolished the response to IL1B significantly. Knockdown of CEBPβ increased the response to IL1B by 1.5-fold whilst c-Jun and c-Fos knockdown reduced the response by half (Figure 3(b)). Since CEBPδ may also bind to the IL8 promoter (see Figure 1), we repeated the experiment using siRNA knockdown of CEBPδ which also increased the response to IL1B by 1.5-fold as with knockdown of CEBPβ (Figure 3(c)).
4. Discussion
NFκB, CEBP, and AP-1 are important transcription factor families that are involved in immune and inflammatory functions as well as in cell growth and differentiation. NFκB, CEBP, and AP-1 are each expressed in human myometrium, and their expression is increased in response to the proinflammatory cytokine, IL1B. Labour-associated genes such as PGHS-2, OTR, IL8, IL6, and TNF-α have been shown to be regulated by either one or a combination of theses transcription factors [14, 16, 20, 21, 24–29].
Targeting activators of inflammation has been proposed as a strategy for prevention or delay of preterm birth and to improve outcome in preterm neonates. Condon et al. have demonstrated a central role for NFκB in the onset of term labour in the mouse, and that inhibition of NFκB can delay parturition [30]. Drugs that act through inhibition of NFκB are available. For example, sulfasalazine is a synthetic anti-inflammatory drug comprising 5-aminosalicylic acid (5-ASA), linked to sulfapyridine, which is routinely used for the treatment of inflammatory bowel disease and rheumatoid arthritis. This has been shown to inhibit labour-associated gene expression in cell culture models [31, 32], although there are currently no animal or clinical studies. In a mouse model of inflammation-induced preterm labour the cyclopentenone prostaglandin J2, which acts via inhibition of NFκB, both delays preterm birth and improves pup survival [33]. Glucocorticoids such as dexamethasone or prednisolone have well-established anti-inflammatory and immunosuppressive activities through their inhibitory effects on AP-1 and NFκB pathways [34] although there is no evidence that these drugs will delay or prevent preterm birth. Progesterone, which, when used clinically, can reduce the risk of preterm labour, has been shown to repress IL1B-induced expression of NFκB regulated genes in human amnion and myometrium [35, 36].
In this study, we examined the role of NFκB, CEBP, and AP-1 in regulation of IL8 expression in human myometrium and used IL1B to mimic the effects of inflammation seen with human labour. We used ChIP to show that IL1B induces the binding of all three transcription factors to the IL8 promoter. This is consistent with the observations of Soloff et al. that IL1B increases both NFκBp65 and CEBPβ binding to the endogenous IL8 promoter in myometrial cells [24]. Studies in other cell types have shown different patterns of binding. For example in a study in human conjunctiva epithelial cells ChIP showed no binding of NFκB, CEBP, or AP-1 alone, but when immunoprecipitation was performed for both NFκB and CEBP simultaneously, ChIP showed binding of both to the IL8 promoter [37]. Conversely treatment of human airway smooth muscle cells with tryptase, which mimics inflammation, induced the binding of all three transcription factors to the promoter [38].
Since we found that, in human myometrial cells all three transcription factors bound to the IL8 promoter, we considered two approaches to identify the relative importance of each transcription factor in IL8 expression. First we used site-directed mutagenesis to alter the binding sites for AP1, CEBP, and NFκB. We found that mutating both the NFκB and AP-1 sites markedly reduced both basal and stimulated IL8 promoter activity. However, mutating the CEBP site did not change the basal promoter activity but did completely inhibit the IL1B-induced increase in promoter activity. These data suggest that the NFκB and AP-1 binding sites are essential for both basal and IL1B-stimulated IL8 expression. In contrast, it appeared that CEBP binding sites are not important for basal promoter activity but are essential for the IL1B-induced increase in promoter activity. Using a similar approach in human amnion and cervical epithelial cells, Elliott et al. found similar results for the NFκB binding site but found no effect of mutating the CEBP or AP-1 binding sites on the IL8 promoter activity [14]. However, site-directed mutagenesis of DNA binding sites gives limited information about the role and importance of transcription factors, particularly where, as in the IL8 promoter, those sites are close to each other or overlap. Therefore in an alternative approach we used the more novel technique of siRNA against AP-1, CEBP, and NFκB to knock down the expression of their endogenous proteins. The data generated by siRNA knockdown is probably the most reliable in terms of the role of individual transcription factors since it is highly specific. In addition, the readout is activity of the endogenous promoter, not a transfected construct. In contrast to the finding from the site-directed mutation studies, basal IL8 levels were not reduced by knockdown of any of the transcription factors. This may be because although we achieved 90% knockdown, this was not complete. However, knockdown of p65 did completely inhibit the IL1B-stimulated increase in IL8 levels. The effects of c-Jun and c-Fos knockdown were less marked but still inhibitory. CEBP knockdown did not inhibit the IL1B-induced increase in IL8 expression and actually tended to increase it. In some cell types CEBP has been shown to inhibit the action of NFκB in regulation of IL8. Our knock-out data suggests that this phenomenon may apply in myometrium. Certainly our data does not point to a central positive role for CEBP in driving IL8 expression.
Our data show that, in human myometrial cells, NFκB is essential for IL8 expression; AP-1 plays a less important but still stimulatory role, while the role of CEBP is less clear. The site-directed mutagenesis studies suggested a positive role in IL8 expression; however, the NFκB and CEBP binding sites are close together, and it remains possible that mutation of the CEBP site may have interfered with NFκB binding or function. The probably more reliable data from siRNA would suggest that pharmacological inhibition of CEBP might enhance IL8 expression and may therefore not be beneficial. The dominant role played by NFκB in IL8 expression confirms that it is an attractive therapeutic target for the prevention of preterm labour.
5. Conclusion
These data show that NFκB is essential for IL8 myometrial expression, AP-1 plays a less important but still stimulatory role, while the role of CEBP is less clear. These results provide further support for the notion that NFκB represents an attractive therapeutic target for the prevention of preterm labour.
Acknowledgment
This study was funded by the Action Medical Research ref. SP4241.
References
- 1.Bollopragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. American Journal of Obstetrics and Gynecology. 2009;200(1):104.e1–104.e11. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Mittal P, Romero R, Tarca AL, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. Journal of Perinatal Medicine. 2010;38(6):617–643. doi: 10.1515/JPM.2010.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tattersall M, Engineer N, Khanjani S, et al. Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction. 2008;135(4):569–579. doi: 10.1530/REP-07-0461. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. American Journal of Obstetrics and Gynecology. 1991;165(4):813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 5.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1β and tumour necrosis factor α in guinea-pigs. Human Reproduction. 1994;9(11):2173–2181. doi: 10.1093/oxfordjournals.humrep.a138413. [DOI] [PubMed] [Google Scholar]
- 6.El Maradny E, Kanayama N, Halim A, Maehara K, Sumimoto K, Terao T. Biochemical changes in the cervical tissue of rabbit induced by interleukin-8, interleukin-1β, dehydroepiandrosterone sulphate and prostaglandin E2: a comparative study. Human Reproduction. 1996;11(5):1099–1104. doi: 10.1093/oxfordjournals.humrep.a019304. [DOI] [PubMed] [Google Scholar]
- 7.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Molecular Human Reproduction. 2003;9(1):41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 8.Hua R, Pease JE, Sooranna SR, et al. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-kappaB activation. Endocrinology. 2012;153:481–491. doi: 10.1210/en.2011-1506. [DOI] [PubMed] [Google Scholar]
- 9.Sooranna SR, Engineer N, Loudon JAZ, Terzidou V, Bennett PR, Johnson MR. The mitogen-activated protein kinase dependent expression of prostaglandin H synthase-2 and interleukin-8 messenger ribonucleic acid by myometrial cells: the differential effect of stretch and interleukin-1β . The Journal of Clinical Endocrinology & Metabolism. 2005;90(6):3517–3527. doi: 10.1210/jc.2004-1390. [DOI] [PubMed] [Google Scholar]
- 10.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-κB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. The Journal of Biological Chemistry. 1990;265(34):21128–21133. [PubMed] [Google Scholar]
- 11.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. Journal of Leukocyte Biology. 1994;56(5):554–558. [PubMed] [Google Scholar]
- 12.Harant H, De Martin R, Andrew PJ, Foglar E, Dittrich C, Lindley IJD. Synergistic activation of interleukin-8 gene transcription by all- trans-retinoic acid and tumor necrosis factor-α involves the transcription factor NF-κB. The Journal of Biological Chemistry. 1996;271(43):26954–26961. doi: 10.1074/jbc.271.43.26954. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. Journal of Leukocyte Biology. 2002;72(5):847–855. [PubMed] [Google Scholar]
- 14.Elliott CL, Allport VC, Loudon JAZ, Wu GD, Bennett PR. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Molecular Human Reproduction. 2001;7(8):787–790. doi: 10.1093/molehr/7.8.787. [DOI] [PubMed] [Google Scholar]
- 15.Edwards MR, Mukaida N, Johnson M, Johnston SL. IL-1β induces IL-8 in bronchial cells via NF-κB and NF-IL6 transcription factors and can be suppressed by glucocorticoids. Pulmonary Pharmacology and Therapeutics. 2005;18(5):337–345. doi: 10.1016/j.pupt.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Shi Q, Le X, Abbruzzese JL, et al. Cooperation between transcription factor AP-1 and NF-κB in the induction of interleukin-8 in human pancreatic adenocarcinoma cells by hypoxia. Journal of Interferon and Cytokine Research. 1999;19(12):1363–1371. doi: 10.1089/107999099312821. [DOI] [PubMed] [Google Scholar]
- 17.Roger T, Out TA, Mukaida N, Matsushima K, Jansen HM, Lutter R. Enhanced AP-1 and NF-κB activities and stability of interleukin 8 (IL-8) transcripts are implicated in IL-8 mRNA superinduction in lung epithelial H292 cells. Biochemical Journal. 1998;330, part 1:429–435. doi: 10.1042/bj3300429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann E, Thiefes A, Buhrow D, et al. MEK1-dependent delayed expression of fos-related antigen-1 counteracts c-Fos and p65 NF-κB-mediated interleukin-8 transcription in response to cytokines or growth factors. The Journal of Biological Chemistry. 2005;280(10):9706–9718. doi: 10.1074/jbc.M407071200. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SA, Tummuru MKR, Blaser MJ, Kerr LD. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. The Journal of Immunology. 1998;160(5):2401–2407. [PubMed] [Google Scholar]
- 20.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (ReIA) and NF-IL-6. The Journal of Immunology. 1994;153(1):153–164. [PubMed] [Google Scholar]
- 21.Matsusaka T, Fujikawa K, Nishio Y, et al. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(21):10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terzidou V, Sooranna SR, Kim LU, Thornton S, Bennett PR, Johnson MR. Mechanical stretch up-regulates the human oxytocin receptor in primary human uterine myocytes. The Journal of Clinical Endocrinology & Metabolism. 2005;90(1):237–246. doi: 10.1210/jc.2004-0277. [DOI] [PubMed] [Google Scholar]
- 23.Loudon JAZ, Sooranna SR, Bennett PR, Johnson MR. Mechanical stretch of human uterine smooth muscle cells increases IL-8 mRNA expression and peptide synthesis. Molecular Human Reproduction. 2004;10(12):895–899. doi: 10.1093/molehr/gah112. [DOI] [PubMed] [Google Scholar]
- 24.Soloff MS, Cook DL, Jr., Jeng YJ, Anderson GD. In situ analysis of interleukin-1-induced transcription of cox-2 and il-8 in cultured human myometrial cells. Endocrinology. 2004;145(3):1248–1254. doi: 10.1210/en.2003-1310. [DOI] [PubMed] [Google Scholar]
- 25.Terzidou V, Lee Y, Lindstrom T, Johnson M, Thornton S, Bennett PR. Regulation of the human oxytocin receptor by nuclear factor-kappaB and CCAAT/enhancer-binding protein-beta. The Journal of Clinical Endocrinology & Metabolism. 2006;91:2317–2326. doi: 10.1210/jc.2005-2649. [DOI] [PubMed] [Google Scholar]
- 26.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Molecular and Cellular Biology. 1990;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanjani S, Kandola MK, Lindstrom TM, et al. NF-κB regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. Journal of Cellular and Molecular Medicine. 2011;15(4):809–824. doi: 10.1111/j.1582-4934.2010.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Molecular and Cellular Biology. 1990;10(4):1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Molecular Human Reproduction. 2004;10(2):109–113. doi: 10.1093/molehr/gah021. [DOI] [PubMed] [Google Scholar]
- 30.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):4978–4983. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor Kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biology of Reproduction. 2002;67(2):668–673. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- 32.Keelan JA, Khan S, Yosaatmadja F, Mitchell MD. Prevention of inflammatory activation of human gestational membranes in an ex vivo model using a pharmacological NF-κB inhibitor. The Journal of Immunology. 2009;183(8):5270–5278. doi: 10.4049/jimmunol.0802660. [DOI] [PubMed] [Google Scholar]
- 33.Pirianov G, Waddington SN, Lindström TM, Terzidou V, Mehmet H, Bennett PR. The cyclopentenone 15-deoxy-δ 12,14-prostaglandin J2 delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2009;150(2):699–706. doi: 10.1210/en.2008-1178. [DOI] [PubMed] [Google Scholar]
- 34.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocrine Reviews. 2003;24(4):488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 35.Loudon JAZ, Elliott CL, Hills F, Bennett PR. Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biology of Reproduction. 2003;69(1):331–337. doi: 10.1095/biolreprod.102.013698. [DOI] [PubMed] [Google Scholar]
- 36.Dodd JM, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth. Cochrane Database of Systematic Reviews. 2006;(1) doi: 10.1002/14651858.CD004947.pub2. Article ID CD004947. [DOI] [PubMed] [Google Scholar]
- 37.Venza I, Cucinotta M, Visalli M, De Grazia G, Oliva S, Teti D. Pseudomonas aeruginosa induces interleukin-8 (IL-8) gene expression in human conjunctiva through the recruitment of both RelA and CCAAT/enhancer-binding protein β to the IL-8 promoter. The Journal of Biological Chemistry. 2009;284(7):4191–4199. doi: 10.1074/jbc.M805429200. [DOI] [PubMed] [Google Scholar]
- 38.Mullan CS, Riley M, Clarke D, et al. β-tryptase regulates IL-8 expression in airway smooth muscle cells by a PAR-2-independent mechanism. American Journal of Respiratory Cell and Molecular Biology. 2008;38(5):600–608. doi: 10.1165/rcmb.2007-0082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


