Abstract
Background
Fish oil, a rich source of omega-3 fatty acids, has never been used as the sole source of lipid in clinical practice for fear of development of essential fatty acid deficiency, as it lacks the believed requisite levels of linoleic acid, an omega-6 fatty acid. The objectives of this study were to establish biochemical standards for fish oil as the sole fat and to test the hypothesis that fish oil contains adequate amounts of omega-6 fatty acids to prevent essential fatty acid deficiency.
Methods
Forty mice were divided into two groups that were either pair fed or allowed to eat ad libitum. In each group, four subgroups of five mice were fed 1%, 5%, and 10% fish oil diets by weight or a control soybean diet for nine weeks. Blood was collected at four time points and fatty acid analysis was performed. Food intake and weight status were monitored.
Results
All groups but the pair fed 1% fish oil group gained weight and the 5% fish oil group showed the highest caloric efficiency in both pair fed and ad libitum groups. Fatty acid profiles for the 1% fish oil group displayed clear essential fatty acid deficiency, 5% fish oil appeared marginal, and 10% and soybean oil diets were found to prevent essential fatty acid deficiency.
Conclusion
Fish oil enhances growth through higher caloric efficiency. We established a total omega-6 fatty acid requirement of between 0.30% and 0.56% of dietary energy, approximately half of the conventionally believed 1% as linoleic acid. This can presumably be attributed to the fact that fish oil contains not only a small amount of linoleic acid, but also arachidonic acid, which has greater efficiency to meet omega-6 fatty acid requirements.
Keywords: menhaden oil, fish oil, omega-3 fatty acid, omega-6 fatty acid, triene-tetraene ratio, essential fatty acid deficiency, arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid
Introduction
Fatty acids are major cellular constituents that form integral parts of the cell membrane and impact the membrane's fluidity and function. Within the plasma lipoprotein particles, fatty acids serve as the major constituents of phospholipids, triglycerides and cholesterol esters. In mammalian cells, there are three important types of fatty acids: omega-3, omega-6, and omega-9. Both the omega-3 and omega-6 fatty acids are considered essential fatty acids (EFAs) in mammals as they cannot insert double bonds at postion-3 and -6 to produce alpha linolenic acid (ALA) and linoleic acid (LA) respectively and thus must be obtained from the diet. Cell membrane composition is determined by dietary intake of either of these EFAs. More importantly LA and ALA are essential nutrients in that all downstream polyunsaturated fatty acids can be synthesized from them. Among these downstream products are several highly physiologically relevant fatty acids: arachidonic acid (AA, derived from LA), docosahexaenoic acid (DHA, derived from ALA), and eicosapentaenoic acid (EPA, derived from ALA). (1) These are considered to be critical metabolites as they are important eicosanoid and prostanoid precursors. Currently the suggested ratio of omega-6 to omega-3 fatty acids is variable and without consensus. Previous recommendation suggest a balance of 10:1 although emerging data indicates that a ratio as low as 2:1 may be optimal. (2,3)
Essential fatty acid deficiency (EFAD) results from low dietary intake, severe malabsorption, and/or increased physical requirements such as growth. (4) In 1971, Holman described the symptoms of EFAD in rats and other mammalian species, including primarily impaired growth and dermatitis, and secondarily steatosis, renal toxicity, pulmonary abnormalities, and increased metabolic rate. (5) In EFAD, tissue levels of both omega-3 and omega-6 fatty acids are diminished. The major biochemical changes of EFAD are decreased arachidonic acid (AA) and increased Mead acid, the latter being a downstream product of oleic acid, an omega-9 fatty acid. Desaturase enzymes display differential activity in the order of preference omega-3 > omega-6 > omega-9. Normally linoleic acid would be converted to AA, a tetraene; however in times of deficiency, de novo lipogenesis occurs, resulting the conversion of oleic acid by elongation and desaturation to Mead acid, a triene. As a result, conversion of oleic acid to Mead acid only occurs when there are low dietary levels of both ALA and LA. This metabolic switch is seen as a compensatory mechanism to maintain the number of double bonds in cell membrane fatty acids. Therefore, elevated Mead acid in conjunction with a lowered AA has been associated with EFAD. (6, 7) Until recently, plasma ratios greater than 0.2 were considered abnormal while levels >0.4 were considered diagnostic for EFAD. (8) Currently, triene-tetraene ratios no greater than 0.2 have been suggested as the average ratio in Western populations, as diets rich in omega-6 fatty acids resulted in ratios that were found to be only 0.1 ± 0.08. (9) The triene-tetraene ratio does not reflect omega-3 fatty acid status. Standard minimum intake to meet LA requirements is set to 1% of total caloric intake in animal studies. In certain conditions, AA, EPA, and DHA may be considered conditionally essential fatty acids, as their production may be inadequate. Young animals deprived of dietary intake of LA and ALA rapidly display adverse effects such as diminished growth, liver and kidney damage, dermatitis and eventually death. Human studies have shown that estimated optimal daily requirements of LA are 1–3% of total caloric intake, increasing proportionally with growth. (5, 10,) The minimum ALA acid concentrations in the diet have been reported to be 0.2–1% of total caloric intake in adults and 0.5% in infants and young children. (11–14) Despite their conditionally essential status, no similar dietary recommendations have been made for AA, EPA or DHA.
Renewed interest in the clinical use of fish oil has appeared due to their high omega-3 fatty acid content. (15) Although the body is able to synthesize AA from LA, and EPA and DHA from ALA, unlike other oils, fish oil appears to be a more efficient source of these fatty acids as it does not rely on conversion from their 18 carbon precursors. However, concerns have emerged that diets exclusive in this fat source may predispose patients to EFAD and subsequent growth failure as fish oil does not fulfill the aforementioned 1% minimum requirement of LA. Recent reports involving parenterally fed pediatric patients receiving diets using fish oil as their only fat source suggest otherwise. (16–18) In each instance, patients with preexisting EFAD had their condition reversed or prevented with fish oil and growth was maintained.
The objectives of this study were to establish biochemical standards and essential fatty acid profiles for diets with differing lipid compositions. We hypothesized that menhaden fish oil contains sufficient amounts of EPA, DHA, and AA to prevent biochemical and clinical EFAD.
Materials and methods
Nutritional model
Animal protocols complied with the NIH Animal Research Advisory Committee guidelines and were approved by the Children’s Hospital Boston Animal Care and Use Committee.
Forty 6–8 week old C57/Bl6 mice (Jackson Laboratories, Bar Harbor, ME) were housed in a barrier room. Prior to the initiation of the study mice were fed a baseline chow (Prolab Isopro, RMH 3000 #25, Prolabs Purina, Richmond, IN). After three days of acclimatization, they were divided into two groups, ad libitum (group I) and pair fed (group II). These groups were further divided into four subgroups of five animals each. These four subgroups were fed identical diets that only differed in fat composition (Table 1). Water was provided ad libitum and none of the groups received any additional source of nutrition. Chow with three different percentages of fish-derived fat (1%, 5%, and 10%) (Dyets Incorporated, Bethlehem, PA) were utilized for the first three subgroups and the fourth subgroup was fed 5% soybean oil chow (Dyets Incorporated, Bethlehem, PA) (Table 1). The base diet used to develop the specialty diets consisted of casein, cornstarch, dextrose, sucrose, cellulose, t-butylhydroquinone, salt mix, vitamin mix, L-cystine, and choline bitartrate. Menhaden oil obtained from the Omega Protein Corporation in Reedville, VA served as the source of the fish oil used to develop these diets. The fat content of the baseline chow was 11.8% and its fatty acid composition is detailed in Table 1, along with the fatty acid composition of the fish oil and soybean oil. In addition to the menhaden oil, the casein and cornstarch components of the diet had a minimal contribution to the total fat content of the chow, which should be noted. Specifically, an additional 0.0357 kcal/gm, 0.0346 kcal/gm, and 0.0309 kcal/gm of energy was derived from the 1%, 5% and 10% menhaden chow respectively. The contribution to total LA acid content from the casein and cornstarch was 0.00781 kcal/gm, 0.00733 kcal/gm, and 0.00562 kcal/gm for the three chow types.
Table 1.
Relevant percentages of fatty acid content between menhaden and soybean oil.
| Fat composition, wt% | Menhaden | Soybean | Baseline Chow |
|---|---|---|---|
| oil | oil | ||
| Palmitic acid (C16:0) | 17.1 | 10.2 | 18.1 |
| Stearic acid (C18:0) | 2.8 | 4.5 | 6.04 |
| Oleic acid (C18:1) | 11.4 | 22.7 | 22.7 |
| Linoleic acid (LA) (C18:2) | 1.5 | 54.8 | 37.7 |
| Arachidonic acid (C20:4) | 0.9 | - | 0.190 |
| α-Linolenic acid (ALA) (C18:3) | 1.6 | 7.8 | 3.71 |
| Eicosapentaenoic acid (C20:5) | 15.5 | - | 1.04 |
| Docosahexaenoic acid (DHA) (C22:6) | 9.1 | - | 1.13 |
All chow was stored at 4° Celsius in accordance with the manufacturer’s instructions to maintain freshness and minimize autooxidation. When present, residual chow in each cage was weighed, discarded, and replaced with fresh chow every 48 hours. Note that the dietary fat percentages are percentages by weight; percentage of energy translates to 2.6%, 12.5% and 23% of energy for 1%, 5% and 10% fish oil (Dyets Incorporated, Bethlehem, PA) respectively and 12.5% of energy in the soybean diet. These discrepancies evolve out of the 2.5 times higher energy value of fish and soybean oil compared to the other energy providers in the diet, cornstarch and sucrose.
Corn starch was utilized to ensure isocaloric diets. The 5% fish oil diet was taken as the caloric point of reference and as such, the control soybean oil content was set to 5%. Using this method, the discrepancies in caloric density between all diets was minimized to ≤ 0.5 kcal/g. The total dietary caloric content was 3.4, 3.6, and 3.9 kcal/g in the 1%, 5%, and 10% fish oil chow, respectively, and 3.6 kcal/g in the 5% soybean oil diet.
To avoid differential food aversion bias amongst the animals and to examine possible metabolic differences, a parallel pair fed and ad libitum feeding model was used. Our technique of pair feeding was based on previously described methods (19, 20). All groups ate ad libitum the first day and the amount of chow ingested was measured. Among the four subgroups, the group with the least amount of chow ingested was identified. To allow for increasing nutritional requirements of growth, 10% was added onto this total to determine the amount of food to be administered to all groups the following day. The time course of the experiment was nine weeks. The ad libitum groups had access to unlimited chow at all times and the amounts eaten were recorded every three days. Both pair fed and ad libitum groups were weighed every three days.
Phlebotomy
At day 0 and weeks 3, 6, and 9 (end date), blood was drawn from all animals by retro-orbital puncture and placed in serum separator tubes. Isoflurane was used as general anesthetic during these procedures. Blood was centrifuged at 800 rpm for 10 minutes after which the serum was separated and stored at −80° C.
Fatty acid analysis
Fatty acid analysis was performed on all serum samples, as previously described (21). Serum samples were transferred to glass tubes, and 30 ml of 1 mg/ml 17:0 was added as an internal standard. Lipids were extracted by adding 6 volumes of chloroform/methanol (2:1). After centrifugation (800 g for 4 min), the lower phase was transferred to a new tube. The samples were then dried under nitrogen gas and methylated as previously described, using boron trifluoride-methanol (21). Fatty acid methyl esters (FAMEs) were quantified using a 5890 Series II Hewlett Packard gas chromatograph equipped with a Supelcowax SP-10 capillary column (Supelco) (Hewlett-Packard 5890, Series II, Palo Alto, CA). FAME mass was determined by comparing the area of each FAME to that of a known amount of 17:0 and the molar values were calculated using the molecular weight of each fatty acid.
Statistical analysis
For statistical analysis of food intake in kilocalories and grams, we performed a paired t-test (Sigmastat 3.0). Calculations were also performed analyzing the amount of double bonds, using a Holm-Sidek comparison (two-way ANOVA).
From the fatty acid analysis profile, we excluded a total of eight fields from our relevant data as outliers using the Extreme Student Deviate (ESD) statistic within the upper 5th percentile (α = 0.05), as described by Rosner (22). Four baseline phospholipid EPA, one baseline triglyceride Mead acid, one 6-week triglyceride triene-tetraene ratio, and two phospholipid triene-tetraene ratios for baseline and 3-week, respectively, were eliminated from the data set before statistical analysis was conducted. Due to loss of phospholipid data in the fatty acid analysis of the baseline 10% ad libitum fish oil group, the retrieved values were insufficient to allow proper statistical analysis. Therefore, we decided to take the average of all baseline animals, not yet influenced by any means, to establish a realistic phospholipid baseline threshold for further comparisons over time within that group.
Indicator fatty acids, including Mead acid, AA, EPA, and DHA, and the triene-tetraene ratio were compared between the fish oil groups and controls using three-way repeated-measures mixed model analysis of variance (ANOVA). This statistical approach accounts for the multiple measurements over time within the same animals (correlated data) and the varying values of animals in the treatment groups (23). Diet and feeding mode (pair fed or ad libitum) were treated as between-subjects factor and time (baseline, 3, 6, and 9 weeks) as a within-subjects factor. Several different covariance structures were compared to determine optimal model fit, including compound symmetry and autoregression, and a diagonal structure fit the data best according to the Bayesian information criterion (BIC) (24). To account for multiple pairwise group comparisons, a Bonferroni adjusted p-value was used to protect against committing false positive errors (type I errors). Data for the triene-tetraene ratio for triglycerides and phospholipids are presented in terms of the estimated marginal means and standard errors. Statistical analysis was performed using the GLM (General Linear Model) procedure in the SPSS software package (version 15.0, SPSS Inc., Chicago, IL). All reported p-values are twotailed. Five mice were randomized to each condition (diet group × mode = 4 × 2 = 8 conditions) for both triglyceride and phospholipid (total N=80 samples). A power analysis indicated that the sample sizes of five mice in each of the diet groups and five controls measured at each of the time points for each of the feeding modes would provide 80% power to detect a mean difference of 0.05 in the triene-tetraene ratio between the fish oil diet groups and soybean controls using mixed-model ANOVA with repeated-measures (version 6.0, nQuery Advisor, Statistical Solutions, Saugus, MA).
Results
Animals
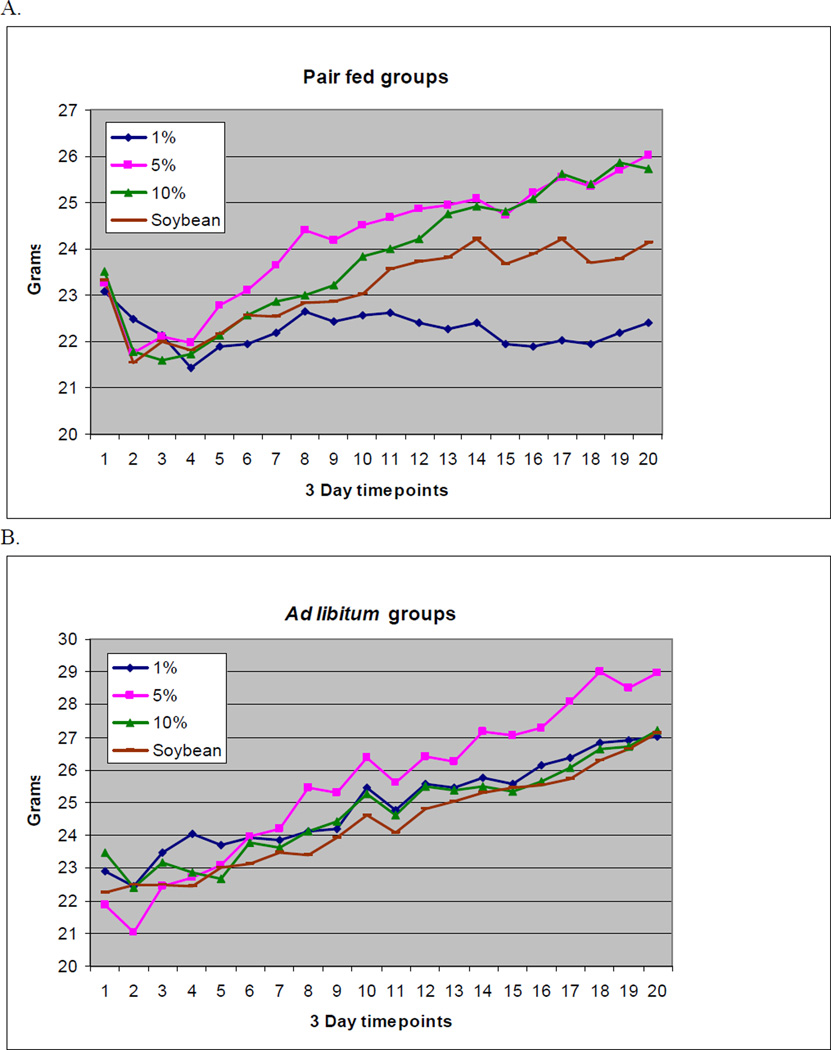
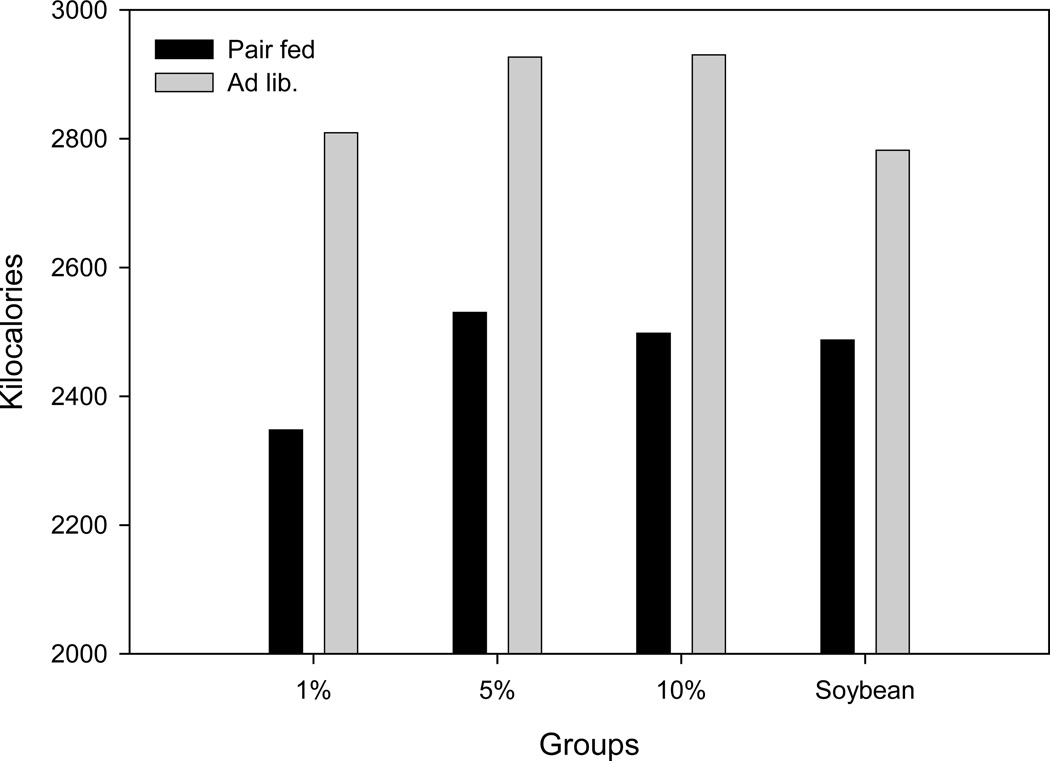
Throughout the nine-week experiment, all animals in both the pair fed and ad libitum groups were clinically well. None of the animals showed any physiologic signs of EFAD, such as dermatitis, alopecia, or infections. Although the development of dermatitis is a valid and sensitive physiologic measure of EFAD, animals in this study were not allowed to develop the extent of EFAD necessary for its development due to institutional animal research regulatory constraints. Growth retardation is another important clinical parameter of EFAD (25–27). Regarding this measure, all groups but the 1% pair fed group gained weight over the course of the study. In both pair fed and ad libitum groups, there was a transient weight loss at the initiation of dietary treatment (baseline). This weight loss was more severe and more prolonged in the pair fed groups (Figure 1). Total average weight differences and standard deviations after nine weeks compared to baseline for the pair fed 1%, 5%, and 10% fish oil groups and the 5% soybean group per mouse were: −0.68 ± 2.02, +2.78 ± 1.60, +2.24 ± 2.10, and +0.82 ± 0.81 g, respectively. For the ad libitum groups, weight change was: +4.12 ± 1.4, +7.1 ± 3.14, +3.74 ± 1.39 and +4.88 ± 0.81 grams for the same groups. In the ad libitum groups, significantly higher values overall were found compared to pair fed groups for intake in grams or intake converted to kilocalories (P = 0.002 for grams, P = 0.002 for kilocalories). In the pair fed groups, the 10% fish oil diet was usually the diet with the most residual chow remaining. Food intake of the pair fed 5%, 10% fish oil groups and soybean 5% groups was relatively isocaloric, whereas the 1% fish oil group intake was slightly decreased (Figure 2).
Figure 1.
Average weights for pair fed (A) and ad libitum (B) groups through the 9 week experiment (1).
(1) Diets are 1%, 5%, and 10% menhaden oil diets and soybean oil diet.
Figure 2.
Total food intake in kilocalories by pair fed and ad libitum groups (1).
(1) Diets are 1%, 5%, and 10% menhaden oil diets and soybean oil diet.
Caloric efficiency
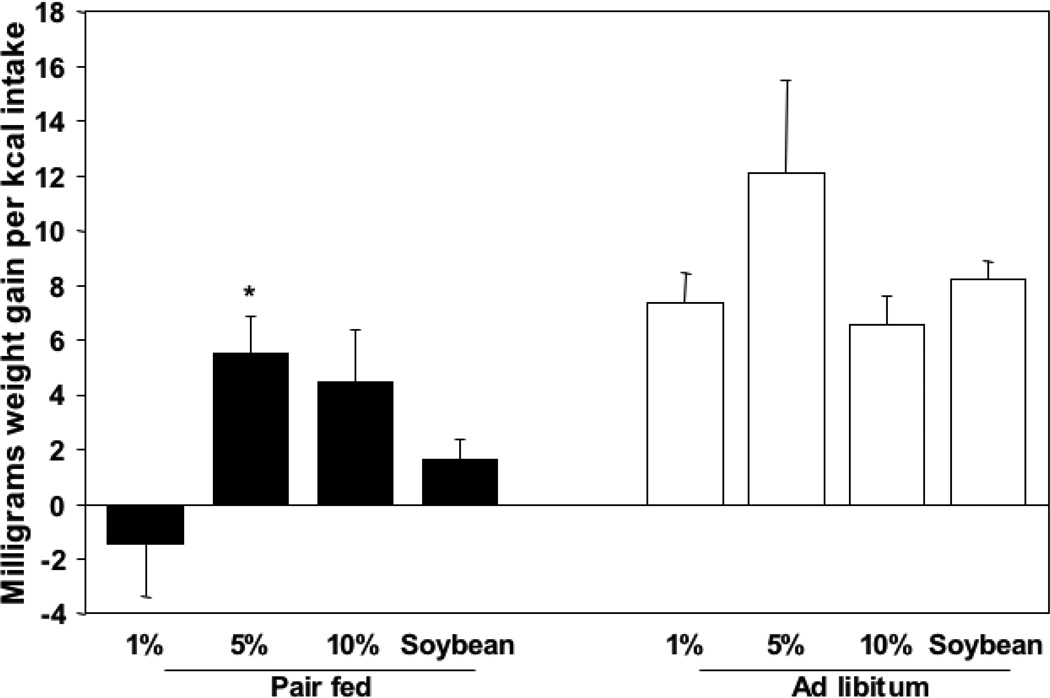
To look at growth parameters for each individual diet over the nine-week experimental period, we divided the total weight gain per group by the total intake in kilocalories of that group. This established a quantification of caloric efficiency per corresponding diet in milligrams per kilocalorie (Figure 3). In the pair fed groups, there was a negative value for the 1% fish oil diet (−1.45 mg/kcal), reflecting the weight loss of this group reported earlier. The pair fed 5% fish oil group showed the highest caloric efficiency at 5.5 mg/kcal, followed by the 10% group at 4.5 mg/kcal. The soybean control diet showed a caloric efficiency of 1.6 mg/kcal. There was a statistically significant difference among all diet groups (P = 0.027, One way ANOVA) and only the 5% fish oil was significantly higher when compared to the soybean oil control (P = 0.042, t-test).
Figure 3.
Caloric efficiency in milligrams weight gain per kilocalorie intake per mouse (1,2).
(1) There is a statistically significant difference among pair fed (P = 0.027) but not among ad libitum groups (P = 0.202) (One way ANOVA).
(2) * Statistically higher than soybean control group (P = 0.042) within pair fed groups (t-test).
The ad libitum group had improved caloric efficiency among all diets. The 5% fish oil group again scored the highest with 12.1 mg/kcal. The 1% fish oil and soybean oil groups had caloric efficiencies of 7.4 and 8.8 mg/kcal, respectively, moderately increased over pair fed, whereas the 10% was 6.4 mg/kcal, minimally increased over pair fed. No statistical significance was shown.
Fatty acid analysis
The fatty acid analysis on the blood samples taken every three weeks allowed for comparisons of changes in the triglyceride and phospholipid levels of the relevant fatty acids as well as the triene-tetraene ratios over time (Table 2).
Table 2.
Fatty acid concentration in nmol/ml of relevant plasma triglycerides at baseline, 3, 6 and 9 week time points for pair fed groups (n=5) and different diets (1,2).
| Triglycerides and Phospholipids in Diets: | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1% TG | 1% PL | 5% TG | 5% PL | 10% TG | 10% PL | Soybean TG | Soybean PL | |
|
Arachidonic acid (20:4) |
||||||||
| Baseline | 0.2873 ± 0.062 | 0.2535 ± 0.012 | 0.2140 ± 0.010 | 0.2468 ± 0.015 | 0.2887 ± 0.056 | 0.2507 ± 0.031 | 0.2651 ± 0.047 | 0.2288 ± 0.025 |
| 3 weeks | 0.1876 ± 0.022 | 0.2037 ± 0.034 d | 0.1545 ± 0.017 d | 0.1461 ± 0.014 d | 0.0690 ± 0.009 d | 0.1484 ± 0.014 d | 0.2708 ± 0.051 b, c | 0.8958 ± 0.081 a, b, c |
| 6 weeks | 0.2196 ± 0.025 d | 0.2378 ± 0.004 d | 0.1115 ± 0.024 d | 0.1334 ± 0.018 d | 0.1449 ± 0.015 d | 0.1574 ± 0.004 d | 0.7150 ± 0.153 a, b, c | 0.7139 ± 0.032 a, b, c |
| 9 weeks | 0.1371 ± 0.010 | 0.1185 ± 0.006 d | 0.1254 ± 0.005 | 0.1466 ± 0.017 d | 0.0719 ± 0.016 d | 0.1264 ± 0.012 d | 0.2721 ± 0.102 c | 0.9973 ± 0.047 a, b, c |
|
Mead acid (20:3n-9) |
||||||||
| Baseline | 0.0024 ± 0.002 | 0.0000 ± 0.000 | 0.0019 ± 0.001 | 0.0369 ± 0.032 | 0.0056 ± 0.004 | 0.0029 ± 0.003 | 0.0155 ± 0.007 | 0.0025 ± 0.003 |
| 3 weeks | 0.0199 ± 0.005 b, c, d | 0.0050 ± 0.003 | 0.0020 ± 0.001 a | 0.0062 ± 0.006 | 0.0017 ± 0.000 a | 0.0023 ± 0.001 | 0.0026 ± 0.001 a | 0.0003 ± 0.000 |
| 6 weeks | 0.0210 ± 0.004 b, c, d | 0.0192 ± 0.006 b, c, d | 0.0020 ± 0.002 a | 0.0046 ± 0.003 a | 0.0015 ± 0.001 a | 0.0005 ± 0.000 a | 0.0000 ± 0.000 a | 0.0031 ± 0.001 a |
| 9 weeks | 0.0307 ± 0.004 b, c, d | 0.0156 ± 0.002 b, c, d | 0.0118 ± 0.007 a | 0.0012 ± 0.001 a | 0.0027 ± 0.002 a | 0.0008 ± 0.000 a | 0.0000 ± 0.000 a | 0.0002 ± 0.000 a |
|
Eicosapentaenoic acid (20:5) |
||||||||
| Baseline | 0.0747 ± 0.020 | 0.0489 ± 0.011 | 0.0408 ± 0.008 | 0.0464 ± 0.001 | 0.0735 ± 0.012 | 0.0597 ± 0.003 | 0.0739 ± 0.015 | 0.0511 ± 0.008 |
| 3 weeks | 0.2195 ± 0.035 d | 0.2462 ± 0.027 b, d | 0.4481 ± 0.064 d | 0.4152 ± 0.049 a, d | 0.2387 ± 0.032 d | 0.4660 ± 0.073 d | 0.0118 ± 0.001 a, b, c | 0.0240 ± 0.014 a, b, c |
| 6 weeks | 0.3691 ± 0.045 b, d | 0.5370 ± 0.027 b, d | 0.4365 ± 0.062 a, c, d | 0.3991 ± 0.062 a, c, d | 0.4509 ± 0.042 b, d | 0.5471 ± 0.045 b, d | 0.0148 ± 0.009 a, b, c | 0.0265 ± 0.003 a, b, c |
| 9 weeks | 0.2470 ± 0.023 b, d | 0.2600 ± 0.026 b, d | 0.3884 ± 0.027 a, c, d | 0.4353 ± 0.046 a, c, d | 0.1984 ± 0.043 b, d | 0.2779 ± 0.031 b, d | 0.0057 ± 0.002 a, b, c | 0.0026 ± 0.002 a, b, c |
|
Docosahexaenoic acid (22:6n-3) |
||||||||
| Baseline | 0.1539 ± 0.037 b | 0.2504 ± 0.042 | 0.0735 ± 0.030 a, c, d | 0.2607 ± 0.022 | 0.1730 ± 0.028 b | 0.2990 ± 0.030 | 0.1534 ± 0.017 b | 0.2967 ± 0.024 |
| 3 weeks | 0.1351 ± 0.016 b, d | 0.4014 ± 0.043 | 0.2582 ± 0.027 a, c, d | 0.4522 ± 0.057 | 0.1260 ± 0.011 b, d | 0.3612 ± 0.074 | 0.0446 ± 0.009 a, b, c | 0.3576 ± 0.036 |
| 6 weeks | 0.2281 ± 0.025 b, d | 0.5006 ± 0.028 d | 0.2630 ± 0.088 a, c, d | 0.4694 ± 0.072 | 0.3200 ± 0.047 b, d | 0.3730 ± 0.054 | 0.1126 ± 0.015 a, b, c | 0.1767 ± 0.033 a |
| 9 weeks | 0.1474 ± 0.012 d | 0.5181 ± 0.024 d | 0.1872 ± 0.007 d | 0.3914 ± 0.061 | 0.1466 ± 0.032 d | 0.4034 ± 0.023 | 0.0372 ± 0.0010 d | 0.3875 ± 0.020 a |
|
Triene-tetraene Ratios |
||||||||
| Baseline | 0.0050 ± 0.005 | 0.0000 ± 0.0000 | 0.0080 ± 0.006 | 0.0024 ± 0.002 | 0.0327 ± 0.025 | 0.0079 ± 0.008 | 0.0594 ± 0.026 | 0.0085 ± 0.009 |
| 3 weeks | 0.1049 ± 0.020 b, c, d | 0.0197 ± 0.0121 b, c, d | 0.0135 ± 0.006 a | 0.0183 ± 0.014 a | 0.0256 ± 0.005 a | 0.0191 ± 0.011 a | 0.0095 ± 0.002 a | 0.0004 ± 0.000 a |
| 6 weeks | 0.0929 ± 0.014 b, c, d | 0.0810 ± 0.0252 b, c, d | 0.0375 ± 0.029 a | 0.0327 ± 0.020 a | 0.0111 ± 0.006 a | 0.0030 ± 0.003 a | 0.0000 ± 0.000 a | 0.0044 ± 0.002 a |
| 9 weeks | 0.2269 ± 0.025 b, c, d | 0.1308 ± 0.0139 b, c, d | 0.0894 ± 0.050 a,d | 0.0066 ± 0.007 a | 0.0301 ± 0.019 a | 0.0062 ± 0.003 a | 0.0000 ± 0.000 a, b | 0.0002 ± 0.000 a |
Values given are means +/− SE in nmol/ml. Only AA, Mead acid, EPA, DHA and triene-tetraene ratios are shown.
Diets are 1%, 5% and 10% Menhaden oil diet and soybean diet.
Value is significantly different from 1% Menhaden oil group within same timepoint and acid (or ratio) group, P< 0.05
Value is significantly different from 5% Menhaden oil group within same timepoint and acid (or ratio) group, P< 0.05
Value is significantly different from 10% Menhaden oil group within same timepoint and acid (or ratio) group, P< 0.05
Value is significantly different from soybean oil group within same timepoint and acid (or ratio) group, P< 0.05
Arachidonic acid (AA)
At baseline, all groups had levels of approximately 0.25 nmol/ml, without any statistical difference between groups. In the serum triglyceride analysis of the pair fed group, the 1% group AA levels stayed relatively high at weeks 3 and 6, where the 5% and 10% groups decreased (Table 2). These values remained elevated in the soybean group peaking at 6 weeks. After 6 weeks, all fish oil groups had significantly lower concentrations of AA than the soybean group. After 9 weeks, all fish oil groups maintained that lower level, although only the 10% fish oil group remained significantly lower.
In the phospholipid analysis, there was a gradual decline of AA levels in the 1% fish oil group as seen in the triglyceride fraction pair fed group (Table 2). At 9 weeks, all fish oil groups again plateaued at the same level (0.11–0.14 nmol/ml) although the 1% fish oil group was the only group to show statistical significance with time (p = 0.01 for 3 weeks compared to 9 weeks). The AA level in the soybean group rapidly elevated and maintained high levels from the 3rd week onward. As in the triglyceride fraction, there were statistically significant differences between fish oil diets and the soybean control diet between weeks 3 and 9.
Mead acid
Typically, Mead acid levels are very low or undetectable. This is supported by the Mead acid levels in all four subgroups at baseline being, or approaching, zero. The triglyceride fraction in the pair fed animals suggest that the 1% fish oil group has statistically significant increasing Mead acid levels over the entire study period while the 5% fish oil group has a slight increase at 9 weeks.
In the phospholipid fraction, the 5% fish oil group had an unusually elevated Mead acid level at baseline. We see a delayed but significant increase in Mead acid levels from 6 weeks onward in the 1% fish oil group with consequential statistical significance when compared to the other groups, where minimal Mead acid concentrations were present at all times.
Eicosapentaenoic acid (EPA)
EPA is one of the two downstream omega-3 fatty acids that is directly provided by fish oil but not present in the soybean oil diet. Soybean oil contains the omega-3 fatty acid ALA which is converted by means of desaturation and elongation to produce EPA.
The triglyceride fraction pair fed profiles showed an EPA increase in all fish oil groups and a decrease in the group fed the soybean diet. The soybean oil group had significantly lower levels of EPA than all fish oil groups from baseline to endpoint. Calculated over time, the 5% fish oil group was significantly higher than both 1% and 10% because of slight declines in EPA levels of the 1% and 10% groups at 9 weeks.
As expected, decreased EPA levels were observed in the phospholipid fraction in the pair fed group for the soybean diet and again, as in the triglyceride fraction, 5% fish oil EPA levels climbed faster than both the 1% and 10% diet fed groups. There was a decrease in EPA concentrations in animals fed fish oil diets in each of the EPA profiles at the 9 week time point. Similarly, the EPA levels in the soybean groups also dropped to very low levels.
Docosahexaenoic acid (DHA)
DHA is the other omega-3 fatty acid derived from ALA, downstream from EPA. We found an unusually low baseline level of DHA in the triglyceride fraction in the pair fed group for the 5% fish oil diet after which this group’s DHA concentration increased to significantly higher levels than all other diets. The soybean diet DHA concentration decreased steadily over time. At 6 weeks, DHA levels in the 1% fish oil group were statistically higher than the soybean group, and eventually at the 9 week endpoint, all fish oil groups reached similar levels, statistically higher than the soybean group (Table 2).
Triene-tetraene ratio
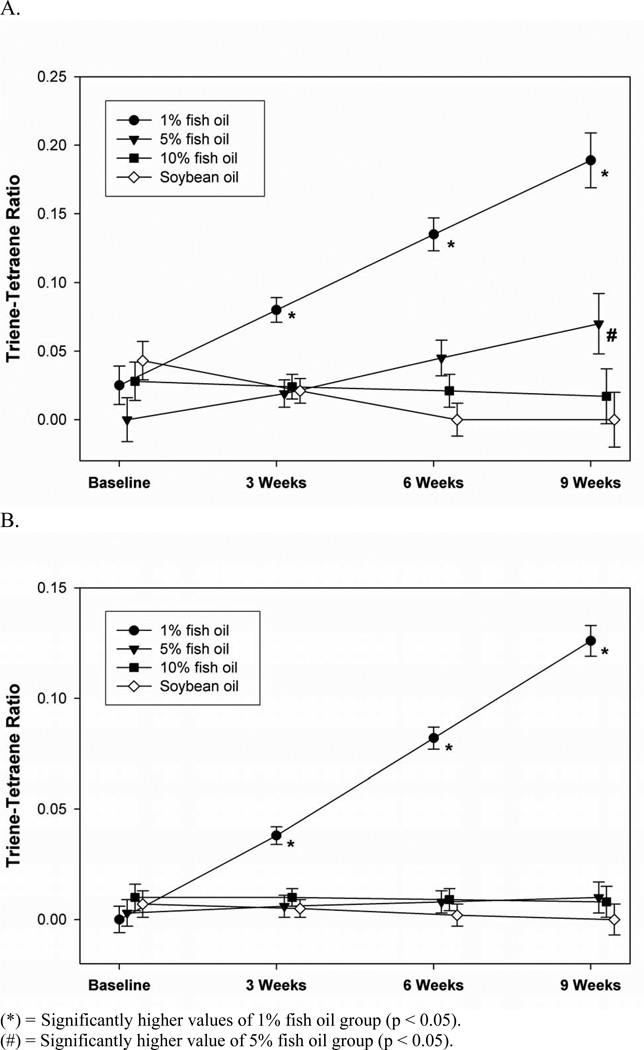
The ratio of Mead acid and arachidonic acid is a reliable measure of EFAD, and is highly correlative with physiologic measures of EFAD such as growth retardation (29). In the triglyceride fraction of the pair fed group, the trienetetraene ratio declined to zero in the soybean diet group and remained stable in the 10% fish oil group. The triene-tetraene ratio in the 1% fish oil group increased steadily to 0.22 nmol/ml, surpassing the critical 0.2 nmol/ml threshold for biochemical determination of EFAD. The triene-tetraene ratio in the 5% fish oil group increased gradually and never exceeded the EFAD threshold. Significantly higher ratios in the 1% fish oil group from 3 weeks on and the increase in the 5% fish oil resulted in statistical significance at 9 weeks (Figure 4a & Table 2).
Figure 4.
Triglyceride analysis pair fed (A) and phospholipid analysis pair fed (B) triene-tetraene ratios over the 9 week experiment.
(*) = Significantly higher values of 1% fish oil group (p < 0.05).
(#) = Significantly higher value of 5% fish oil group (p < 0.05).
In the phospholipid fraction of the pair fed cohorts, all groups except the 1% fish oil displayed very low ratios during the entire experiment. The 1% fish oil group had significantly higher ratios, although levels did not reach the 0.2 nmol/ml threshold (Figure 4b & Table 2).
Discussion
Parenteral nutrition (PN) has been used for many years to provide calories and essential nutrients to patients who are unable to obtain them through the enteral route. Among the first major obstacles associated with the use of PN was the induction of EFAD in recipients of this therapy. (28, 29, 30) In response, a soybean emulsion was introduced to provide essential fatty acids and thus prevent deficiency (31). Recent studies have shown that lipid metabolism is altered by its route of administration; infusions of intravenous fat emulsions bypasses the entero-hepatic circuit, causing fat accumulation in the liver and predisposing patients to PN associated liver disease (32). Based on animal models, intravenous fat emulsions derived from fish oil may prevent such complications through inhibition of de novo lipogenesis (17). However, the administration of parenteral fish oil intravenous fat emulsion is intended to be as a supplement and has never been investigated as the sole source of fat because of concern for EFAD. Gura et al reported on an EFAD, PN-dependent patient who was treated with an intravenous fish oil fat emulsion because of an inability to tolerate conventional soybean based fat emulsions due to soy allergy; biochemical markers improved within a week after the start of therapy and clinical signs of EFAD resolved shortly thereafter (16). More recently, two infants were administered parenteral fish oil as monotherapy in an attempt to reverse PN associated cholestasis and again no signs of EFAD were noted. (17).
Despite these promising preliminary results, concerns about the EFA content in fish oil remain. Indeed, the manufacturer of one such product has stated that the fish oil contained in their lipid emulsion does not have sufficient omega-6 fatty acids to prevent EFAD (33). This continued skepticism, despite recently published clinical evidence, prompted us to investigate the development of EFAD utilizing different lipid sources and thereby establish biochemical standards.
Cunanne et al reports about minimal LA and AA requirements separately as the sole sources of fat (34). However, a comprehensive biochemical EFAD study of fish oil, which contains EPA and DHA and small amounts of LA and AA, has never been done.
The energy percentages provided by omega-6 fatty acids (LA and AA) per diet are needed for adequate comparison to conventional requirement beliefs. By weight, fish oil (as menhaden oil) contains 1.5% LA and 0.9% AA, whereas soybean oil contains 54.8% and 0%, of these fats respectively. We calculated the total energy provided by the different diets (Table 3). The relative amounts of omega-3 fatty acids have also been listed (Table 1).
Table 3.
Percentages of energy provided by LA, AA, and total omega-6 fatty acids for the menhaden and soybean oil diets (1,2).
| % of total energy | Linoleic acid % | Arachidonic acid % | Total omega-6 % |
|---|---|---|---|
| 1% menhaden oil (2.6%) | 0.0393 | 0.0236 | 0.0629 |
| 5% menhaden oil (12.5%) | 0.196 | 0.118 | 0.314 |
| 10% menhaden oil (23%) | 0.393 | 0.236 | 0.629 |
| Soybean oil (12.5%) | 6.7 | - | 6.7 |
Menhaden oil percentages are named by weight, energy percentages in parentheses.
Standard requirement beliefs are 1% of energy for omega-6 fatty acids.
A parallel set-up was chosen with treatment of the four diets by either pair feeding or ad libitum feeding technique. Pair feeding eliminates the variable of differential food intake and ensures adequate metabolic comparison. The ad libitum feeding model allows the determination of whether fish oil decreases food intake and also serves as a control. Remarkably, in the ad libitum groups, all fish oil groups took in more calories than the soybean oil group throughout the 9 week experiment, removing the concern that fish oil palatability would bias food intake.
A limitation of our pair feeding model was that the amount of chow given was based on chow weight per day, rather than chow calories per day. Consequently, although in the 5%, 10% fish oil and soybean oil groups pair feeding was successful (2510 kcal ± 1%), the 1% fish oil group had decreased caloric intake (2347 kcal), associated with the only report of weight loss. The animals consuming the ad libitum 1% fish oil actually ate the most by weight, perhaps to compensate for diminished caloric intake and/or the lack of EFA.
In the pair fed groups, caloric efficiency was higher in the 5% and 10% fish oil groups when compared to soybean oil. The most appropriate comparison was between the 5% fish oil group and the control soybean oil group, as they shared the same caloric value (3.6 kcal/g). The only instance of statistical significance in caloric efficiency improvement was 5% fish oil compared to the soybean oil diet (P = 0.042). Therefore, fish oil proved to have higher caloric efficiency in both the pair fed and the ad libitum groups. (Figure 3)
We have shown that fish oil actually enhances growth when compared to soybean oil (Figure 1). This is in contradistinction to earlier reports about the negative effect of fish oil on growth in infants (35–38). It is important to note that omega-3 fatty acids in those studies were used as supplements as opposed to the sole source of fat, which leads to a substantial difference in actual amounts of LA and relative amounts of LA to AA intake when fish oil is the only source of fat in the diet. The principal effects of EPA on AA levels in serum phospholipid membranes in supplementation is through inhibition of elongase and desaturase-mediated conversion of dietary LA to AA, as well as increased competition for transacylation into tissue lipids.
Ad libitum data were very similar to pair fed, deviating only through accelerated data differences due to the faster metabolic switch resulting from increased food intake. Some discrepancies with the pair fed groups could be attributed to the different caloric intake among all diets rather then the fatty acid content.
In the AA profiles of both triglyceride and phospholipid fractions, only the soybean group displayed significantly higher levels after the ‘wash out’ period despite vast differences in AA content amongst the fish oil diets (Table 1). Together with the direct provision of AA, sufficient LA was converted to AA to in each of the fish oil groups to maintain stable levels. As expected, the levels of AA were much higher in the soybean oil group with higher levels of LA and no EPA to both inhibit the conversion of LA to AA and compete for transacylase insertion.
The increasing levels of Mead acid in the 1% fish oil group signaled the development of EFAD with a delay in phospholipid levels when compared to the triglyceride fraction. Additionally, at nine weeks, the increased Mead acid levels reported in the 5% fish oil group for the triglyceride fraction levels suggested instability. This implies that 5% fish oil as the sole source of fat might be marginal over time to prevent EFAD with greater synthesis of Mead acid, although it may still be sufficient to maintain the triene-tetraene ratio within the normal range.
EPA profiles showed an increase in all fish oil groups and a decrease in the soybean groups. The 5% fish oil levels of EPA climbed significantly faster than the other fish oil groups in both triglyceride and phospholipid fractions. This is also seen in DHA triglyceride levels. These findings correlate with our caloric efficiency data suggesting highly effective fatty acid metabolism for the 5% fish oil diet. The DHA data show only a slight increase and decrease for fish oil and soybean phospholipids, respectively. The more profound changes in EPA profiles compared to DHA can be attributed to the higher concentration of EPA in fish oil and to the higher baseline levels for DHA after weaning from baseline chow. Notably, the EPA levels, and to a lesser extent DHA, only in the triglyceride analysis, dropped to low levels in the soybean group when compared to baseline. This was likely a consequence of the minimal amounts of ALA accompanied by large amounts of LA in the soybean diet. EPA metabolites serve as important anti-inflammatory eicosanoids resulting in a less inflammatory cytokine profile, whereas AA metabolites are thought to be pro-inflammatory (39). This has been suggested in clinical studies where children supplemented with conventional soybean oil fat emulsions may be more susceptible to steatohepatitis and PN-associated liver disease (17).
The triene-tetraene ratio indicates whether the decrease of AA in all fish oil groups led to EFAD. Because none of the AA levels showed any significant difference, any changes in the ratio were solely dependent on the conversion of oleic acid to Mead acid. The 1% fish oil diet did not contain sufficient EFAs to prevent EFAD (Figure 4), a conclusion that was supported by growth data. Conversely, the 10% fish oil group never showed any clinical or biochemical signs of EFAD, suggesting that it could be safely used as the sole source of fat. The 5% fish oil diet seemed to display a pivotal point in the spectrum between 1% and 10%.
The threshold for fish oil as the sole source of fat in prevention of EFAD in mice lies between 5% and 10%. This suggests an omega-6 fatty acid requirement between 0.314% and 0.629% of dietary energy when given in as a combination of LA and AA, roughly half of what is conventionally believed (1%). This is likely due to the small amounts of AA present in fish oil that have been found to be three times more effective than LA in preventing EFAD (10). Therefore this requirement would presumably be even lower if omega-6 fatty acids were entirely provided by AA. AA status is usually tightly regulated as it is a source of bioactive eicosanoids and prostaglandins. Our data suggests that fish oil at 5 and 10% maintained AA levels at these lower levels. The 1% fish oil group was the only group that showed significantly decreasing values over time (Table 2). This suggests that there were sufficient omega-6 fatty acids to satisfy the regulatory needs of AA in 5% and 10% but not in 1% fish oil.
Our animal model suggests that fish oil, when used at sufficient concentrations, can be used as the sole source of fat, displaying no adverse effects on growth and no evidence of EFAD. In addition, fish oil as the sole fat source may provide the added benefit of a more profound anti-inflammatory effect due to the very low AA levels achieved without the development of EFAD. At the very least, the widely held notion that a fish oil based lipid cannot be used as the sole source of parenteral fat should be reevaluated.
Acknowledgements
Mr. Strijbosch was funded by Trustfonds Erasmus Universiteit Rotterdam (Rotterdam, The Netherlands), Dr. Saal van Zwanenberg Stichting (Oss, The Netherlands), VSB fonds (Den Haag, The Netherlands), and Michaël van Vloten fonds (Venray, The Netherlands).
Dr. Puder is funded by NIH grant DK069621-03 and the Children’s Hospital Surgical Foundation (Boston, MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. Journal of Pediatrics. 2003;143(4 Suppl):S1–S8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- 2.Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet. 2003;92:1–22. doi: 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- 3.Simopoulos AP. n-3 fatty acid-enriched eggs, lipids, and Western diet: time for change. Nutrition. 1993;9:561–562. [PubMed] [Google Scholar]
- 4.Jeppesen PB, Hoy CE, Mortensen PB. Essential fatty acid deficiency in patients receiving home parenteral nutrition. American Journal of Clinical Nutrition. 1998;68(1):126–133. doi: 10.1093/ajcn/68.1.126. [DOI] [PubMed] [Google Scholar]
- 5.Holman R. Essential fatty acid deficiency. Progress in the Chemistry of Fats and Other Lipids. 1971;9:275–348. [Google Scholar]
- 6.Smit EN, Muskiet FA, Boersma ER. The possible role of essential fatty acids in the pathophysiology of malnutrition: a review. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2004;71(4):241–250. doi: 10.1016/j.plefa.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Farrell PM, Gutcher GR, Palta M, DeMets D. Essential fatty acid deficiency in premature infants. American Journal of Clinical Nutrition. 1988;48(2):220–229. doi: 10.1093/ajcn/48.2.220. [DOI] [PubMed] [Google Scholar]
- 8.Holman R. The ratio of trienoic:tetranoic acids in tissue lipids as a measure of essential fatty acid requirements. J Nutr. 1970;60:405–410. doi: 10.1093/jn/70.3.405. [DOI] [PubMed] [Google Scholar]
- 9.Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. National Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- 10.Mohrhauer H, Holman RT. The effect of dose level of essential fatty acids upon fatty acid composition of the rat liver. Journal of Lipid Research. 1963;4:151–159. [PubMed] [Google Scholar]
- 11.Innis SM. Essential fatty acids in growth and development. Progress in Lipid Research. 1991;30(1):39–103. doi: 10.1016/0163-7827(91)90006-q. [DOI] [PubMed] [Google Scholar]
- 12.Bjerve KS, Fischer S, Alme K. Alpha-linolenic acid deficiency in man: effect of ethyl linolenate on plasma and erythrocyte fatty acid composition and biosynthesis of prostanoids. American Journal of Clinical Nutrition. 1987;46(4):570–576. doi: 10.1093/ajcn/46.4.570. [DOI] [PubMed] [Google Scholar]
- 13.Bjerve KS, Fischer S, Wammer F, Egeland T. Alpha-linolenic acid and long-chain omega-3 fatty acid supplementation in three patients with omega-3 fatty acid deficiency: effect of lymphocyte function, plasma and red cell lipids, and prostanoid formation. American Journal of Clinical Nutrition. 1989;49(2):290–300. doi: 10.1093/ajcn/49.2.290. [DOI] [PubMed] [Google Scholar]
- 14.Bjerve KS, Mostad IL, Thoresen L. Alpha-linolenic acid deficiency in patients on long-term gastric-tube feeding: estimation of linolenic acid and long-chain unsaturated n-3 fatty acid requirement in man. American Journal of Clinical Nutrition. 1987;45(1):66–77. doi: 10.1093/ajcn/45.1.66. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Gura KM, Kim S, Arsenault DA, Bistrian BR, Puder M. Current clinical applications of omega-6 and omega-3 fatty acids. Nutrition in Clinical Practice. 2006;21(4):323–341. doi: 10.1177/0115426506021004323. [DOI] [PubMed] [Google Scholar]
- 16.Gura KM, Parsons SK, Bechard LJ, et al. Use of a fish oil-based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clinical Nutrition. 2005;24(5):839–847. doi: 10.1016/j.clnu.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Gura KM, Duggan CP, Collier SB, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118(1):e197–e201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 18.Gura K, Strijbosch R, Arnold S, McPherson C, Puder M. The role of an intravenous fat emulsion composed of fish oil in a parenteral nutrition-dependent patient with hypertriglyceridemia. Nutr Clin Pract. 2007 Dec;22(6):664–672. doi: 10.1177/0115426507022006664. [DOI] [PubMed] [Google Scholar]
- 19.Maynard LA. The Determination of Digestibility. McGraw-Hill: 1962. Feeding Experiments. [Google Scholar]
- 20.Chen H, Hansen MJ, Jones JE, et al. Cigarette smoke exposure reprograms the hypothalamic neuropeptide Y axis to promote weight loss. American Journal of Respiratory Critical Care Medicine. 2006;173(11):1248–1254. doi: 10.1164/rccm.200506-977OC. [DOI] [PubMed] [Google Scholar]
- 21.Alwayn IP, Gura K, Nose V, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatric Research. 2005;57(3):445–452. doi: 10.1203/01.PDR.0000153672.43030.75. [DOI] [PubMed] [Google Scholar]
- 22.Rosner B. Fundamentals of Biostatistics. 6th ed. Thompson; 2005. [Google Scholar]
- 23.Khuri AI, Sinha BK. Statistical tests for mixed linear models. New York: John Wiley; 1998. pp. 95–118. [Google Scholar]
- 24.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 25.Henry CJ, Ghusain-Choueiri A, Payne PR. Protein utilization, growth and survival in essential-fatty-acid-deficient rats. British Journal of Nutrition. 1996;75(2):237–248. doi: 10.1079/bjn19960127. [DOI] [PubMed] [Google Scholar]
- 26.Phinney SD, Clarke SD, Odin RS, Moldawer LL, Blackburn GL, Bistrian BR. Thermogenesis secondary to transdermal water loss causes growth retardation in essential fatty acid-deficient rats. Metabolism. 1993;42(8):1022–1026. doi: 10.1016/0026-0495(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 27.Goubern M, Yazbeck J, Senault C, Portet R. Non-shivering thermogenesis and brown fat adipose tissue activity in essential fatty acid deficient rats. Archives Internationales de Physiologie et de Biochimie. 1990;98(4):193–199. doi: 10.3109/13813459009113977. [DOI] [PubMed] [Google Scholar]
- 28.Paulsrud JR, Pensler L, Whitten CF, et al. Essential fatty acid deficiency in infants induced by fat-free intravenous feeding. The American Journal of Clinical Nutrition. 1972;25:897–904. doi: 10.1093/ajcn/25.9.897. [DOI] [PubMed] [Google Scholar]
- 29.Conner WE. Pathogenesis and frequency of essential fatty acid deficiency during total parenteral nutrition. Annals of Internal Medicine. 1975;83(6):895–896. doi: 10.7326/0003-4819-83-6-895. [DOI] [PubMed] [Google Scholar]
- 30.Barr LH, Dunn GD, Brennan MF. Essential fatty acid deficiency during total parenteral nutrition. Annals of Surgery. 1981;193(3):304–311. doi: 10.1097/00000658-198103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wene JD, Connor WE, DenBesten L. The development of essential fatty acid deficiency in healthy men fed fat-free diets intravenously and orally. Journal of Clinical Investigation. 1975;56:127–134. doi: 10.1172/JCI108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javid PJ, Greene AK, Garza J, et al. The route of lipid administration affects parenteral nutrition-induced hepatic steatosis in a mouse model. Journal of Pediatric Surgery. 2005;40(9):1446–1453. doi: 10.1016/j.jpedsurg.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 33.Marcus AD. A Doctor’s Push for Drug Pits Him Against Its Maker. The Wall Street Journal. 2006 Nov 13;:A1, A15. [Google Scholar]
- 34.Cunnane SC. Problems with essential fatty acids: time for a new paradigm? Progress in Lipid Research. 2003;42(6):544–568. doi: 10.1016/s0163-7827(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 35.Carlson SE, Cooke RJ, Werkman SH, Tolley EA. First year of growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids. 1992;27(11):901–907. doi: 10.1007/BF02535870. [DOI] [PubMed] [Google Scholar]
- 36.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proceedings of the National Academy of Sciences USA. 1993;90(3):1073–1077. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlson SE, Werkman SH, Tolley EA. Effect of long-chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. American Journal of Clinical Nutrition. 1996;63(5):687–697. doi: 10.1093/ajcn/63.5.687. [DOI] [PubMed] [Google Scholar]
- 38.Ryan AS, Montalto MB, Groh-Wargo S, et al. Effect of DHA-containing formula on growth of preterm infants to 59 weeks postmenstrual age. American Journal of Human Biology. 1999;11(4):457–467. doi: 10.1002/(SICI)1520-6300(1999)11:4<457::AID-AJHB5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 39.Das Essential fatty acids: biochemistry, physiology and pathology. Biotechnology Journal. 2006;1:420–439. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]