Abstract
Context:
Pompholyx (called dyshidrosis by some) is one of the most common conditions and its immune response is presently poorly understood.
Case report:
We describe a 58 year old African American female with a clinical history of rheumatoid arthritis and type II diabetes who presented a chronic five-year, itchy vesicular/blistering rash involving her hands and feet. A lesional skin biopsy was taken for hematoxylin and eosin (H & E) analysis. In addition, a multicolor direct immunofluorescence (MDIF) and immunohistochemistry (IHC) studies were performed. The major findings to be reported were: the H & E examination revealed spongiotic dermatitis and pompholix. IHC and MDIF studies demonstrated focally deposits of positive CD45, CD3, CD8, anti myeloperoxidase (MPO), and anti-human IgE, C3C, C3D and anti-human-fibrinogen within the epidermal spongiotic process, as well as around the blood vessels surrounding the inflammatory process especially at the sweat glands and respective ductus. The patient began mycophenolate mofetil therapy, with successful clearing of the palms and soles.
Conclusion:
The significance of our findings indicates a complex immunological process including complement, MPO and T-cell immune response. In addition, possibly a secondary allergic process for the presence of IgE immune response and possibly aggravation by application of other medicines. Further immunological studies on pompholyx are needed. (Abreu-Velez AM, Pinto FJ, Howard MS. North Am J Med Sci 2009; 1: 117-120).
Keywords: Sweat glands, dyshidrotic eczema, pompholyx, immunohistochemistry, immunofluoresence, CD3, CD45, CD8
Introduction
Pompholyx (called dyshidrosis by some) is a form of vesicobullous eczema that affects the palms and soles. It could be an acute, chronic, or recurrent dermatosis of the fingers, palms, and soles, characterized by the sudden onset of many deep-seated pruritic, clear “sagolike” vesicles; later, scaling, fissures, and some times leads to lichenification of the skin.[1–3]. Pompholyx is more common in warm weather, some patients are attacked annually in summer and these patients have intensely pruritic or burning, vesicular eruption on their palms, and soles[1–3]. The course of this disease may range from mild or may progress to a severe debilitating disease and can occur at intervals of 3 or 4 weeks for months or years, or at long irregular intervals.[1–3]. Much like other forms of eczema, this is a benign, chronic, inflammatory disease that causes a decline in the quality of life rather than impacting survival. Most cases are idiopathic and, for severe cases, there are few effective treatment options[1–3].
Because it is so common a disease, very few studies have reported focusing on the relevance to the immune response in situ. This case report's focal point is to contribute to filling this gap in knowledge.
Case Report
We describe a 58 year old African American female with rheumatoid arthritis and type II diabetes who consulted for a 5 years old chronic, vesicular/blistering itchy rash involving her hands and feet. The patient also reported a distant, possible past clinical diagnosis of lupus erythematosus and rheumatoid arthritis (RA), which have not been confirmed by proper examination. The hand and foot rash initially presented in 2003. The patient was treated unsuccessfully by several physicians with terbinafine hydrochloride. In August, 2008, the patient re-presented to a second dermatology practice. At the time of the August presentation, the patient had also been evaluated by a podiatrist, and had been treated topically and orally with naftifine HCl 1% cream and clobetasol propionate Her concurrent medications on presentation to the dermatologist included Amavil©, sitagliptin and abatacept, and quinapril hydrochloride tablets for her hypertension.
The physical exam revealed the presence of several hyperpigmented macules and a few patches, in addition to tense microvesicles observed on the palms and soles. Laboratory analysis of the presentation included a normal potassium hydroxide (KOH) test normal, thiopurine methyltransferase (45.3 U/Ml) (normal range 40.0-65.0 U/Ml), normal glomerular filtration rate calculated for African American female 98 (normal range >60). The red blood cell distribution width, (RDW) was slightly elevated at 16.8 % (normal range11.5-14.0 %). The majority of her comprehensive metabolic panel including calcium, blood urea nitrogen (BUN), creatinine, total protein, albumin, total bilirubin, sodium, potassium, carbon dioxide and chloride was also within normal limits. The alkaline phosphatase was within normal limits; however, the aspartate aminotransferase) (AST) (SGOT) was slightly elevated (153 MG/DL) (normal range 30-120 MG/DL), and the glucose as well (175 MG/DL) (normal range 70-100 MG/DL). The laboratory examination also revealed several extractable nuclear antigens such us SSA/Ro at 19.5 (normal range 0-16), and an elevated SSB/La at 71.9 (normal range 0-16). In addition, an elevated double-stranded DNA (dsDNA) was noted at 29.7 (normal range 0-25), as well as an elevated anti-nuclear antibody (ANA) at 1:160, and an elevated rheumatoid factor (RF) at 221.1 (normal range 0-14).The anti-extractable nuclear antigens Sm/RNP were normal.
Histologically: Review of the H&E slides demonstrated diffuse, intraepidermal vesicles, moderate epidermal spongiosis with the presence of epidermal Langerhans cell microabcesses. (Figs 1, 2) No subepidermal blisters were noted, and the dermis displayed a moderately florid superficial perivascular infiltrate of lymphocytes, histiocytes and occasional eosinophils. Evidence of a vascular allergic component was noted, i.e., focal perivascular leukocytoclastic debris without frank vasculitis was appreciated. The hematoxylin and eosin (H & E) diagnosis was consistent with spongiotic dermatitis with dyshidrotic eczema. However, given the previously described vascular findings, the diagnosis of a concomitant allergic drug component was suggested.
Fig. 1.
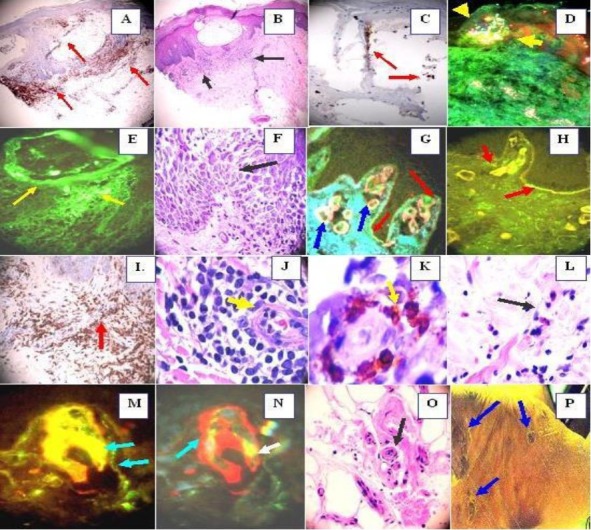
IHC (a, c, i, k), MDIF (d, e, g, h, m, n). H 7& E (b, f, j, o). Positive stain with CD45 around the superficial vessels and inside the blister (a) (red arrows) (brown stain). b. Intra-epidermal spongiotic vesicle, surrounded in the dermis by a moderately florid superficial perivascular infiltrate of lymphocytes, histiocytes and some eosinophils (black arrows) (200X). c Positive stain with MPO inside the blister (red arrows). d Positive IgA secretory FITC conjugated mixed with lipofuscin on the external duct-isthmus of the acrosyringium of one sweat gland, resembling a “firework explosion” (mixed colors) (yellow arrows). e. DIF showing deposits of anti-human IgA FITC conjugated around the intra-epithelial vesicle (yellow arrows). f. Spongiotic epidermis in several keratynocytes (black arrow). g, Positive deposits of anti-human IgE FITC conjugated in the dermal papillary vessels, as well at some areas of the base membrane zone (BMZ) (red arrows). The vessels are positive to anti-human fibrinogen conjugated with Texas red (red stain) (blue arrows). h. Positive stain with anti-human fibrinogen to superficial vessels and some areas of the BMZ (red arrows). i. Positive CD45 around the vessels of the sweat glands ductus (red arrow). j Perivascular infiltration of predominantly lymphocytes and histiocytes with focal perivascular leukocytoclastic debris, but no frank vasculitis (yellow arrow). k Positive CD8 stain around the vessel (yellow arrow). l. A group of 3 eosinophils as part of the superficial infiltrate (black arrows) in the adjacent dermis subjacent to the spongiotic phenomena. m, and e, positive anti-human IgE FITC conjugated in the dermal papillary vessels and some at the BMZ (yellowish-orange stain) (aqua arrows), and in n using both anti-human fribrinogen Texas red conjugated (red staining) (aqua arrow) and anti IgE-FITC conjugated (yellow stain) (white arrow) o. Shows a detail of the perivascular infiltrate around the central vessels, as well as a mild infiltrate around the deep dermal vessels (black arrow). p. Shows some indurate hyperpigmented plaques in the sole after the treatment with mycophenolate mofetil.
Fig. 2.
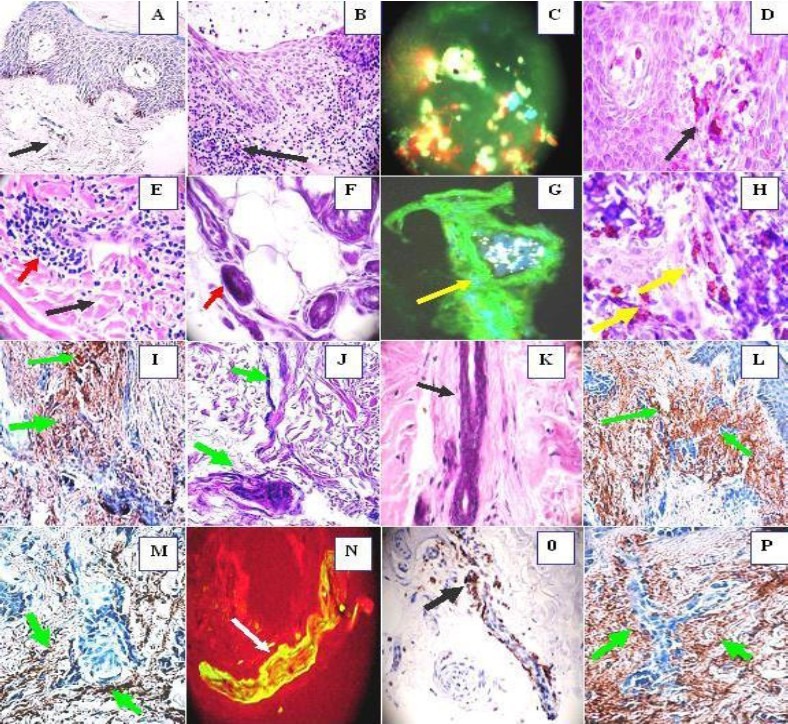
IHC (a, d ,h ,i, l, m, o, p), MDIF (c, g, n), H & E (b, e, k). a, Show weak positive stains in the vessels using anti HLA DR, DP, DQ (black arrow). b, Epidermal spongiosis and infiltration of predominantly lymphocytes and histiocytes (black arrow). c. Acrosyringium secretion of IgA (yellow), some nuclei cells debris with Dapi (blue) and positive IgE (green). d. CD8 positive around the superficial vessels (black arrow). e. Sclerodermoid alterations around one sweat glands ductus (black arrow). The red arrows show infiltration of predominantly lymphocytes and histiocytes. f. Necrosis of the sweat glands (red arrow). g. Positive stain of the sweat glands with anti-human fibrinogen FITC conjugated (yellow arrow) (green stain). The white material is self-fluoresce lipofuscin. h, CD8 positive stain around the ecrine ductus under the base membrane zone (yellow arrows). i. Positive stain with anti-human fibrinogen around all the ecrine ductus and sweat glands (brown stain) green arrows. j. Sclerosis of the sweat glands and its ductus (green arrows). K. Narrowing of the sweat glands ductus (black arrow). l. Strong stain (brown) around the sweat glands ductus and its vessels with complement C3C (green arrows). m. Positive stain around the sweat glands coiled portion using complement C3D (brown stain) (green arrows). n. Positive IgE-FITC conjugated in the vessels (yellow stain) (white arrow). o. Positive CD 45 stain along the sweat ductus (black arrow). p. Strong stain (brown) around the sweat glands ductus with complement C3D (green arrows).
Immunohistochemistry (IHC): To study the possible immune response in situ we performed IHC by using a dual endogenous peroxidase blockage, according to the Dako (Denmark) insert, with the addition of Envision dual link. Furthermore, we applied 3, 3 diaminobenzidine and counterstained with hematoxylin. The samples were run in a Dako Autostainer Universal Staining System. We tested for mouse anti-human CD3, CD4, CD8, CD45, HLA DP, DR, DQ, myeloperoxidase (MPO), and rabbit anti-human IgM, and anti-human fibrinogen. Our tests showed positive staining with several antibodies by IHC of the sweat glands and its ductus as well as to some vessels in proximity where the main inflammatory process was found (Figs 1, 2).
Multicolor direct immunofluorescence (MDIF): In brief, perilesional skin in Michel's medium (Newcomer Supply Parview Road, Middleton, WI, USA) was washed with PBS; 4 um thickness skin cryosections were incubated with FITC-conjugated antibodies. The following antibodies were utilized: a), rabbit anti-human IgG (γ chain), b), rabbit anti-human IgA (α chains) c) rabbit anti-human IgM (μ-chain), (all at 1:20 dilutions), rabbit anti-human C3 and d) rabbit anti-human fibrinogen, and e) rabbit anti-human albumin (both at 1:40 dilutions, and all from Dako, Carpinteria, California, USA). In addition, we also used goat anti-human IgE antiserum conjugated with fluorescein isothiocyanate (FITC) (Vector Laboratories, Bridgeport New Jersey, USA). Unconjugated rabbit anti-human IgE antiserum was also utilized. The slides were then counterstained with 4’, 6-diamidino-2-phenylindole (Dapi) (Pierce, Rockford, Illinois, USA). In addition, a mouse anti human collagen IV (H & L) at 2mg/ml from Invitrogen (Carlsbad, California, USA) was utilized, with Alexa Fluor® 555 from Invitrogen as its secondary. The DIF examination revealed the presence of anti-human IgE (+++) surrounding several papillary and deep dermal blood vessels, and overexpression of collagen IV within those vessel walls. In addition, focal, linear BMZ deposits of C3 (++), IgE (++), IgA (+), albumin (++) and fibrinogen (++) were observed, specifically noted along the BMZ in patchy areas near the prominent, previously described vascular deposits of IgE (i.e., in close proximity to selected superficial papillary blood vessels) (Figs. 1, 2).
Discussion
The terminology of eruptive, symmetric, vesicular, and/or bullous dermatitis on the palms and/or palmar aspects or sides of the fingers includes the terms pompholyx, dyshidrosis, and dyshidrotic eczema. It usually presents as a recurrent vesicular hand and feet dermatosis[1,2]. This is one of the most common medical consultations in medicine and it is usually relapsing and very recalcitrant. The three main types of hand/foot dermatitis are irritant contact dermatitis, allergic contact dermatitis, and dyshidrotic eczema, which require long treatments, ideally patch testing to discard other concomitant cause[1,2]. The typical and unique morphology of the clear “sago-like” vesicles, their longer duration and severe burning, itching, and pain may be due to the specific anatomy of the glabrous skin of the palms and soles characterized by a thick epidermis with a compact stratum corneum.
To date, the pathogenesis of pompholyx is still unknown although there are many theories about the etiology of pompholyx. It is very likely that several aggravating factors, such as atopy, contact allergy, allergy to ingested metals, psychological stress, dermatophyte infection, and drug eruption, influence the development and formation of pompholyx in a predisposed individual. To our surprise by reviewing the literature, very few studies have been reported studying in situ the immune response in this disease. Based on the combined clinical, histologic and in situ immunological examination in this case, we evaluated several differential diagnoses for this patient. We found a strong T-cell immune response, with the concomitant presence of MPO and some possible allergic component to concomitant medications.
We first considered the diagnosis of systemic lupus erythematosus (SLE) based on the patient's distant previous history, and the current rheumatologic laboratory panel[3]. Our overall histologic MDIF and IHC examination data were inconsistent with either SLE or RA. Both, RA and SLE could be playing a role in the immunologic process involving the autoreactivity seen in the blood vessels[3].Contrary to the predicted findings on skin biopsies for RA or SLE, the main immune response in the vessels was seen by IgE[3]. Another possible diagnosis we considered was a rare variant of bullous pemphigoid (BP), dyshidrosiform pemphigoid (DP)[4,5]. DP represented a diagnostic consideration based on our patient's clinical history, and on the fact that DP can display some non specific histologic changes, and/or reveal subepidermal blisters[4,5].
Our patient did not display subepidermal blisters on histologic examination. Classic DP also differs from our case given that deposits of BMZ of immunoglobulins in DP are classically evenly distributed, and not present in a patchy form along the BMZ (following same pattern than BP)[4,5]. Other differential diagnosis of pompholix include pustular psoriasis, however in pustular psoriasis of the palms and soles, there are usually no clear vesicles, although this is not invariably so, and the pustules are sterile on culture. Furthermore, contact dermatitis, pemphigoid, linear IgA disease, and pemphigoid gestationis should also be differentiated from pompholyx Thus, a diagnostic difficulty in our case was present, given the presence of 1) a spongiotic dermatitis with eosinophils detected by H and E, 2) the laboratory autoimmune test profile, and 3) our MDIF results. Spongiotic dermatoses (including eczema and its clinicopathologic variants) are capable of presenting in a variety of clinical patterns, and are pathologically defined by the presence of epithelial intercellular edema that may rarely form blisters.
The mechanisms which underlie the pathogenesis of dyshidrotic dermatoses are not well understood, and to our surprise few MDIF or IHC studies are reported[6]. Other authors have observed the presence of anti-CD45 cells in a similar distribution, in patients suffering from other spongiotic dermatoses, including atopic dermatitis. Langerhans cells were also increased in number[7]. We detected strong presence of CD3, CD8, CD45, anti-human fibrinogen anti-MPO, anti human fibrinogen, as well as anti-human IgE antibodies where the main lesions were present. For our findings and the fact that pompholyx is a chronic relapsing dermatosis belonging to the spectrum of eczema, we may hypothesize that more immunological studies are needed to better approach the treatment of this disease.
Among the new developments, topical calcineurin inhibitors (TCI) and botulinum toxin A (BTXA) seem to be effective against pompholyx. The major drawback of BTXA is the need for injections, but efforts are being made to develop a topical form of application. Bexaroten gel has been used for chronic hand dermatitis, with good efficacy in the hyperkeratotic type. Further studies on pompholyx are needed.
Acknowledge
For excellent technical assistance to Mister Jonathan Jones at GDA.
Footnotes
Conflicts of interest: None.
Declaration of all funding sources
This work was performed with the funds of Georgia Dermatopathology Associates (GDA).
Short Title: Dyshidrotic eczema, T cell response and IgE.
References
- 1.Veien NK. Acute and recurrent vesicular hand dermatitis. Dermatol Clin. 2009;3:337–53. doi: 10.1016/j.det.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Wollina U. Pompholyx: what's new? Expert Opin Investig Drugs. 2008;6:897–904. doi: 10.1517/13543784.17.6.897. [DOI] [PubMed] [Google Scholar]
- 3.Schroeter AL, Conn DL, Jordon RE. Immunoglobulin and complement deposition in skin of rheumatoid arthritis and systemic lupus erythematosus patients. Ann Rheum Dis. 1976;4:321–326. doi: 10.1136/ard.35.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimura C, Katsuura J, Moriue T, Matsuoka Y, Kubota Y. Dyshidrosiform pemphigoid: report of a case. J Dermatol. 2003;7:525–529. doi: 10.1111/j.1346-8138.2003.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 5.Beylot-Barry M, Doutre MS, Beylot C. Dyshidrotic pemphigoid. Ann Dermatol Venereol. 1995;3:81–83. [PubMed] [Google Scholar]
- 6.Houck G, Saeed S, Stevens GL, Morgan MB. Eczema and the spongiotic dermatoses: a histologic and pathogenic update. Semin Cutan Med Surg. 2004;1:39–45. doi: 10.1016/s1085-5629(03)00086-5. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K, Kondo N, Fukutomi O, Takami T, Agata H, Orii T. Characterization of infiltrating CD4+ cells in atopic dermatitis using CD45R and CD29 monoclonal antibodies. Ann Allergy. 1994;1:39–44. [PubMed] [Google Scholar]


