Abstract
Background:
Type 1 diabetes is an autoimmune disease. Genetics as well as environmental factors seem to play a role in the pathogenesis of type 1 diabetes.
Aims:
We sought to investigate the possible relationship between migration from Sardinia to a low incidence area of type 1 diabetes (Lombardy) and the prevalence of autoantibody positivity.
Methods:
We enrolled 554 Sardinian immigrants and 226 of their offspring. All subjects underwent a complete anamnestic evaluation. Fasting blood glucose, HbA1c, GADA and IA-2 were measured in all study participants. Additionally, the presence of risk haplotypes (HLA-DR3 –DR4 and DQB1/0302) was determined. After a seven-year follow-up, high genetic risk and/or autoantibody positivity subjects were re-evaluated.
Results:
Among Sardinian immigrants, the prevalence of type 1 diabetes was 0.9%, while in the offspring group, the prevalence was 0.4%. After removing type 1 diabetic patients, the GADA prevalence was 2.4% in the immigrant group and 3.8% among their offspring. Among Sardinian immigrants, the IA-2 prevalence was 0.7%, while all offspring were IA-2 negative. After a seven-year follow-up, 85.7% of GADA-positive migrants had persistent GADA positivity. Two GADA-negative offspring subjects turned positive. None of the study participants developed diabetes during the follow-up.
Conclusions:
The present study showed a higher prevalence of GADA positivity within Sardinian immigrants at high genetic risk; GADA positivity may represent the first detectable phase of type 1 diabetes. After a seven-year follow-up, none of the high genetic/antibody risk group subjects developed type 1 diabetes. However, it seems reasonable to strictly control high-risk individuals in order to diagnose subclinical diabetes.
Keywords: Type 1 diabetes, autoimmunity, autoantibodies, immigration
Introduction
Type 1 diabetes is characterized by an autoimmune destruction of the pancreatic ß-cells. Despite several efforts, its etiology is not yet fully elucidated. Although a strong genetic component is believed to play a pivotal role in the pathogenesis of type 1 diabetes, it has been suggested that environmental factors such as viruses, bacteria or toxins may be important risk factors for the development and progression of the disease.
From an epidemiological point of view, Scandinavian countries have the highest incidence of type 1 diabetes in Europe. For example, in Finland the total incidence per 100,000/year in the under-15 population was 40.9, in Sweden 27.5, in Norway 20.8 and in Denmark 16.6. Anglo-Saxon countries (Canada, USA, UK and Australia) come immediately after. Interestingly, Sardinia presents the highest incidence in Italy (37.8 per 100,000/year) and the second highest incidence worldwide, thus being the only region with a risk of type 1 diabetes approaching that of Finland. In general, it is accepted that Caucasoid populations have a higher incidence of type 1 diabetes than Negroids or Mongoloids[1].
Studies concerning immigrant populations may represent a powerful tool for evaluating genetic predisposition; several reports regarding Sardinian immigrants showed results in contrast with the “environmental hypothesis”. In fact, after migration to Turin[2], Lazio[3], and Lombardy[4], children of two Sardinian parents seemed to have the same level of risk of type 1 diabetes as residents of Sardinia (those who had not migrated to elsewhere), while children of one Sardinian parent showed an intermediate incidence (though significantly higher)[5].
Another recent study evaluates changes in type 1 diabetes incidence after migration from low-incidence areas to Sweden, considered a high-incidence area. Parental country of birth has been demonstrated to play a key role in the development of type 1 diabetes in the offspring. Indeed, offspring of migrant populations presented similar incidence rates when compared to offspring of native populations[6].
In type 1 diabetes, as well as in other autoimmune conditions, the presence of specific autoantibodies can be revealed in serum before the clinical onset of the disease. Four different autoantibodies are considered predictive markers of type 1 diabetes and represent the autoimmune response against pancreatic β-cells[7]: Islet Cell Antibodies (ICA), Glutamic Acid Decarboxylase Auto-antibodies (GADA), autoantibodies to Insulin (IAA) and to tyrosine phosphatase-like protein (IA-2A). It is as yet unclear which stressors are responsible for autoantibody production, though it was hypothesized that viruses can be involved in the outbreak of the autoimmune response[8].
A study from Kulmala et al.[9] has shown that offspring of type 1 diabetes patients had a significantly higher risk of the disease (70%) in the presence of persistent positivity for at least three or four autoantibodies. Moreover, it was observed that the same risk dramatically decreased to 25%, 2% and 0.8% in siblings with, respectively, two, one, or no autoantibodies positivities.
Several reports[10–12] showed that the most important predictors for the identification of first-degree relatives with an elevated disease risk were GADA and IA–2 antibodies. Accordingly, current guidelines[13] for the primary testing of this population recommend the measurement of GADA along with either IA–2 or autoantibodies against insulin (IAA). However, it has been proven that antibody tests give elevated but not absolute predictive specificity and sensitivity. To increase sensitivity it is possible to screen the presence of human leukocyte antigen (HLA)-DRB1/DQB1 genotypes associated with diabetes, like HLA-DRB1*03 (DQB1 0201) and DRB1*04/DQB1 0302[14].
In the present study, we sought to determine whether there was an association between migration to a geographical area of lower incidence of type 1 diabetes and the prevalence of GADA and IA-2A in a group of Sardinian migrants and their offspring. Additionally, the prevalence of other autoimmune disease, such as thyroiditis, thalassemia, multiple sclerosis, celiac disease and G6PD deficiency, was recorded.
Materials and Methods
In 1997, according to the Lombardy population-based registry, the number of Sardinian migrants living in the province of Pavia was 2808. All the study participants were recruited by means of letters or phone calls. Of the 2808 subjects of the original sample, only 2261 received the questionnaire and 1193 completed it. Questionnaire data have been previously reported[15]. In 2001, among the responders (N=1193), 787 individuals were enrolled by letters. Residents in Pavia and proximities were referred to the Department of Preventive Medicine in the University of Pavia, whereas people from other cities in the province were referred to the local hospitals of Vigevano, Voghera and Stradella.
Seven participants were excluded from the sample because they were not of Sardinian descent. The final sample included 554 subjects born in Sardinia and 226 offspring of Sardinian migrants. The latter were divided into two subgroups: 49 (21.7%) whose parents were both Sardinian and 177 (78.3%) who have only one parent from Sardinia (Figure 1). Study participants underwent a complete anamnestic evaluation through questionnaires. All subjects were asked about family history of type 1 diabetes, presence of thalassemia, G6PD deficiency, autoimmune diseases (thyroiditis, multiple sclerosis and celiac disease), and about lifestyle and nutrition. Fasting blood samples were drawn from all participants and collected in Vacutainer tubes (Becton-95 Dickinson, Meylan Cedex, France) containing 0.12mL (0.34 mol/L) EDTA solution and stored at -20°C. Glycaemia and HbA1c were determined using standard laboratory measurements (Glu-cinet Sclavo and NycoCard HbA1c, respectively). Diagnosis of diabetes mellitus was based on two fasting plasma glucose levels ≥ 126 mg/dl and HbA1c ≥ 7%.
Figure 1.
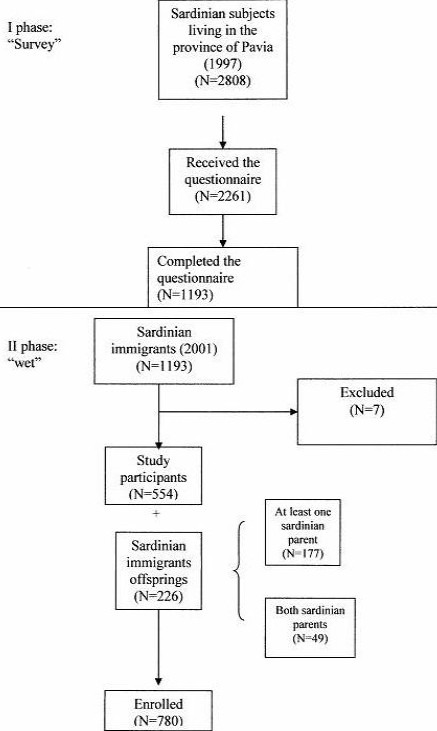
Study flow-chart
Samples for HLA genotyping and GADA and IA-2 analyses were sent to an external laboratory at the San Raffaele Hospital in Milan. HLA class I antigens were determined using a standard microlymphocytotoxicity technique[16]. HLA class II genotyping was performed using PCR with amplification with sequence-specific primers.
Accordingly with their HLA class II genotype, all subjects were divided into three groups using a simplified classification: high risk (presence of both HLA-DR3 -DR4 and DQB1/0302), moderate risk (presence of HLA-DR3 or HLA-DR4) and low risk (presence of neither haplotype).
RIA method was performed in order to evaluate GADA and IA-2 levels. The following ranges were used to assess GADA positivity, according to DASP data[17]:
-
➢
< 0.9 U/ml = negative;
-
➢
0.9 ÷ 1.25 U/ml = weakly positive;
-
➢
≥ 1.25 U/ml = positive.
IA-2 positivity was defined as follows:
-
➢
< 0.75 U/ml = negative;
-
➢
0.75 ÷ 1.10 U/ml = weakly positive;
-
➢
≥ 1.10 U/ml = positive.
After a seven-year follow-up, subjects with GADA positivity and/or high genetic risk were reassessed and fasting blood glucose, HbA1c, GADA and IA-2 levels were measured.
Data were analyzed by the Kolmogorov–Smirnov test to determine distribution. All continuous variables were expressed as mean ± standard deviation (SD) and analyzed by means of Student's t-test. Categorical data were expressed as proportions and were analyzed by the χ2 test. We calculated a 95% confidence interval for all data. The SPSS 11.0 statistical package (SPSS Inc., Chicago, IL, USA) was used in all statistical analysis. Significance tests for comparison were two-tailed, and results were considered significant at p<0.05.
Results
Sardinian Migrants Results
Subgroup characteristics are reported in table 1. The 554 study participants included 238 males and 316 females. Mean ± SD age was 49.6 ± 15.27 years; mean ± SD age at the time of migration was 18.6 ± 10.6 years. Autoimmune diseases and genetic disorders were reported in 20.57% of the sample (thyroiditis 6.5%, celiac disease 0.4%, thalassemia 6.7% and G6PD deficiency 7.0%).
Table 1.
Characteristics of the Sardinian Migrants Subgroup
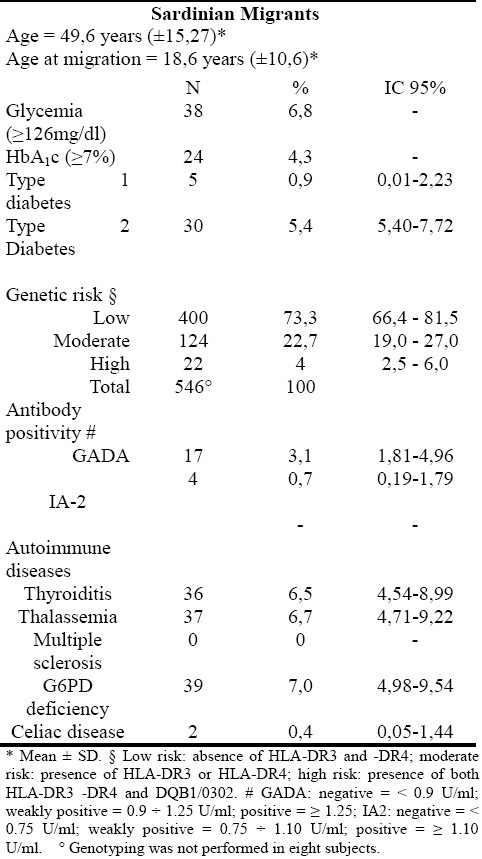
Prevalence of type 1 diabetes was 0.9%, whereas type 2 diabetes prevalence was 5.4%.
Subjects included in low, moderate and high genetic risk groups were 73.3%, 22.7% and 4.0%, respectively.
GADA positivity was found in 3.1% of the sample, whereas IA-2 positivity was present in 0.7%. After removing type 1 diabetic patients from the analysis, GADA prevalence decreased to 2.4% with a mean ± SD age of GADA-positive subjects of 43.69 ± 19.40 years. Among the high genetic risk group, subjects showing GADA positivity were 10.0% (p<0.03; table 2).
Table 2.
Comparison between GADA positivity and genetic risk (high/low) in the Sardinian migrants subgroup, after removing type 1 diabetes subjects
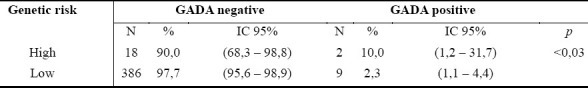
The major findings of the study are presented as Sardinian Migrants results, Offspring results and Follow-up results. The final sample size was 780 (parents=554, offspring=226).
Offspring Results
Major subgroup characteristics are shown in table 3. The 226 offspring included 93 males and 133 females. Mean ± SD age was 22.1 ± 10.99 years. Four participants were affected by thyroiditis, seven had thalassemia, four had G6PD deficiency and only one girl had multiple sclerosis.
Table 3.
Characteristics of the Offspring Subgroup
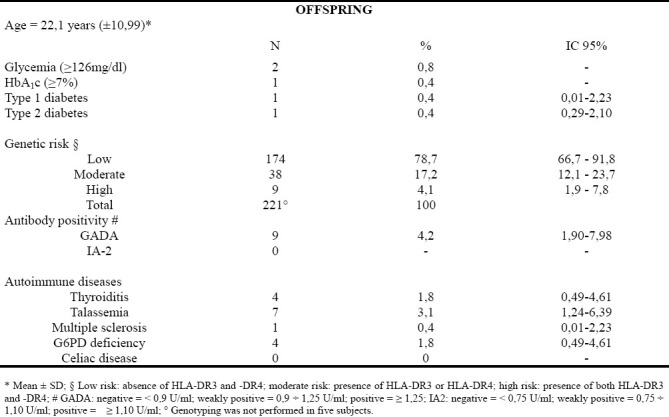
Prevalence of type 1 and type 2 diabetes were both 0.4% (one subject for each type of diabetes).
Offspring included in low, moderate and high genetic risk groups were 78.7%, 17.2% and 4.1%, respectively. GADA positivity was found in 4.2% of the sample, whereas no subject presents IA-2 positivity. After removing the type 1 diabetic patient from the analysis, GADA prevalence decreased to 3.8%. Mean ± SD age of GADA-positive subjects was 21.67 ± 8.42 years.
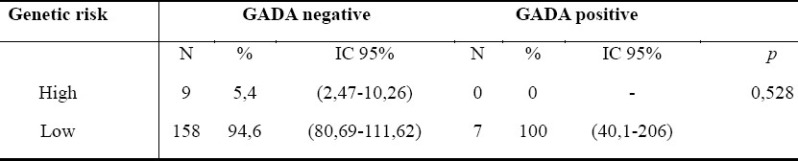
Among the offspring high genetic risk group, all subjects were GADA negative (table 4).
Table 4.
Comparison between GADA positivity and genetic risk (high/low) in the non-type 1 diabetes offspring subgroup
Follow-up Results
The second part of the study took place after a seven-year follow-up. We evaluated 31 subjects (22 Sardinian migrants and 9 offspring) with high genetic risk and/or GADA and/or IA-2 positivity in order to determine whether there was an increase in antibody levels or a change in autoantibody positivity. Among Sardinian migrants, 11 (50%) were lost during follow-up.
In 2001, none of the study participants was positive to both autoantibodies. Of seven migrant subjects positive in 2001, 6 (85.7%) had persistent GADA positivity whereas one (11%) became negative (transient). In two positive offspring, GADA positivity persisted, and two negative offspring presented GADA positivity in 2008. Only one offspring with a type 1 diabetic father presented an increase in GADA levels (from 0.10 to 49 U), thus becoming positive. Moreover, he became positive for IA-2 (IA-2>100U).
None of the GADA-positive subjects have developed type 1 diabetes, although two late-adult subjects (one GADA-positive and at low risk and the other autoantibody-negative, but at high risk) may have developed impaired glucose tolerance (fasting glucose levels≥110 mg/dl and HbA1c≥7%).
Within the eight offspring who had persistent GADA, none developed diabetes nor have they shown impaired fasting blood glucose and HbA1c≥7%.
Discussion
The aim of the present study was to evaluate the relationship between genetic risk and GAD and IA2 levels in the Sardinian migrant population living in the province of Pavia.
During the first phase (Survey, Figure 1), study participation reached 53%. Unfortunately, in the second part of the study (called “wet”, Figure 1) participation rate decreased to 46.4%. One reason for declining participation could be blood sampling in the wet phase. This growing refusal to participate should not be attributed to the study design or to challenges in finding and contacting study participants who previously received questionnaires and were subsequently reached by mail or phone. In fact, migrants and their offspring not living in Pavia could refer to local hospitals or, in some cases, blood was drawn domiciliary. Among responders, prevalence of high genetic risk individuals was similar to that of native Sardinian populations (4% and 5.9%, respectively)[18], but higher as compared with Caucasian populations in general (0.77%)[19]. Prevalence of moderate genetic risk was also higher than within Caucasoids (22.7% vs. 12.75%)[19].
In current literature, there are no studies investigating GADA positivity in the native Sardinian population. However, Loviselli et al.[20] observed the ICA positivity rate to vary between 2.5% and 6.1% in a sample of 6463 Sardinian children of 6-14 years of age. A Finnish study[10] reported GADA and IA-2 positivity rates of 1% and 0.6% in the general population. Our data showed a higher prevalence of autoantibodies positivity (GADA 2.4%) in a non-diabetic population. Moreover, GADA positivity in non-diabetic offspring in our study was higher than in the Finnish population. This finding might support the hypothesis of a prevalent genetic component in the pathogenesis of type 1 diabetes.
Among other autoimmune diseases recorded in this study, G6PD deficiency was found in 7% of the sample compared to 15% of the Sardinian population[21]. However, prevalence among Sardinians was determined by means of G6PD dosage in 846 newborns[21], while our data were only anamnestic. Additionally, we could not exclude the “healthy migrant effect”, i.e., first-generation immigrants are often healthier than native populations which share a similar ethnic background[22]. Prevalence of autoimmune thyroiditis was higher among Sardinian migrants but irrelevant among their offspring. This latter result could be due to the young age of offspring[20]. In line with these findings, Salabè-Lotz et al.[23] investigated the presence of anti-thyroid antibodies (ATA) in 4 samples of the general populations from Bologna, Napoli, Palermo, Rome and Cagliari. The authors found a higher prevalence of ATA positivity in the Cagliari sample. The present study has shown a higher prevalence of GADA positivity within Sardinian migrants at high genetic risk; GADA positivity may represent the first detectable phase of the autoimmune cascade leading to the development of type 1 diabetes in migrants and their offspring[18]. This finding may suggest a leading role of genetic susceptibility in type 1 diabetes compared with hypothetical environmental risk factors.
Follow-up of high genetic/antibody risk individuals involved a very small sample of participants. In this group, none of the subjects developed type 1 diabetes, as also reported in a study from LaGasse et al.[24]. As in the DAISY study[12], we also observed transient positivities. Longitudinal[12,24] studies showed that type 1 diabetes risk was positively correlated with the follow-up time and the number of positive subjects. However, it seems questionable to perform follow-up of individuals at high risk for the disease in order to diagnose subclinical type 1 diabetes and to prevent the development of complications.
Acknowledgments
Conflict of interest disclosures: no conflict of interest.
References
- 1.The DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabetic Medicine. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 2.Bruno G, Pagano G, Faggiano F, De Salvia A, Merletti F. Effect of Sardinian heritage on risk and age at onset of type 1 diabetes: a demographic case-control study of Sardinian migrants. International Journal of Epidemiology. 2000;29:532–535. [PubMed] [Google Scholar]
- 3.Muntoni S, Fonte MT, Stoduto S, Marietti G, Bizzarri C, Crinò A, Ciampalini P, Multari G, Suppa MA, Matteoli MC, Lucentini L, Sebastiani LM, Visalli N, Pozzilli P, Boscherini B, Muntoni S. Incidence of insulin-dependent diabetes mellitus among Sardinian-heritage children born in Lazio region, Italy. Lancet. 1997;349:160–162. doi: 10.1016/s0140-6736(96)04241-9. [DOI] [PubMed] [Google Scholar]
- 4.Calori G, Gallus G, Bognetti E, Chiumello G. Insulin-dependent diabetes mellitus in Sardinian-heritage children living in Lombardy. Lancet. 1998;351:263–264. doi: 10.1016/S0140-6736(98)24004-9. [DOI] [PubMed] [Google Scholar]
- 5.Songini M, Casu A. Epidemiology of childhood diabetes. Acta Biomedica. 2005;76(suppl.3):19–25. [PubMed] [Google Scholar]
- 6.Hjern A, Söderström U. Parental country of birth is a major determinant of childhood type 1 diabetes in Sweden. Pediatric Diabetes. 2007;101:1399–1404. doi: 10.1111/j.1399-5448.2007.00267.x. [DOI] [PubMed] [Google Scholar]
- 7.Bizzarro N. The Predictive Significance of Autoantibodies in Organ-Specific Autoimmune Diseases. Clinic Rev Allerg Immunol. 2008;34(3):326–331. doi: 10.1007/s12016-007-8059-5. [DOI] [PubMed] [Google Scholar]
- 8.Tenconi MT, Martinetti M. Infectious diseases and Type 1 diabetes in children. Pediatric Health. 2008;2(5):583–594. [Google Scholar]
- 9.Kulmala P, Savola K, Petersen JS, Vähäsalo P, Karjalainen J, Löppönen T. Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes. A population-based study. J Clin Invest. 1998;101:327–336. doi: 10.1172/JCI119879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siljander HT, Veijola R, Reunanen A, Virtanen SM, Åkerblom HK, Knip M. Prediction of type 1 diabetes among siblings of affected children and in the general population. Diabetologia. 2007;50:2272–2275. doi: 10.1007/s00125-007-0799-5. [DOI] [PubMed] [Google Scholar]
- 11.Dittler J, Seidel D, Schenker M, Ziegler AG. GADIA2- combi determination as first line screening for improved prediction of type 1 diabetes in relatives. Diabetes. 1998;47:592–597. doi: 10.2337/diabetes.47.4.592. [DOI] [PubMed] [Google Scholar]
- 12.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, Eisenbarth GS, Rewers M. Diabetes Autoimmunity Study in the Young.Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY) J Clin Endocrinol Metab. 2004;89(8):3896–3902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]
- 13.Bingley PJ, Bonifacio E, Ziegler AG, Schatz DA, Atkinson MA, Eisenbarth GS. Proposed guidelines on screening for risk of type 1 diabetes. Diabetes Care. 2001;24:398. doi: 10.2337/diacare.24.2.398. [DOI] [PubMed] [Google Scholar]
- 14.Thorsby E, Ronningen KS. Particular HLA-DQ molecules play a dominant role in determining susceptibility or resistance to Type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:371–377. doi: 10.1007/BF00402270. [DOI] [PubMed] [Google Scholar]
- 15.Tenconi MT, Devoti G, Nasetti G, Piazza M, Songini M, Bottazzo GF. Diabete mellito di tipo 1 nei sardi emigrati in provincia di Pavia. Il Diabete. 1999;11(suppl.2):102–103. [Google Scholar]
- 16.Muro M, Marín L, Torío A, Moya-Quiles MR, Minguela A, Rosique-Roman J, Sanchis MJ, Garcia-Calatayud MC, García-Alonso AM, Alvarez-López MR. HLA polymorphism in the Murcia Population (Spain) in the Cradle of the Archaeologic Iberians. Hum Immunol. 2001;62:910–921. doi: 10.1016/s0198-8859(01)00290-7. [DOI] [PubMed] [Google Scholar]
- 17.Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: First Assay Proficiency Evaluation. Diabetes. 2003;52:1128–1136. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- 18.La Nasa G, Carcassi C, Cirillo R, Mulargia M, Leone AL, Vacca A, Pizzati A, Boero R, Arras M, Porcella R. Serological and molecular studies of HLA in insulin-dependent diabetes mellitus in Sardinia. Dis Markers. 1990;8(6):333–340. [PubMed] [Google Scholar]
- 19.Lorini R, Minicucci L, Napoli F, Padovani P, Bazzigaluppi E, Tortoioli C, Cherubini V, Bottazzo G, Pozzilli P, Falorni A, Buzzetti R. Screening for type 1 diabetes genetic risk in newborns of continental Italy.Primary prevention (Prevefin Italy)-preliminary data. Acta Biomed. 2005;76(3):31–35. [PubMed] [Google Scholar]
- 20.Loviselli A, Velluzzi F, Locatelli M. Immunological prediction for type 1 diabetes (T1D) in a school children population living in Sardinia Island. J Endocrinol Invest. 2000;23(6):23–31. [Google Scholar]
- 21.Rapallo M, Zulato AM, Matzeu G. Frequenza del deficit di G6PD nel Sarcidano e Barbagia di Seulo e ricoveri per favismo nell’ospedale di Isili. Epidemiologia in Sardegna. 2000;4:45–54. [Google Scholar]
- 22.Muening P, Fahs M. Health status and hospital utilization among immigrants to New York City. Prev Med. 2002;35:225–232. doi: 10.1006/pmed.2002.1072. [DOI] [PubMed] [Google Scholar]
- 23.Salabè-Lotz H, Salabè GB. Population survey of thyroid autoimmunity in Italy.Three year follow up. Thyroidology. 1990;2(3):107–112. [PubMed] [Google Scholar]
- 24.LaGasse JM, Brantley MS, Leech NJ, Rowe RE, Monks S, Palmer JP, Nepom GT, McCulloch DK, Hagopian WA. Washington State Diabetes Prediction Study.Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State Diabetes Prediction Study. Diabetes Care. 2002;25(3):505–511. doi: 10.2337/diacare.25.3.505. [DOI] [PubMed] [Google Scholar]


