Abstract
Puberty is a time of significant change in preparation for adulthood. Here, we examined how stressful experience affects cognitive and related hormonal responses in male and female rats prior to, during and after puberty. Groups were exposed to an acute stressor of brief periodic tailshocks and tested 24 h later in an associative memory task of trace eyeblink conditioning. Exposure to the stressor did not alter conditioning in males or females prior to puberty but enhanced conditioning in both males and females during puberty. The enhancement occurred in pubescent females irrespective of the estrous cycle. In adulthood, sex differences in trace conditioning and the response to stress emerged: females outperformed males under unstressed conditions, but after stressor exposure, trace conditioning in females was impaired whereas that in males was enhanced. These differences were not related to changes in gross motor activity or other nonspecific measures of performance. The effects of acute stress on corticosterone, estradiol, progesterone, and testosterone were also measured. Stressor exposure increased the concentration of corticosterone in all age groups, although sex differences were only evident in adults. All reproductive hormones except estradiol increased with age in a predictable and sex dependent fashion and none were affected by stressor exposure. Estradiol decreased in male rats across age, and remained stable for female rats. Together, these data indicate that males and female respond similarly to learning opportunities and stressful experience before and during puberty; it is in adulthood that sex differences and the opposite responses to stress arise.
Keywords: Development, Memory, Eyeblink, Sex difference, Age, Gender, Depression, Corticosterone, Estrogen, Testosterone
Introduction
Clinical studies document that gender differences in mental disorders emerge during or shortly after puberty and many of these disorders are stress related. For example, depression, anxiety, eating, and post-traumatic stress disorders are all more prevalent in women than men, but only after puberty (Kaltiala-Heino et al., 2003; Kessler, 2003; Piccinelli and Wilkinson, 2000; Ruiz et al., 2000). These studies suggest that puberty is a time when women become most vulnerable to stressful experience and some types of psychopathology. Nevertheless, the literature addressing stress and how it affects behavior during puberty is very limited (McCormick et al., 2004). This is particularly surprising given the recent controversies surrounding the prescription of psychotropic drugs to children and adolescents for these disorders.
In humans, the onset of puberty is defined by first menstruation in girls, and first ejaculation/nocturnal emission in boys (Kaltiala-Heino et al., 2003). In rats, puberty is a relatively short period between about 33 and 56 days of age (Gabriel et al., 1992; Ojeda and Urbanski, 1994) and is indicated by canalization of the vagina in females and balanopreputial separation of the foreskin from the glans of the penis in males (Ojeda and Urbanski, 1994). Puberty in most mammalian species is associated with dramatic increases in circulating sex hormones. Anatomically, these changes induce secondary sexual characteristics and behaviorally they alter peer interaction and risk taking behavior (Laviola et al., 2002; Spear, 2000a,b). Within the brain, puberty is associated with changes in neurotransmitter release and concentration (Badr et al., 1989; Choi and Kellogg, 1996; Choi et al., 1997; Insel et al., 1990) and gross changes in volume and white matter organization that continue well into the late teens in humans (Gogtay et al., 2004; Paus et al., 1999). It is thus reasonable to assume that these changes in hormone concentration and anatomical structure are accompanied by changes in nonreproductive behaviors such as those associated with learning and memory.
In previous studies, we documented sex differences in learning. Specifically, we observed that female rats outperform males during acquisition of an associative learning task, classical eyeblink conditioning (Wood and Shors, 1998; Wood et al., 2001). These sex differences must emerge before, during or after puberty since males and females condition at similar rates prior to puberty (Ivkovich et al., 2000). Others have found similar effects for spatial learning (Kanit et al., 2000; Krasnoff and Weston, 1976). Together, these data suggest that sex differences in learning arise as the capacity for reproduction is being established. However, little is known about how sex differences affect learning during puberty itself.
In addition to sex differences in learning, we have observed robust sex differences in response to stressful experience. In particular, we have shown that exposure to an acute stressful experience of periodic tail shocks or inescapable swimming has opposite effects on classical eyeblink conditioning in male versus female rats (Wood and Shors, 1998; Wood et al., 2001). Exposure to the stressful event enhances conditioning in males, whereas exposure to the same event impairs conditioning in females. These effects of stress on later conditioning are not specific to hippocampal-dependent types of learning tasks and occur during training on hippocampal-independent tasks (Wood et al., 2001). They are also not dependent on sex differences in learning itself since they occur even when performance is similar between unstressed males and unstressed females (Wood and Shors, 1998). They also do not appear to reflect performance effects since stress does not alter the magnitude of the unconditioned response (UR), that is, the blink response to the eyelid stimulation (Bangasser and Shors, 2004; Servatius et al., 2001). Nor does it affect pain, sensitivity, or gross motor activity (Shors, 2001). These effects are, however, dependent on the psychological aspects of the stressful event since the opportunity to control the stressor eliminates both the enhanced learning in males and the deficit in females (Leuner et al., 2004).
The opposite effects of stress on learning in males versus females are organized by the presence of sex hormones during very early development and in utero. Thus, manipulations of testosterone in utero and shortly after birth eliminate the effects of stress on learning in males, and reverse the effects in females, as expressed in adulthood (Shors and Miesegaes, 2002). In adulthood, the expression of these behaviors is dependent on the activating effects of ovarian hormones in females and corticosterone in males (Beylin and Shors, 2003; Wood and Shors, 1998). Thus, the critical hormonal and psychological determinants of these sex differences have been described. However, we do not know when during development these sex differences emerge. Therefore, in the present studies, we examined sex differences in learning and the response to acute stress in rats before, during, and after puberty. We also examined how stress alters the production of corticosterone and the reproductive hormones, estrogen, testosterone, and progesterone in males and females before, during, and after puberty.
Material and methods
Subjects
Prepubescent (25–29 days), pubescent (35–40 days), and adult (60 days and older) Sprague–Dawley rats were weaned at 21 days and housed individually prior to and after surgery in the Department of Psychology animal facility at Rutgers University. They were given ad libitum access to water and laboratory chow and maintained on a 12:12 light/dark cycle with light onset at 8 am. For the conditioning studies, groups consisted of prepubescent males (stress = 9; no stress = 10), prepubescent females (stress = 9; no stress = 9), pubescent males (stress = 8; no stress = 11), pubescent females that were trained in diestrus/proestrus = (stress = 7; no stress = 10) or trained in estrus (stress = 8; no stress = 11), adult males (stress = 10; no stress = 11), adult females (stress = 9; no stress = 10). We also obtained blood from separate groups of animals in order to detect hormonal responses to the stressor immediately after its cessation. These groups consisted of male prepubescents (n = 10), female prepubescents (n = 10), male pubescents (n = 10), female pubescents sacrificed in estrus (n = 5) or in proestrus/diestrus (n = 7), male adults (n = 10), and adult females sacrificed in diestrus 2 (n = 12).
Vaginal cytology and determination of estrous cycle
Following vaginal canalization (Ojeda and Urbanski, 1994), smears were collected daily in the morning. A Q-tip immersed in physiological saline was inserted into the vaginal tract and rotated to remove loose cells. Cells were rolled onto a slide, dried and stained with 1% Toluidine Blue. Only adult females with a regular 4–5 days cycle including proestrus, estrus, diestrus 1, and 2 were included. Pubescent females alternated between a cytology consistent with estrus and another with characteristics of proestrus and diestrus 1. To evaluate the effects of the two stages in pubescent females, they were either stressed and trained 24 h later in estrus, or stressed and trained 24 h later in proestrus. As in previous studies, adult females were exposed to the stressor during diestrus 2 and trained 24 h later in proestrus (Wood and Shors, 1998; Wood et al., 2001).
Surgical procedures for trace eyeblink conditioning
Animals were anesthetized through inhalation of isoflur-ane and oxygen. Four electrodes (stainless steel wire .005 in diameter) were implanted around the eyelid for eyeblink conditioning. This occurred via an incision in the skull skin through which wires were drawn, and placed in the upper ridge of the eyelid. Electrodes were attached to a headstage that was anchored to the skull with 4 surgical screws and dental acrylic. After surgery, animals were provided one oral dose of acetaminophen (0.1 mg/kg) and placed in a heated recovery box until full consciousness was regained. Animals were then returned to their home cages for at least 48 h of recovery before testing.
Stressor exposure, trace eyeblink conditioning, and activity measurements
Rats were taken directly from their home cage to the conditioning environment, which consisted of conditioning boxes in lit (7.5 wt) sound-attenuating chambers and connected by their headstage to a cable that allowed free movement within the chamber. They remained there for 45 min during which spontaneous eyeblinks were recorded. After this acclimation period, rats in the stress group were taken into a separate context, placed in a Plexiglas restraining tube contained within a dark sound attenuating chamber. They were exposed to 30, 1 s, 1 mA, 60 Hz tailshocks at the rate of 1 per min and then returned to their home cages. Unstressed rats were returned to their home cage immediately after acclimation.
Twenty-four hours later, rats were returned to the conditioning chamber. Prior to training rats were given a 10-min period of acclimation. They were then exposed to a neutral stimulus (10, 250 ms bursts of 82 dB white noise, 5 ms rise/fall time). Eyeblinks immediately following the white noise were recorded as sensitized responses. Rats were then exposed to 300 trials of paired stimuli using a trace paradigm. A conditioned stimulus (CS—the previously described white noise) was followed by a 500-ms stimulus-free trace interval and a 100 ms (0.55 mA) periorbital unconditioned stimulus (US). Trials were administered in sets of 10 in the following order: 1 CS alone trial, 4 paired trials, 1 US alone trial, 4 paired trials. Inter-trial intervals were randomized at 25 ± 5 s. Eyeblinks were indicated by changes in EMG activity as recorded from the obicularis oculi muscle. Eyelid electrodes were connected to a differential amplifier with a 300- to 500-Hz band pass filter and amplified by 10 K. EMG signals were digitized at 1 kHz with a 16-bit A/D card (Keithley-Metrabyte, Taunton, MA) and relayed for analysis via computers. Eyeblinks during the trace interval were considered CRs and if they persisted for >3 ms and were >4 times the standard deviation of responses during a 250-ms stimulus-free period which was recorded prior to CS onset.
Twenty-four hours after conditioning, rats were transferred into a different and dimly lit room (single 40 wt bulb) and placed in Plexiglas activity chamber (30 cm3) for 30 min. Activity in the chamber was monitored with 8 photobeams at 4 cm intervals. Breaks in the beams were converted to horizontal and vertical movements.
Hormone concentrations
As noted, we obtained blood from separate groups of animals to detect immediate hormonal responses to the stressor. These groups were either exposed to the stressor of restraint and periodic tail shocks (30, 1 s, 1 mA, 60 Hz tailshocks, 1 per min) or left unstressed. Within 5 min of stressor exposure, rats were injected with a lethal dose of pentobarbital (0.25 ml/kg) and cardiac blood was collected at the time of sacrifice (10 am–1 pm). Blood was collected in test tubes with heparin and centrifuged for 20 min at 3000 rpm. Plasma aliquots were stored frozen until analysis. Circulating levels of corticosterone, estradiol, progesterone, and testosterone were analyzed using a solid-phase radio-immunoassay (RIA) system (Coat-A-Count, Diagnostic Products, Corp.) Assay sensitivities for corticosterone, estradiol, progesterone, and testosterone were 5.7 ng/mL, 8 pg/mL, 0.02 ng/mL, and 4 ng/dL, respectively. Intra-assay variabilities for corticosterone, estradiol, progesterone and testosterone were 4.3%, 5.8%, 6.1%, and 12%, respectively. Inter-assay variabilities for corticosterone, estradiol, progesterone, and testosterone were 5.8%, 7.4%, 7.1%, and 12%, respectively. Samples for all subjects were assayed for each specific hormone in tandem using the same materials.
Results
Trace conditioning and the response to acute stress before puberty
To determine the effects of stress on eyeblink conditioning in males versus females for each age group, we used a repeated measures and between subject analysis of variance (ANOVA) with sex and stress as independent variables and percentage of conditioned responses (% CRs) over 3 blocks of 100 trials as the dependent measure. Prior to puberty, there were no main effects of stress [F(1,33) = 3.41; P > 0.05] or sex differences [F(1,33) = 0.01; P > 0.05] (Fig. 1A). Prepubescent animals did learn as evidenced by an increase in responding to the CS over blocks of trials [F(2,66) = 6.65; P < 0.01]. To demonstrate that there was an association formed we also applied two-factor ANOVAs (sex by stressor exposure) to the % CRs on CS alone trials. Thus, we analyzed responses to the CS on the 30 trials in which a CS was presented in the absence of a reinforcing US. Prior to puberty, there were no interactions between stressor exposure and sex [F(1,33) = 0.955; P > 0.05], nor were there any main effects of stress [F(1,33) = 3.08; P > 0.05] or sex [F(1,33) = 0.008; P > 0.05] on these responses (Fig. 1D). Thus, prior to puberty, males and females did learn but there were no sex differences and they did not respond to the stressor.
Fig. 1.
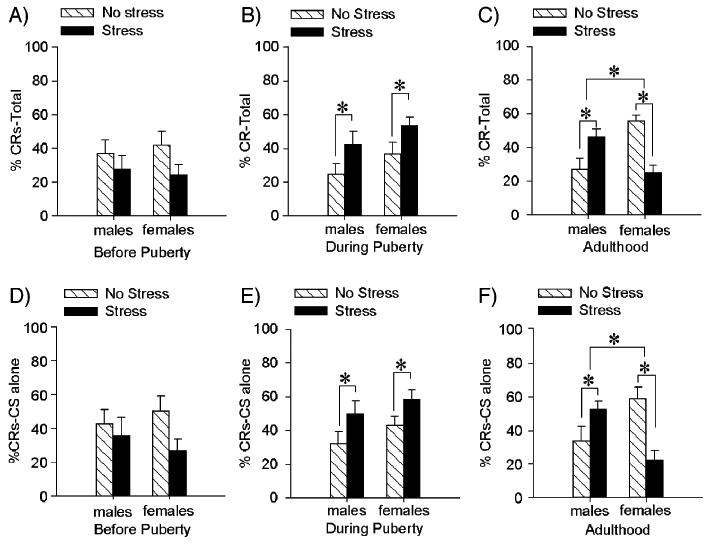
Effects of stress on trace eyeblink conditioning before, during and after puberty. For A–C, the bars represent the mean (±SEM) percentage of CRs across the 300 trials of training. For D–F, the bars represent the mean (±SEM) percentage of CRs on CS alone trials. (A) Prior to puberty, there were no differences in conditioning between males and females and no effect of stress on trace conditioning as measured 24 h after exposure to an acute stressor of brief, periodic tailshocks. (B) During puberty, there were no sex differences in trace conditioning, although exposure to the stressful event enhanced levels of conditioning in males and females. (C) In adulthood, females emitted more CRs than did males and exposure to the stressful event enhanced conditioned responding in males and impaired responding in females. (D) Prior to puberty, there were no sex differences or effects of stress on the responses to the CS on CS alone trials. (E) During puberty, stress enhanced these responses regardless of sex. (F) In adulthood, females emitted more CRs than did males but in response to stressor emitted fewer. In contrast, males emitted more CRs on CS alone trials if they had been exposed to the acute stressor.
Trace conditioning and the response to acute stress during puberty
For animals that were tested during puberty, there was a main effect of stressor exposure [F(1, 57) = 8.88; P < 0.01], indicating that males and females responded more to the CS 1 day after exposure to the stressful stimulus of periodic tailshocks. There was no difference in conditioned responding between unstressed males and females [F(1,57) = 2.83; P > 0.05] and no interaction between sex and stressor exposure [F(1,57) = 0.05; P > 0.05] (Fig. 1B). Both males and females emitted more conditioned responses across blocks of training trials indicating they did learn [F(2,114) = 21.25; P < 0.001]. However, there was no sex difference in responding [F(2,114) = 0.83; P > 0.05]. Furthermore during puberty stressor exposure increased responding to the CS on CS alone trials [F(1,55) = 5.60; P < 0.05]. There were no sex differences between pubescent males and females [F(1,55) = 2.30; P > 0.05] and no interaction between stressor exposure and sex [F(1,55) = 0.07; P > 0.05] (Fig. 1E). Thus, males and females learned similarly during puberty and both showed an increase in conditioned responding after stressor exposure.
As noted, females in puberty cycle through two stages, one resembling proestrus/diestrus and the estrus. To determine whether the stage of the estrous cycle interacts with the enhanced conditioning during puberty, we analyzed these females according to what stage they were in during the training procedure. As shown in Fig. 2, pubescent females responded similarly irrespective of stage [F(1,32) = 0.006; P > 0.05]. There was no main effect of estrous cycle on conditioning [F(1, 32) = .218; P > 0.05], and only a main effect of stress, as noted previously [F(1,32) = 6.47; p < 0.05] (Fig. 2A). Pubescent females in both stages of their estrous cycle demonstrated increases in % CRs across blocks of trials regardless of treatment [F(2,64) = 17.74; P < 0.001]. In addition, stress increased responding to the CS alone for pubescent females regardless of cycle [F(1,31) = 4.20; P < 0.05]. There was no effect of stage of estrus in pubescent females [F(1,31) = 0.67; P > 0.05] nor any interaction between stage and stress on eyeblink responses to the CS on CS alone trials [F(1,31) = 0.11; P > 0.05] (Fig. 2B).
Fig. 2.
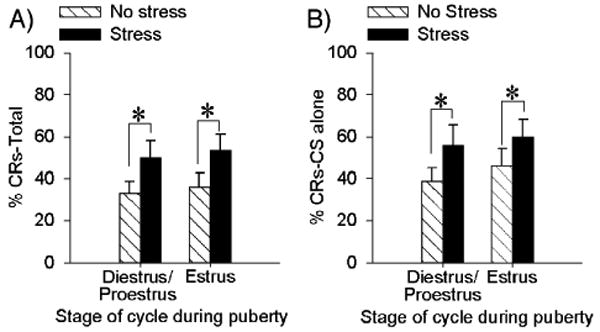
Stress enhanced conditioning in pubescent females irrespective of stage of estrus. (A) Bars represent the mean (±SEM) percentage of CRs across 300 trials of trace eyeblink conditioning in pubescent females that were trained either in diestrus or estrus. (B) Bars represent the mean (±SEM) percentage of CRs on CS alone trials in these same pubescent females.
Trace conditioning and the response to acute stress during adulthood
The results in adults were similar to those that we have reported previously (Leuner et al., 2004; Shors and Miesegaes, 2002; Wood and Shors, 1998). An ANOVA on the percentage of CRs over all blocks of trials indicated an interaction between sex and stressor exposure [F(1, 36) = 27.88; P < 0.01]. Post hoc analysis with NewmanKeuls indicated that unstressed females in proestrus emitted a greater percentage of CRs over all trials of training (P < 0.001). However, in response to the acute stressor of periodic tail shocks, males responded more (P < 0.05) and females responded less (P < 0.05). Thus, as shown previously, adult females trace condition more than do males but respond in opposite directions to the acute stressful event (Fig. 1C). Both males and females demonstrated an increase in responding over blocks of trials regardless of stressor exposure [F(2,72) = 15.54; P < 0.001]. For adults, there was also an interaction between stressor exposure and sex on the % CRs on CS alone trials [F(1,31) = 16.16, P < 0.001] (Fig. 1F). Post hoc analysis demonstrated that unstressed females produced more CRs than unstressed males (P < 0.05) and stressed females (P < 0.01). As with the response on paired trials, exposure to the stressor was associated with decreased responding in females and enhanced responding in males (P < 0.05).
Activity levels and spontaneous blinking as a function of age, sex and stress
In these analyses, we used a 3-factor ANOVA with sex, stress and age as independent variables. It indicated no effect of stress on spontaneous blinking [F(2, 126) = 0.60; P > 0.05] or responses to the white noise prior to training [F(2,126) = 0.35; P > 0.05]. There was a very slight increase in the number of spontaneous blinks emitted during the recording period (total = 15 s) in adults since they blinked on average, one more time than did prepubescent and pubescent animals [F(2,126) = 12.02; P < 0.05]; however there were no interactions between sex and age [F(2,126) = 0.15; P > 0.05], stress and age[F(2,126) = 1.19; P > 0.05] or sex, stress and age [F(2,126) = 0.59; P > 0.05]. Age also affected responses to the white noise prior to training [F(2,126) = 4.38; P < 0.05]. Post hoc analysis indicated that pubescent rats produced fewer sensitized responses than other age groups (P values < 0.05), although these differences were negligible and only varied between one versus two blinks out of ten. In terms of gross motor activity, we analyzed a combined number of horizontal and vertical movements. After ANOVA, we found no interactions between sex, stressor exposure or age [F(2,88) = 0.16; P > 0.05] and no main effects of stress [F(1,88) = 0.32; P > 0.05], sex [F(1,88) = 1.79; P > 0.05] or age [F(2,88) = 1.38; P > 0.05].
Corticosterone responses by age, sex, and in response to stress
For analyses of corticosterone concentrations, we used an ANOVA, again with 3 independent variables of stress, sex and age. There was a sex by age interaction [F(2,49) = 3.88; P < 0.05] indicating that adult females had higher corticosterone concentrations than any other age group of either sex (P values < 0.05). There was also an interaction between stressor exposure and sex across age [F(1,49) = 4.16; P < 0.05] and as expected, post hoc analysis with Newman– Keuls indicated that stressed females expressed higher corticosterone concentrations than stressed males (P < 0.001) and unstressed females (P < 0.001). Stressed males had higher concentrations than unstressed males (P < 0.001), however, unstressed males did not differ from unstressed females (P = 0.12). There were no interactions between stressor exposure and age [F(2, 49) = 0.34; P > 0.05] and no 3-way interaction [F(2, 49) = 1.48; P > 0.05]. There was a main effect of stress [F(1,49) = 157.87; P < 0.001] with stressed animals expressing higher concentrations than unstressed animals regardless of age or sex. There was a main effect of sex [F(1, 49) = 17.47; P < 0.001] with females expressing higher concentrations than males, and a main effect of age [F(2,49) = 4.98; P < 0.05) with adults expressing higher concentrations than the prepubescents (P < 0.001) (Fig. 3).
Fig. 3.
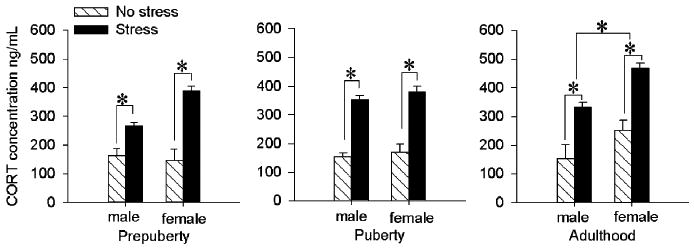
Corticosterone concentrations immediately after stressor exposure were elevated in all groups. Bars represent the mean (±SEM) corticosterone concentration (ng/mL). Prior to puberty, exposure to the stressor of brief periodic tailshock increased corticosterone concentrations in males and females and did so more in females than males. There were no sex differences under unstressed conditions. During puberty, exposure to the stressor increased corticosterone concentrations in both males and females. In adulthood, exposure to the stressor increased corticosterone concentrations in males and females. Females had higher levels of corticosterone than males in both basal and stressed conditions.
Gonadal hormonal responses by age, sex, and in response to stress
To determine the effects of sex, stressor exposure and age on gonadal hormones, we assayed estradiol, progesterone, and testosterone (Table 1). A three-factor ANOVA indicated that there was an interaction between sex and age on estradiol concentrations [F(2,52) = 26.44; P < 0.01]. Post hoc analysis with Newman–Keuls indicated that prepubescent male rats had elevated concentrations of estradiol compared to pubescent males (P < 0.05) and adult males (P < 0.01). Prepubescent males did not differ from any of the female age groups (P values > 0.05), nor did the females differ from each other at any age (P values > 0.05). All females had higher estradiol concentrations than pubescent and adult males (P values < 0.05). During puberty, estradiol levels did not differ according to cycle [F(1,8) = 0.001; P > 0.05]. Exposure to the stressor did not alter estradiol concentrations in any group [F(1,52) = 0.02; P > 0.05]. Progesterone concentrations were elevated in adult females [F(2,50) = 5.52; P < 0.01] and more so than any other group (P values < 0.05). There was no effect of stressor exposure on progesterone concentrations for any group [F(1,50) = 3.61; P > 0.05]. Finally, testosterone concentrations were elevated in adult males as demonstrated by an age by sex interaction [F(2,49) = 32.15; P < 0.05]. Post hoc analysis indicated that adult males had higher concentrations of testosterone than any other group (P values < 0.05). Stressor exposure did not alter concentrations of testosterone [F(1,49) = 2.5; P > 0.05].
Table 1. Mean (±SEM) hormonal concentrations for estradiol, progesterone and testosterone collapsed across stressor exposure (stress/no stress) at 3 ages; prepubescent (25–29 days), pubescent (35–40 days), and adult (>60 days) male and female rats.
| Pre-pubescent | Pubescent | Adult | |
|---|---|---|---|
| Male estradiol pg/mL | 13.20 ± 1.81* | 7.40 ± 0.61 | 4.55 ± 1.07 |
| Female estradiol pg/mL | 12.62 ± 1.70* | 16.17 ± 1.60* | 15.67 ± 1.81* |
| Male progesterone ng/mL | 6.28 ± 1.5 | 3.04 ± 0.91 | 5.89 ± 1.34 |
| Female progesterone ng/mL | 4.57 ± 1.20 | 11.83 ± 2.53 | 18.51 ± 3.78* |
| Male testosterone ng/dL | 15.03 ± 5.39 | 7.61 ± 2.51 | 189.80 ± 36.81* |
| Female testosterone ng/dL | 2.15 ± 0.77 | 0.75 ± 0.21 | 1.0 ± 0.44 |
Asterisk within a hormonal assay indicates a significant difference (P < 0.05) from the unmarked means.
Discussion
In previous studies, we found that male and female rats learn at different rates and can respond in opposite directions to the same stressful event (Shors, 2004). In the present studies, we established when during development these sex differences emerge. As shown, females that were trained with trace conditioning during proestrus emitted more learned responses than did males under unstressed conditions (Wood and Shors, 1998; Wood et al., 2001). However, this sex difference in trace conditioning was only apparent in adults. Prior to and during puberty, sex differences were not evident. These findings are consistent with others that report no sex difference in learning prior to puberty (Ivkovich et al., 2000; Kanit et al., 2000; Krasnoff and Weston, 1976). More critically, the effects of stress on trace conditioning were very different before, during and after puberty. As shown previously, adult males emitted more conditioned responses after exposure to the stressor whereas adult females emitted fewer (Shors, 1998; Wood and Shors, 1998; Wood et al., 2001). However, prior to puberty, there was no detectable effect of stress on conditioning in either sex, and during puberty, both sexes emitted more responses after exposure to the stressful event. This response pattern in pubescent females was somewhat unexpected since it is similar to that expressed by adult males. Taken together, these data suggest that stress affects this type of memory formation differently before, during, and after puberty.
Overall, these effects of stress on trace conditioning appear to reflect processes involved in learning since they were evident not only when examining responses to paired training but also in the response to the CS alone which was presented periodically during the training regime. Also, the differences in conditioned responding were not associated with sex- or stress-related changes in gross motor activity or nonspecific responses to the conditioned stimuli. There was a slight but significant difference in spontaneous blinking and sensitized responses to the white noise according to age. Adult rats emitted about one more spontaneous blink than prepubescent and pubescent rats during the same period. These differences were not related to differences in conditioning and were not altered by stressor exposure. Overall, these data indicate that exposure to an acute stressful event has negligible effects on performance and no effect on classical conditioning prior to puberty, but has enhancing effects on conditioning in males and females during puberty and opposite effects in males and females as adults. Some of the behavioral changes are related to hormone levels in response to stress whereas others appear not. Each is discussed in turn below.
Effect of stress on learning before puberty
Exposure to the stressor did not alter trace eyeblink conditioning in animals that had yet to enter puberty. This effect might have been predicted since many species do not show a robust stress response very early in development. This period is referred to as the stress hyporesponsive period and often detected as a reduced hypothalamic– pituitary–adrenal (HPA) response to a stressful stimulus (Levine, 2001). However, the hyporesponsive period reportedly only persists for about 14 days after birth in rats (Okimoto et al., 2002) and our prepubescent animals were tested well beyond that at 25–29 days after birth. Moreover, we observed a very robust HPA response to the stressor of periodic tailshocks in all age groups. Therefore, the absence of a stress effect on trace conditioning prior to puberty is not directly attributable to diminished HPA activity. However, it has been demonstrated that elevated levels of corticosterone in response to stress do persist longer in rats that have yet to enter puberty (Romeo et al., 2004). It is possible that a persistent effect of corticosterone contributes to the absence of a stress effect on conditioning prior to puberty.
A reduction in norepinephrine could also contribute to these effects, since it is involved in the stress response and its release is reduced in male prepubescent rats after stressor exposure (Choi and Kellogg, 1996; Choi et al., 1997). Also, plasma levels of norepinephrine increase from puberty into adulthood (Weise et al., 2002). Anatomically, noradrenergic neurons in the locus coeruleus project to the hippocampus and the cerebellum, both structures of which are critically involved in the acquisition of trace memories (for review, see Christian and Thompson, 2003; Shors, 2004). Moreover, these neurons are sensitive to changes in estrogens and androgens and become sexually dimorphic as animals reach adulthood (Borsody and Weiss, 1996; Palamarchouk et al., 2002; Pinos et al., 2001). Other possible neural candidates include NMDA receptors, since their activation is necessary for acquisition of the classically conditioned eyeblink response as well as the enhanced conditioning after stressor exposure (Servatius and Shors, 1996; Shors and Mathew, 1998). These receptors are differentially regulated by glucocorticoids across development (Lee et al., 2003) and their affinity is modulated by the presence of sex hormones. For example, estrogen enhances the expression of several NMDA receptor subunits more as females approach puberty (Kanamaru et al., 2001).
Effects of stress on learning from puberty through adulthood
During puberty, both males and females emitted more learned responses after exposure to the stressful event. Thus, upon entry into this new stage of development, a stress effect did emerge and it was similar in males and females. The enhancement was evident in females irrespective of which stages of the estrous cycle they were in, estrus or proestrus/diestrus. Interestingly, estradiol levels did not differ between pubescent females estrus and proestrus/diestrus (16 ± 2 pg/ml), although their vaginal cytology was different. As a group, however, estradiol concentrations were elevated relative to those in pubescent and adult males (<10 pg/ml). Thus, overall levels of trace conditioning and the response to stress were similar between pubescent males and females even though estradiol levels were higher in females. These data suggest that enhanced levels of estradiol may be necessary for expression of a performance deficit in adulthood (Wood and Shors, 1998) but are insufficient during puberty. It is also noted that although estrogen levels in this study are consistent across ages in females, they would be higher in the adult females at the time of training (the blood from one group was obtained after stressor exposure and during diestrus 2, but the group which were conditioned but were trained at a different time point in proestrus).
Finally, we note that the enhanced conditioning observed in adult unstressed females during proestrus may interact with the effects of stress on conditioning. Specifically, it may be that stress does not so much impair learning in adult females as it prevents the enhanced learning that normally occurs during proestrus. The present pubescent data are consistent with this hypothesis since performance was similar in males and females under unstressed conditions and they responded similarly to stress. This hypothesis is not supported by other data however. For example, females that were chronically treated with the antidepressant Prozac were not adversely affected by stress and yet still conditioned more than males (Leuner et al., 2004). Given these various findings, it would be interesting to determine whether antidepressant treatment would prevent the enhanced conditioning observed after stressor exposure in the pubescent females, as it does the impairment in adult females. This is a timely topic since the Federal Drug Agency recently advocated strict warnings regarding suicidal thoughts and behaviors expressed in children and adolescents treated with antidepressants (FDA (US Food and Drug Administration), 2004).
In the end, these data do not indicate the mechanism whereby stress enhances learning in females during puberty. It may operate via a stress-induced release of corticosterone, as it does in males (Beylin and Shors, 2003) or alternatively via a disruption in the development of estrus. There are data in humans to suggest that trauma, such as that occurring during war and physical hardship, can delay the onset of menstruation (Tahirovic, 1998). There are also reports that menstruation can be prematurely induced by psychosocial stress in the home environment (Ellis and Garber, 2000). In our previous studies, we found no evidence that exposure to the stressor of periodic tailshock alters the cycle in adult female rats but we have not evaluated the effects of stress on the emergence of the estrous cycle. However, since animals in the wild encounter stressful stimuli on a nearly daily basis, it seems unlikely that it would be so sensitive to disruption.
Hormonal correlates of stress and learning
In a separate experiment, we assayed blood levels of hormones in groups of animals immediately after stressor exposure. We did this in order to evaluate any age and sex differences in response to the stressor itself. In previous studies, we have measured corticosterone in animals that were stressed and then trained 24 h later and in general, after 24 h, there is no difference in corticosterone between stressed and unstressed animals (Shors, 2001; Shors and Miesegaes, 2002). Here, we wanted to know which hormones were directly and immediately affected by stressor exposure. Corticosterone levels increased immediately after stressor exposure in all groups irrespective of age or sex. Sex differences for basal corticosterone concentrations emerged in adulthood and were higher in females (Shors and Miesegaes, 2002; Viau and Meaney, 1991). With respect to changes in reproductive hormones, estrogen levels were as expected and higher in females than in pubescent and adult males. Interestingly, levels of estradiol were elevated before puberty in males and similar to the levels in females. Since stress did not enhance conditioning in males prior to puberty, as it does during and after, it could be that the presence of estrogen at this time may act to suppress the effect that we typically observe in older males. With respect to progesterone, concentrations were highest in adult females. Since they are the only group to be adversely affected by stress, the presence of high levels of progesterone may be involved somehow in the decrement in learning. Indeed, others have connected progesterone with stress-induced effects on behavior and particularly those interacting via glucocorticoid receptors (Ahima et al., 1992; Duncan and Duncan, 1979). Clinically, progesterone has been associated with negative symptoms of depression and other stress-related mental illness such as premenstrual dysmorphic disorder (Roca et al., 2003; Young and Altemus, 2004).
As expected, testosterone concentrations increased with age in males and were most elevated in adulthood. Since stress has similar enhancing effects on learning in pubescent and adult males even though testosterone levels were quite different, it would seem that testosterone does not directly mediate the enhancement. These data are consistent with others indicating that castration at birth has no consequence for the enhanced performance in adulthood (Shors and Miesegaes, 2002). On the other hand, exposure to a testosterone antagonist in utero did prevent the enhanced performance after stress in adulthood. Thus, the contribution of testosterone to these effects of stress on learning appears organizational rather than activational.
Conclusion
Even though most appreciate puberty as a time of great change, there is relatively limited research in this age group, especially with respect to the effects of stress on learning. A recent search on MedLine for publications with stress, learning and puberty as key words revealed only ten reports in the past 35 years. There are perhaps even fewer studies that have assessed stress and learning during puberty itself. The present data illustrate that learning in males and females prior to puberty is not particularly responsive to stress but that during puberty, a robust stress response develops, one that is similar in males and females. It is in adulthood that sex differences in learning arise as well as the opposite effect of stress on conditioning in males versus females. The findings indicate that the behaviors expressed during puberty can be quite distinct from those related to reproduction or those associated with adulthood and should be studied in their own right.
Acknowledgments
This work was supported by the National Institute of Mental Health (59970) and the National Alliance for Research on Depression and Schizophrenia to TJS.
References
- Ahima RS, Laswason ANL, Osei SYS, Harlan RE. Sexual dimorphism in regulation of type II corticosteroid receptor immunoreactivity in the rat hippocampus. Endocrinology. 1992;131:1409–1416. doi: 10.1210/endo.131.3.1505471. [DOI] [PubMed] [Google Scholar]
- Badr M, Marchetti B, Pelletier G. Changes in hippocampal LH-RH receptor density during maturation and aging in the rat. Brain Res Dev Brain Res. 1989;45:179–184. doi: 10.1016/0165-3806(89)90037-0. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. Acute stress impairs trace eyeblink conditioning in females without altering the unconditioned response. Neurobiol Learn Mem. 2004;82:57–60. doi: 10.1016/j.nlm.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav. 2003;43:124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsody MK, Weiss JM. Influence of corticotropin-releasing hormone on electrophysiological activity of locus querulous neurons. Brain Res. 1996;724:149–168. doi: 10.1016/0006-8993(96)00199-0. [DOI] [PubMed] [Google Scholar]
- Choi S, Kellogg CK. Adolescent development influences functional responsiveness of noradrenergic projections to the hypothalamus in male rats. Brain Res Dev Brain Res. 1996;94:144–151. doi: 10.1016/s0165-3806(96)80005-8. [DOI] [PubMed] [Google Scholar]
- Choi S, Weisberg SN, Kellogg CK. Control of endogenous norepinephrine release in the hypothalamus of male rats changes over adolescent development. Brain Res Dev Brain Res. 1997;98:134–141. doi: 10.1016/s0165-3806(96)00179-4. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Duncan GR. An in vivo study of the action of antiglucocorticoids on thymus weight, antibody titre and the adrenal–pituitary–hypothalamus axis. J Steroid Biochem. 1979;10:245–259. doi: 10.1016/0022-4731(79)90250-4. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Garber J. Psychosocial antecedents of variation in girls' pubertal timing: maternal depression, stepfather presence, and marital and family stress. Child Dev. 2000;71:485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- FDA (US Food and Drug Administration) Recommendations of the psychopharmacologic drugs and pediatric advisory committees. Food and Drug Administration Website; 2004. [Google Scholar]
- Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992;56:619–628. doi: 10.1159/000126284. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain—I. NMDA and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Paczkowski C, Stanton ME. Ontogeny of delay versus trace eyeblink conditioning in the rat. Dev Psychobiol. 2000;36:148–160. doi: 10.1002/(sici)1098-2302(200003)36:2<148::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpela M. Early puberty is associated with mental health problems in middle adolescence. Soc Sci Med. 2003;57:1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Kanamaru H, Kakeyama M, Seki T, Arai Y. Estrogen potentiates N-methyl-d-aspartate receptor subunit R2B MRNA expression during the late prepubertal period in female rats. Neurosci Lett. 2001;300:9–12. doi: 10.1016/s0304-3940(01)01527-0. [DOI] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Yilmaz ÖA, Balkan B, Demirgören, Furedy JJ, Pögün S. Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res Bull. 2000;52:243–248. doi: 10.1016/s0361-9230(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Krasnoff A, Weston LM. Pubertal status and sex differences: activity and maze behavior in rats. Dev Psychobiol. 1976;9:261–269. doi: 10.1002/dev.420090310. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Morley-Fletcher S, Terranova M. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res. 2002;130:117–125. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady D, Koenig JI. Corticosterone alters N-methyl-d-aspartate receptor subunit MRNA expression before puberty. Brain Res Mol Brain Res. 2003;115:55–62. doi: 10.1016/s0169-328x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry. 2004;56:964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male rats. Horm Behav. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2nd. Raven Press; New York: 1994. pp. 1699–1737. [Google Scholar]
- Okimoto DK, Blaus A, Schmidt M, Gordon MK, Dent GW, Levine S. Differential expression of c-fos and tyrosine hydroxylase mRNA in the adrenal gland of the infant rat: evidence for an adrenal hyporesponsive period. Endocrinology. 2002;143:1717–1725. doi: 10.1210/endo.143.5.8819. [DOI] [PubMed] [Google Scholar]
- Palamarchouk VS, Swiergiel AH, Dunn AJ. Hippocampal noradrenergic responses to CRF injected into the locus coeruleus of unanesthetized rats. Brain Res. 2002;950:31–38. doi: 10.1016/s0006-8993(02)02983-9. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Br J Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Pinos H, Collado P, Rodriguez-Zafra M, Rodriguez C, Segovia S, Guilamon A. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56:73–78. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Deusfer P, Danaceau MA, Putnam K, Altemus M. Differential menstrual cycle regulation of hypothalamic–pituitary–adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhau N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Blanco R, Santander J, Miranda E. Relationship between sex differences in onset of schizophrenia and puberty. J Psychiatr Res. 2000;34:349–353. doi: 10.1016/s0022-3956(00)00030-3. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Shors TJ. Early acquisition but not retention, of the classically conditioned eyeblink response is N-methyl-d-aspartate (NMDA) receptor dependent. Behav Neurosci. 1996;110:1040–1048. doi: 10.1037//0735-7044.110.5.1040. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Brennan FX, Beck KD, Beldowicz D, Coyle-DiNorcia K. Stress facilitates acquisition of the classically conditioned eyeblink response at both long and short interstimulus intervals. Learn Motiv. 2001;32:178–192. [Google Scholar]
- Shors TJ. Stress and sex effects on associative learning: for better or worse. Neuroscientist. 1998;4:353–364. [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Learning during stressful times. Learn Mem. 2004;11:137–144. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mathew PR. NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learn Mem. 1998;5:220–230. [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G. Testosterone in utero and birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci. 2002;99:13955–13960. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Curr Dir Psychol Sci. 2000a;9:111–114. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000b;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tahirovic HF. Menarchal age and stress of war: an example from Bosnia. Eur Jof Ped. 1998;157:978–980. doi: 10.1007/s004310050981. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic–pituitary– adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Weise M, Eisenhofer G, Merkr DP. Pubertal and gender-related changes in the sympathoadrenal system in healthy children. J Clin Endocrinol Metab. 2002;87:5038–5043. doi: 10.1210/jc.2002-020590. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]


