Abstract
Context:
Hidradenocarcinoma is a rare carcinoma of high malignant potential. It most metastasizes to regional lymph nodes and distant viscera.
Case report:
We report a case of 52-year-old woman who presented with an invasive hidradenocarcinoma of the finger, treated with surgical excision. The patient presented with skin and lymph node metastases four years after, treated by chemotherapy.
Conclusion:
Hidradenocarcinoma is an aggressive tumor. It seems important to use adjuvant therapies particularly for recurrent and metastatic forms.
Keywords: Hidradenocarcinoma, metastases, chemotherapy.
Introduction
Hidradenocarcinoma is a rare malignant adnexal tumor that represented less than 0,001%[1,2]. It is an Aggressive tumor that most metastasizes to regional lymph nodes and distant viscera. It has been reported most frequently on the head and neck and rarely on the extremities[3]. There is no consensus treatment for metastatic hidradenocarcinoma since it is a rare and aggressive tumor. We report a case of a metastatic hidradenocarcinoma with discussion of the literature and problems concerning differential diagnosis and treatment.
Case Report
We report a case of 52-year-old white woman, without past medical history, who presented with an asymptomatic nodule of the left hand (fourth finger) of 3 months duration (Fig. 1). Physical examination revealed a 3 cm firm nodule without features of inflammation. The regional lymph node examination was negative. A surgical excision of the nodule was performed. Histological examination showed a poorly circumscribed lobulated dermal mass with scalloped borders that may extend to the fat with connection to the epidermis (Fig. 2). Tumor lobules were composed by nodules, focal tubular lumina and cystic spaces. Tumor cells were mostly polygonal or fusiform with basophilic elongated nuclei, rarely they were polyhedral clear cells. Cytological atypia and mitotic activity were remarkable with focal necrosis (Fig. 3).
Fig. 1.
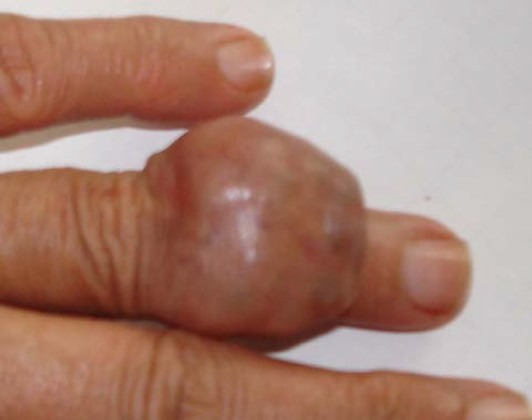
cutaneous nodule of the fourth finger.
Fig. 2.
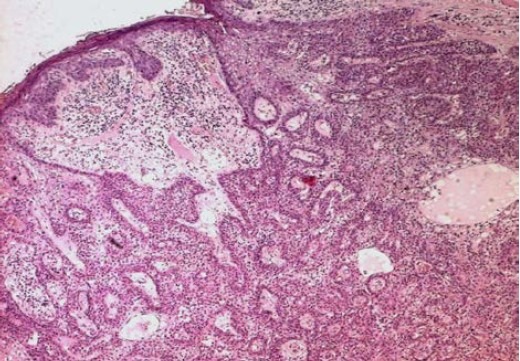
Poorly circumscribed lobulated dermal tumor (HE × 200).
Fig. 3.
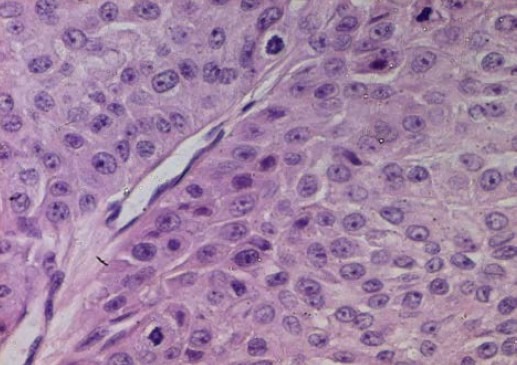
Polygonal tumor cells with remarkable atypia and mitotic activity (HE × 400)
However, angiolymphatic and perineural invasion were absent. Immunohistochemically showed immunoreactivity for EMA (epithelial membrane antigen) and Ki 67 (Fig. 4). The diagnosis of hidradenocarcinoma was performed. The patient has not received any adjuvant therapy. Four years later, the lesion recurred with multiple cutaneous metastases located in the left arm after completely excision (Fig. 5). Systemic chemotherapy was started and consisted on intravenous 5 fluouracil: 425 mg/m2/day (day 1 to day 5). Four cycles after, physical examination revealed stable disease. Second line chemotherapy was done based on intravenous doxorubim: 75 mg/m2 (day 1) and intravenous platine regimen: 100mg/m2 (day 1). Four cycles after, the evaluation revealed partial response with decrease of the size of the cutaneous metastases.
Fig. 4.
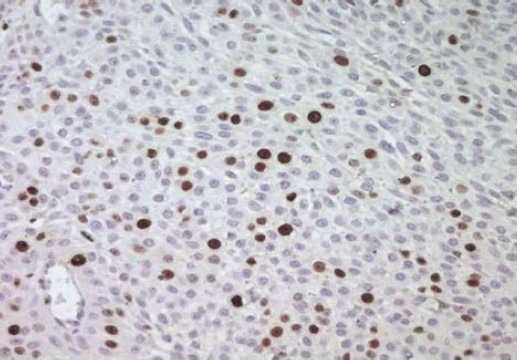
Immunoreactivity for Ki 67 (IHC × 400).
Fig. 5.
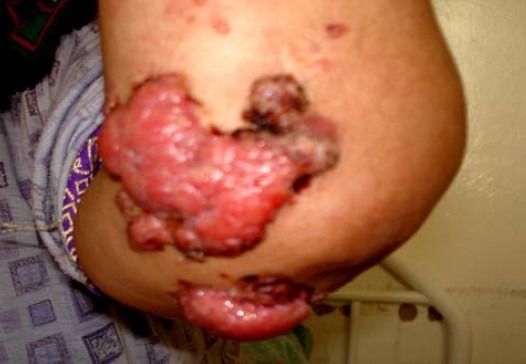
Multiple cutaneous metastases after surgical excision.
Discussion
Hidradenocarcinoma is a rare malignant adnexal tumor that represented less than 0.001%[1,2]. Clinical presentation was typically a firm, subcutaneous nodule or plaque. It may appear flesh-colored, red, violet or gray with normal overlying skin[3]. Hidradenocarcinoma can be mistaken clinically with the more common infundibular and pilar cysts; other entities to consider are cutaneous tuberculosis or dermatofibrosarcoma protuberans[3]. Hidradenocarcinoma has been reported most frequently on the head and neck[3] and rarely on the extremities like our case.
On pathologic examination, hidradenocarcinoma showed two distinct cell types: darker fusiform cells with eosinophilic cytoplasm and larger cells exhibit nuclear pleomorphism and atypical mitotic figures. Cystic spaces and ductal structures are rarely present[4]. Other criteria may be present such as perineural invasion, vascular invasion, deep extension and necrosis[4]. Hidradenocarcinoma can be difficult to distinguish histologically from hidradenoma, which is a benign tumor. Histological criteria used to distinguish these two entities include greater mitotic activity, mitoses in clear cells, angiolymphatic invasion into surrounding tissue and loss of circumscription[5,2]. However, the absence of these criteria should not reassure the pathologist, because the diagnostic of hidradenocarcinoma may rest solely on the presence of tumor cords invading peripherally. Standard treatment for hidradenocarcinoma is surgical excision, but local recurrence rates range from 10% to 50%[6]. Adjuvant therapies included radiation has been used in the setting of positive margins, complete resection of large tumors for “nodal sterilization” and recurrent tumors and positive lymph nodes when further surgery has not been an option[7]. Various chemotherapy regimens have also been reported[8,9]; drugs used was 5 fluouracil based regime, capecitabine (oral 5 fluouracil) was also reported[9]. We used for our patient intravenous 5 fluouracil on first line, without response. We used intravenous doxorubim and intravenous platine on second line since its proved efficiency in the treatment of cutaneous squamous cell carcinoma.
Targect therapies like Herceptin which is effective for treatment of various solid cancers, is also used in the treatment of metastatic hidradenocarcinoma with stabilization of the disease[8,10].
There is no consensus treatment for hidradenocarcinoma since it is a rare and aggressive tumor. It seems important to use adjuvant therapies particularly for recurrent and metastatic forms.
References
- 1.Santa Cruz DJ. Sweet gland carcinomas a comprehensive review. Sen Diagn Pathol. 1987;4:38–74. [PubMed] [Google Scholar]
- 2.Mehregan AH, Hashimoto K, Rahbari H. eccrine adenocarcinoma: a clinicopathologic study of 35 cases. Arch Dermatol. 1983;119:104–114. doi: 10.1001/archderm.119.2.104. [DOI] [PubMed] [Google Scholar]
- 3.Yavel R, Hinshaw M, Rao V, Hartig GK, Harari PM, Stewart D, Snow S N. Hidradenoma and hidradenocarcinoma of the scalp. Managed using Mohs micrographic surgery and a multidisciplinary surgery and a multidisciplinary approach: case reports and review of the literature. Dermatol Surg. 2009;35:273–281. doi: 10.1111/j.1524-4725.2008.34424.x. [DOI] [PubMed] [Google Scholar]
- 4.KO JC, Cochran AJ, Eng W, Binder SW. hidradenocarcinoma: a histological and immunohistochemical study. J Cutan Pathol. 2006;33:726–730. doi: 10.1111/j.1600-0560.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 5.Lim SC, Lee MJ, Lee MS, Kee KH, Suh CH. Giant hidradenocarcinoma: a report of malignant transformation from nodular hidradenoma. Pathol Int. 1998;48:818–823. doi: 10.1111/j.1440-1827.1998.tb03843.x. [DOI] [PubMed] [Google Scholar]
- 6.Ashley I, Smith-Reed M, Chernys A. Sweet gland carcinoma case report and review of the literature. Dermatol Surg. 1997;23:129–133. [PubMed] [Google Scholar]
- 7.Harari PM, Shimm DS, Bangert JL, Cassady JR. The role of radiotherapy in the treatment of malignant sweat gland neoplasms. Cancer. 1990;65:1737–1740. doi: 10.1002/1097-0142(19900415)65:8<1737::aid-cncr2820650813>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Nash JW, Barrett TL, Kies M, Ross M, Sneige N, Diwan AH, Lazar AJF. Metastatic hidradenocarcinoma with demonstration of Her-2/neu gene amplification by fluorescence in situ hybridation: potential treatment implications. J Cutan Pathol. 2007;34:49–54. doi: 10.1111/j.1600-0560.2006.00570.x. [DOI] [PubMed] [Google Scholar]
- 9.Jouary T, Kaiafa A, Lipinski P, Vergier B, Lepreux S, Delaunay M, Taïeb Metastatic hidradenocarcinoma: efficacy of capecitabine. Arch Dermatol. 2006;142:1366–1367. doi: 10.1001/archderm.142.10.1366. [DOI] [PubMed] [Google Scholar]
- 10.Battistella M, Mateus C, Lassau N, Chami L, Boukoucha M, Duvillard P, Cribier B, Robert C. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2009 doi: 10.1111/j.1468-3083.2009.03301.x. (in print) [DOI] [PubMed] [Google Scholar]


