Abstract
Self-injurious behavior (SIB) is a spontaneous behavior that threatens the health and wellbeing of multiple species. In humans, the opioid antagonist naltrexone hydrochloride has been used successfully to modulate the endogenous opioid system and reduce the occurrence of SIB. This study is the first to assess the efficacy of extended-release naltrexone in the pharmacologic treatment of SIB in rhesus macaques (Macaca mulatta). In an acute pharmacokinetic study of 4 macaques, we determined the mean naltrexone plasma concentration was maintained above the therapeutic level (2 ng/mL) after administration of a single dose (20 mg/kg) of 28-d extended-release naltrexone throughout the release period. For a subsequent treatment study, we selected 8 singly housed macaques known to engage in SIB. The study comprised a 4-wk baseline phase; an 8-wk treatment phase, during which each macaque received 2 doses of extended-release naltrexone 28 d apart; and a 4-wk posttreatment phase. Plasma samples were collected and analyzed weekly for naltrexone concentrations throughout the treatment and posttreatment phases. In addition, total of 6 h of video was analyzed per animal per phase of the study. Compared with baseline phases, both the frequency and the percentage of time spent displaying SIB decreased during the treatment phase, and the percentage of time remained decreased during the posttreatment phase. In contrast, extended-release naltrexone did not alter the expression of other abnormal, anxiety-related, or agonistic behaviors nor were levels of inactivity affected. The present study supports the use of naltrexone in the treatment of SIB in rhesus macaques.
Abbreviations: SIB, self-injurious behavior
Self-injurious behavior (SIB), a multifactorial abnormal behavior with a poorly understood etiology, is defined as any behavior involving potentially damaging manipulation of or deliberate infliction of direct physical harm to one's own body without suicidal intent.2,22 In humans, the condition often is associated with neurodevelopmental disorders, including mental retardation, autism, borderline personality disorder, and Lesch–Nyhan syndrome, with expression in 4% to 20% of persons who are affected by these disorders.10 SIB also is expressed by approximately 4% of persons who are not affected by neurodevelopmental disorders.11,35,,60 SIB is not a uniquely human phenomenon; the disorder also affects many captive nonhuman primate species. To date, the most widely studied species is the rhesus macaque, with 5% to 13% of the caged population spontaneously demonstrating SIB in the form of self-biting. 5,6,48 Self-biting often appears severe to an observer but typically does not result in trauma to the skin. However, some macaques engage in a more extreme form of self-injury and inflict wounds that require veterinary intervention.
Although no proposed cause of SIB has been accepted widely,48 serotonergic, dopaminergic, and opioidergic organic mechanisms have been implicated in the development and maintenance of the behavior in both human and nonhuman primates.9,14,24,29,40,47,55,56,65 Limited evidence suggests that altered or reduced serotonin function plays a role,12,28 and therapeutic intervention with serotonin reuptake inhibitors such as fluoxetine, buspirone, and the serotonin precursor L-tryptophan have had mixed success in reducing the incidence of SIB.23,43,46,63 However, other studies have been unsuccessful in definitively identifying direct evidence that either decreased synthesis or function of the neurotransmitter likely results in SIB.33,64 Similar mixed findings were described with respect to the dopamine system,25,24,64,67 and, as with serotonin, there is little direct evidence of its role in spontaneous SIB.39,64
More recently, there has been a concerted focus on disturbances in the endogenous opioid system.40,51,52,55,53,61 A prevailing hypothesis is that at least a subpopulation of subjects engages in SIB as a means to stimulate the release of endogenous opioids, resulting in a euphoric state that seeds an addictive mechanism for maintenance of the behavior once it is established. In addition, SIB in humans shares features of addiction including compulsive and ritualistic (or stereotypic) patterns that either comprise or surround the self-injuring acts.53 In rhesus macaques, there is a similar association between stereotypic floating limb and self-biting behaviors.7
In humans, SIB often is coexpressed with stereotypy, anxiety, aggressive, and obsessive– compulsive behaviors in a variety of neurodevelopmental disorders.3,11,20,38,44,59,62 In addition, although there have been limited investigations into the relationship between anxiety and the incidence of SIB,34,35 physiologic evidence of anxiety attenuation in the form of reductions in heart rate and blood pressure after SIB bouts has been reported to occur in both human and nonhuman primates.8,41,42 Treatment with the anxiolytic drug diazepam successfully reduced self-biting behavior in a subset of rhesus macaques known to engage in this behavior. However, some subjects responded with a significant increase in self-biting.65
While there are currently no widely accepted treatments available for SIB,the nonselective opioid antagonist naltrexone hydrochloride,71 used to treat alcoholism and opioid dependence, may be effective in reducing SIB in humans52,53 and nonhuman primates.50 The proposed mechanism of action for attenuation of SIB by naltrexone is the competitive blockade of opioid receptors, which limits the euphoria or analgesia that results from an SIB episode.53 It is theorized that reduction of positive feedback mechanisms removes the motivation for maintenance of the behavior, thereby reducing the incidence.53,65 A review of studies in the human literature reported that 80% of developmentally disabled patients responded positively to treatment with oral naltrexone, and 47% showed reductions of 50% or greater in the expression of the target behavior.61 Similar effects were noted at the Tulane National Primate Research Center, where 85% of nonhuman primate subjects exhibiting self-wounding prior to pharmacologic intervention had decreased SIB after initiating oral naltrexone therapy. Of the animals that responded positively, 38% showed a reduction in the frequency of SIB behavior of at least 50%.50
Although the aforementioned results are positive, inherent difficulties complicate definitive assessment of the efficacy of naltrexone given orally. The drug has a short half-life, and direct drug effects may be obscured when an animal occasionally refuses daily oral administration. In this scenario, plasma concentrations cannot be maintained above the putative therapeutic level of 2 to 5 ng/mL.68 Therefore, we extended the investigation of the use of naltrexone to include an extended-release, parenteral formulation.
To our knowledge, a pharmacokinetic study of extended-release naltrexone by intramuscular administration in rhesus macaques has not been described in the literature. The first goal of the present study was to determine that a therapeutic plasma concentration of naltrexone could be maintained for 28 d by using a single 20-mg/kg dosage of an extended-release formulation. Once that goal was achieved, we pursued our primary objective of examining the efficacy of extended-release naltrexone in the treatment of self-biting behavior in rhesus macaques. We also evaluated the drug's effects on other abnormal, anxiety-related, and agonistic behaviors, which are often associated or coexpressed with SIB in humans.
Materials and Methods
Subjects.
Rhesus macaques (Macaca mulatta) of either Indian or Chinese origin were obtained from the AAALAC-accredited Tulane National Primate Research Center conventional and SPF (antibody- and virus-negative for simian retrovirus and seronegative for simian T-lymphotrophic virus 1, SIV, and Macacine herpesvirus 1) breeding colonies as well as from AAALAC-accredited vendor sources. Macaques were housed in accordance with the standards set forth by the Public Health Service policy as outlined in the Guide for the Care and Use of Laboratory Animals32 and the US Department of Agriculture's Animal Welfare Regulations.1 Animal rooms were maintained on a 12:12-h light:dark cycle with a relative humidity of 30% to 70% and an ambient temperature of 64 to 72 °F (17.8 to 22.2 °C). Macaques weighing less than 10 kg were individually housed in cages measuring 36 in. (91.4 cm) in height with 4.3 ft2 (0.4 m2) of floor space, whereas subjects weighing 10 kg or more were housed in cages with floor space measuring 8.6 ft2 (0.8 m2) and a height of 36 in (91.4 cm). Macaques were fed a commercial nonhuman primate diet (Purina Lab Fiber-Plus Monkey Diet, Richmond, IN) twice daily and fresh produce or foraging mix at least once daily 5 times each week, with access to water ad libitum. All macaques were housed with visual access to several conspecifics at all times. Each cage contained 2 perches and a toy. In addition, a mirror and an additional toy were hung on the outside of each cage. All study procedures and methods were preapproved by the Tulane University IACUC.
Acute pharmacokinetic study.
Extended-release naltrexone (Vivitrol, Alkermes, Waltham, MA) is naltrexone incorporated into 75:25 biodegradable poly(d,l-lactide-cogycolide) microspheres at a concentration of 337 mg of naltrexone per gram of microspheres. The microspheres are suspended in a diluent composed of carboxymethylcellulose sodium salt, polysorbate 20, sodium chloride, and water prior to injection. A dosage of 20 mg/kg (naltrexone) was selected on the basis of a published recommendation.18 The authors previously determined that the plasma concentration of naltrexone remained above the putative therapeutic threshold of 2 ng/mL68 throughout the 28-d release phase after subcutaneous administration of extended-release naltrexone in rhesus macaques.18
The current study included 4 healthy, adult male rhesus macaques of Indian origin ranging in age from 5 to 10 y (mean ± 1 SD, 7.5 ± 2.4 y). Subjects had not previously been inoculated with infectious agents and had never been observed to engage in self-biting or abnormal behaviors. All procedures were performed while macaques were anesthetized with ketamine hydrochloride (10 mg/kg). Prior to enrollment in the pharmacokinetic study, macaques were weighed, a physical examination was performed, and blood was collected for CBC and serum biochemistry analysis. On the first day of the pharmacokinetic study, the dose of extended-release naltrexone was administered and divided equally into 2 intramuscular injections, 1 each into the left and right quadriceps muscles. Blood samples for pharmacokinetic analysis were collected into EDTA anticoagulant containing tubes and obtained at 0, 2, and 8 h and 1, 2, 5, 7, 10, 14, 17, 21, 25, and 28 d after injection. At each time point, macaques were weighed and examined. Blood samples were placed on wet ice and processed within 30 min to separate plasma by spinning the samples in a centrifuge (Allegra 6R, Beckman Coulter, Fullerton, CA) at 2600 g for 10 min at 2 to 5 °C. The resulting plasma was split into 2 approximately equal aliquots (A [primary samples] and B [back-up]), transferred to individual polypropylene tubes in a 96-well plate format, and stored at approximately −80 °C until shipment for analysis. Plasma concentrations of naltrexone were determined by using liquid chromatography–tandem mass spectrometry.19 Disposition and metabolism of naltrexone were not examined.
Naltrexone study.
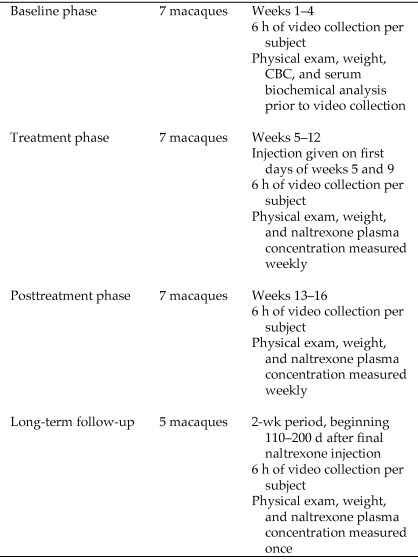
Three male and 5 female rhesus macaques of either Chinese or Indian origin, ranging in age from 3 to 10 y (mean ± 1 SD, 6.5 ± 2.8 y), were selected as subjects on the basis of their high frequency of self-biting (more than 4 bouts per hour). Each animal was singly housed and served as its own control. As part of ongoing infectious disease studies at our institution, 5 of the subjects had previously been inoculated with SHIV-RT virus and one subject with SIVmac239 virus. One of these subjects began to show clinical signs of illness associated with SHIV-RT disease status prior to the completion of the study. Subsequently, data from this animal were excluded from statistical analysis. The remaining 2 subjects had not been inoculated with infectious agents. Prior to study assignment, macaques were anesthetized, weighed, and given physical examinations, and blood was collected for CBC and serum biochemical analysis. After confirmation of normal health status, macaques were enrolled in the 4-wk baseline phase, during which behavioral data were collected (see below). This phase was followed by an 8-wk treatment phase, during which macaques each received 2 extended-release naltrexone injections as described earlier, on the first days of weeks 5 and 9 of the study (Figure 1). Collection of behavioral data began no sooner than 5 d after the initial injection of extended-release naltrexone. This pause in data collection allowed subjects to adjust to the blockade of endogenous opioids by extended-release naltrexone. Physical examinations were performed, weights were obtained, and blood was collected for naltrexone plasma concentration analysis once weekly. Analysis of naltrexone plasma concentration was performed to ensure that subjects were maintained above the putative therapeutic threshold for naltrexone (2 ng/mL)68 and to compare the naltrexone plasma concentration with the response to treatment. The third phase consisted of a 4-wk posttreatment phase, during which behavioral data were collected, blood was sampled, body weights were obtained, and physical exams were performed in the same manner as during the treatment phase. In this phase, naltrexone plasma concentration was analyzed to confirm that detectable drug levels were no longer present.
Figure 1.
Naltrexone study design.
Subsequently, we followed and evaluated the long-term posttreatment effects of naltrexone in 5 subjects for a 2-wk period that began 110 to 200 d after the final injection of extended-release naltrexone. Behavioral data were collected as described below. On day 7 of this 2-wk long-term follow-up time period, macaques were given a physical examination and weighed, and a single blood sample was collected for analysis of naltrexone plasma concentration as previously described. Because only 5 of the 7 subjects were followed during this period, the long-term extended-release naltrexone effects observed were not included in the statistical analysis of the data but are described.
Behavioral data.
During each study phase, a total of 6 h of focal animal sampling per subject was obtained via videotaping. Personnel at our institution use video cameras frequently and most indoor-housed animals are acclimated to their presence. Video-recording sessions were scheduled between the hours of 1100 to 1300 and 1400 to 1600 to reduce variability and avoid possible confounds that may have been introduced by interactions between animals and staff during routine husbandry, daily feedings, and research-associated procedures. Each session varied from 30 to 120 min in length, as dictated by the timing of these procedures, but achieved the same mean (± SE) during all phases (baseline phase: 87.5 ± 21.9 min; treatment phase: 87.3 ± 20.1 min; posttreatment phase: 84.5 ± 15.8 min; long-term follow-up period: 90.0 ± 1.27 min). Behavioral observations collected during the long-term follow-up period were obtained 1 wk prior to and 1 wk after the single blood sample collection.
Data were coded and quantified by a single observer (DJK) using Observer XT 10.0 software (Noldus Information Technology, Leesburg, VA). An exhaustive, mutually exclusive ethogram that included a total of 77 behaviors was used. Behaviors of interest were categorized into 7 behavioral categories for analysis: self-biting, floating limb, abnormal locomotor, abnormal nonlocomotor, anxiety-related, agonistic, and inactive (see Figure 2 for operational definitions). Levels of inactivity were measured for the purpose of evaluating whether effects of extended-release naltrexone were due to antagonism of the endogenous opioid system or were secondary to sedation. All behaviors excluding inactivity were sampled continuously. Instances of self-biting behavior were recorded and calculated as frequency per hour as well as percentage of time animals were observed performing the behavior. By analyzing both frequency and percentage, we were able to assess how many times the animals performed self-biting episodes and the total percentage of time that the subjects dedicated to the behavior. In addition, abnormal and anxiety-related behavior levels were calculated as percentages of time that subjects spent performing the behaviors. Agonistic behaviors were quantified as frequency per hour, owing to the brief duration of these behaviors. Levels of inactivity were quantified by using instantaneous sampling collected at 30-s intersample intervals. The total number of samples during which inactivity was recorded was divided by the total number of samples recorded during the phase.
Figure 2.
Operational definitions of abnormal behavior.
Behavioral and physiologic data analysis.
Statistical analyses were performed by using 2-tailed Wilcoxon matched-pairs tests with Bonferroni correction (Statistica, Statsoft, Tulsa, OK) to compare baseline values with treatment and posttreatment values. Tests were conducted by using data from 7 subjects and excluded the animal that became clinically ill during the study period. All analyses were completed with the α level set to 0.025 to control for multiple comparisons. Data derived from the long-term follow-up period were not tested for statistical significance, because only 5 subjects could be studied during this period. As such, the findings from the long-term follow-up period are presented only descriptively.
We calculated the percentage reduction in self-biting behavior after treatment with extended-release naltrexone and analyzed these data for correlation with the naltrexone plasma concentrations by using a Spearman rank-order test with an α level of 0.05.
Results
Acute pharmacokinetic study.
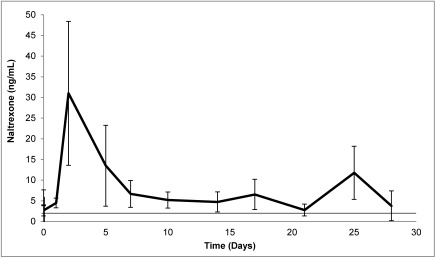
Plasma concentrations of naltrexone remained pharmacologically relevant (greater than 2 ng/mL) for the duration of the 28-d study (Figure 3), with the exception of a single measurement from one macaque on day 21 (1.5 ng/mL) and a single measurement from a different macaque on day 28 (1.3 ng/mL). A mean peak plasma concentration of 31.0 ng/mL was reached on day 2. A small secondary peak of 11.7 ng/mL was observed on day 25 as the polymeric microspheres encapsulating extended-release naltrexone degraded.
Figure 3.
Acute pharmacokinetic study: mean naltrexone plasma concentrations (bar, 1 SD) over the 28-d study period. The horizontal black line represents the therapeutic level (2 ng/mL) of naltrexone.
Extended-release naltrexone was well tolerated by macaques, with neither injection site reactions nor adverse drug effects being observed. Body weights remained stable over the course of the study, with a mean (± SE) animal weight of 12.6 ± 1.9 kg at commencement of the study and 11.9 ± 1.7 kg at the end of the study.
Naltrexone study.
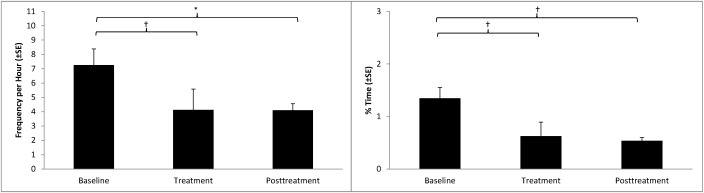
Descriptively, 7 of the 8 macaques responded positively to treatment with extended-release naltrexone as measured by reductions in either frequency of or percentage of time spent self-biting (Table 1). For the subjects included in the analysis (macaques 1 through 7), the mean reduction was significant both in respect to frequency (Wilcoxon ranked-pairs test; P < 0.025, Figure 4) and percentage of time (P < 0.025, Figure 4). Naltrexone plasma concentration remained greater than the therapeutic level of 2 ng/mL for all subjects during the treatment phase. In the posttreatment phase, the reduction in frequency of self-biting compared with baseline was not significant, but the percentage of time remained so (P < 0.025, Figure 4). Values during long-term follow-up were not tested for significance but are provided in Table 1.
Table 1.
Responses (% reduction in frequency and duration [time] SIB) of individual macaques to extended-release naltrexone
| Treatment |
Posttreatment |
Long-term follow-up |
||||
| Frequency | Time | Frequency | Time | Frequency | Time | |
| 1 | 38%a | 57%a | +17%a,b | 39%a | 33% | 46% |
| 2 | 55%a | 37%a | 30%a | 22%a | 58% | 43% |
| 3 | 90%a | 97%a | 76%a | 87%a | 90% | 97% |
| 4 | 35%a | 44%a | 48%a | 53%a | 52% | 55% |
| 5 | 81%a | 94%a | 8%a | 49%a | 19% | 61% |
| 6 | 44%a | 56%a | 44%a | 72%a | — | — |
| 7 | 4%a | 4%a | 59%a | 49%a | — | — |
| 8 | 77% | 89% | — | — | — | — |
| Reductions are relative to baseline values; macaques 1 through 6 were considered to be responders. Data from macaque 8 were excluded from statistical analysis due to clinical illness.aP < 0.025 compared with baseline value.bThis value represents an increase in the frequency of SIB. | ||||||
Figure 4.
Self-biting behavior presented as frequency per hour (mean ± SE) and percentage of time spent performing the behavior (mean ± SE). †, P < 0.025; *, P < 0.05.
Among the 7 subjects included in the analysis, self-biting frequency was attenuated in 86% of subjects. Of those that responded positively, half showed a reduction of 50% or greater. Overall, a significant (P < 0.025, Figure 4) mean frequency reduction of 49% was observed during the treatment phase compared with baseline values. A trend (P < 0.05) toward maintenance of this positive response persisted after complete naltrexone metabolism, with a frequency reduction of 36% in the posttreatment phase as compared with the baseline phase. The percentage of time that the macaques engaged in self-biting behavior was significantly reduced by 54% and 60% (P < 0.25, Figure 4) during the treatment and posttreatment phases as compared with the baseline phase, respectively. Descriptively, the eighth subject, which was excluded from the statistical analysis, not only reduced the frequency of self-biting by 75% but also reduced the percentage of time engaged in self-biting behavior by 85% in the treatment phase as compared with the baseline phase.
Data from this macaque were not available for the posttreatment phase.
We investigated the relationship between response to extended-release naltrexone treatment for self-biting behavior and naltrexone plasma concentrations during the treatment phase. A correlation between naltrexone plasma concentrations and reductions in the frequency of or percentage of time spent self-biting was not observed.
The long-term follow-up period included 5 of the 7 subjects that responded to extended-release naltrexone treatment. The positive effects of naltrexone therapy appeared to persist well beyond the posttreatment phase (Table 1). The percentage of time that the macaques engaged in self-biting behavior over the 6 h of video collected per phase was reduced by 66% during the treatment phase, 50% in the posttreatment phase, and 60% during the long-term follow-up period as compared with baseline levels. Although not as dramatic, reductions in self-biting frequency were present also. The frequency of self-biting was reduced by 60% in the treatment phase, 30% during the posttreatment phase, and 37% in the long-term follow-up period as compared with baseline, after 2 subjects did not exhibit long-term effects of naltrexone on self-biting during the posttreatment phase or the long-term follow-up period.
Significant changes were not detected in the frequency of agonistic behavior or in the levels of expression of floating limb, abnormal locomotor, abnormal nonlocomotor, and anxiety-related behaviors between the baseline and treatment phases. Levels of inactivity did not change from baseline levels throughout the study. As in the acute pharmacokinetic study, animal weights did not change significantly. The mean weight (± SE) of the 7 subjects was 6.9 ± 2.4 kg on the first day of the study, 7.1 ± 2.4 kg on the last day of the treatment phase, and 7.0 ± 2.6 kg on the last day of the posttreatment phase. The remaining subject, which was not included in the statistical analysis, began with a weight of 7.8 kg and weighed 7.6 kg on the final day of the treatment phase before it began showing signs of clinical illness. The mean weights obtained from the subset of 5 subjects included in the long-term follow-up period similarly did not change significantly and were 7.6 ± 2.5 kg on the first day of the study and 8.0 ± 2.6 kg during the follow-up period.
Discussion
In the current study, the hypothesized opioid-driven maintenance of self-biting behavior was pharmacologically targeted with naltrexone, a nonselective opioid antagonist. We elected to evaluate both the frequency of and percentage of time spent expressing self-biting behavior among our macaque subjects, because bout length can vary greatly between individual subjects as well as by each self-biting event expressed by a subject. Therefore, to better evaluate drug efficacy, it was crucial to measure how frequently subjects engaged in the behavior and what percentage of time was spent expressing the behavior. In the current study, extended-release naltrexone was effective in producing a significant mean reduction in the percentage of time that macaques dedicated to self-biting during the treatment and posttreatment phases as compared with the baseline phase, represented by reductions of 54% and 60% (Figure 4), respectively. The response significantly persisted in the absence of detectable naltrexone plasma concentrations during the posttreatment phase. In addition, a significant reduction of 49% (Figure 4) in frequency was achieved during the treatment phase compared with baseline, and a trend toward maintenance of this positive response was observed during the posttreatment phase, when frequency remained 36% below the level expressed during the baseline phase.
The results of this study and the previous oral naltrexone study in nonhuman primates conducted at our institution50 parallel those reported in a quantitative synthesis of peer-reviewed published literature describing naltrexone effects on self-injurious behavior in humans.61 In that review, the authors reported 80% of human subjects improved relative to baseline and that of those who improved, 47% showed reductions in SIB of 50% or greater.61 In the previous study from our institution,50 85% of nonhuman primate subjects decreased their frequency of self-biting after treatment with oral naltrexone. Of those subjects that responded, 38% had decreases in frequency of 50% or greater.50 Similarly, in the current study, 86% of subjects reduced self-biting frequency, and of those subjects that responded positively, 50% showed reductions in frequency of 50% or greater.
Descriptively, reductions of self-biting persisted into the long-term follow-up period for a subset of 5 subjects (Table 1). Similar persistent positive effects of naltrexone on SIB were seen in a subset of human patients one year after acute exposure and complete metabolism of the drug.52 In our study, only 5 subjects were included in the follow-up period, and a larger sample size may have been more effective in demonstrating meaningful response by providing more statistical power. However, these subjects were moved to new primary enclosures in different rooms and, in some cases, different buildings, 2 to 6 times during the period between the posttreatment phase and follow-up period. The expression of self-biting in animals known to engage in SIB can increase after relocations.17 That the reductions in levels of self-biting appeared to persist even under these conditions underscores the potential for naltrexone not only to have long-term positive effects but to have the potential to exert a robust effect even in the face of environmental perturbations that can exacerbate expression of the behavior. Nonetheless, additional studies are needed to test long-term positive effects and investigate the potential of pulse or intermittent acute-dosing regimens of naltrexone in nonhuman primates.
Body weight and levels of inactivity were not significantly altered across phases. These findings indicate that the action of extended-release naltrexone was not due to sedation and that adverse effects could not be detected. Rather, our results support the hypothesis that mechanisms associated with self-injury engage the endogenous opioid system in nonhuman primates.
We also investigated the effects of extended-release naltrexone on behaviors that may be coexpressed with SIB in rhesus macaques. Floating limb, abnormal locomotor, abnormal nonlocomotor, anxiety-related, and agonistic behaviors can be coexpressed with SIB in rhesus macaques.5,7,16,45 However, they were not influenced by extended-release naltrexone, suggesting that their expression may relate to mechanisms other than the endogenous opioid system. Another consideration is that only a single dosage of extended-release naltrexone (20 mg/kg) was evaluated in this study. The therapeutic dosage of naltrexone is well-accepted to result in essentially complete blockade of the µ-opioid receptor69,72 and less-than-complete blockade of the δ- and κ-opioid receptors.69,70,72 In addition, the antagonism of κ-opioid receptors by naltrexone occurs in a dose- and time- dependent manner,57 such that as the dosage and treatment duration increase, κ-opioid receptors become antagonized.57,72 Given that κ-opioid receptors are associated with dysphoria,49 anxiety,37 and depressive-like behavioral signals,13,21 studies investigating the effects of increased naltrexone dosages on these behaviors may be indicated.
The hypothalamic–pituitary–adrenocortical axis has particular relevance for self-injurious and associated behaviors. This neuroendocrine system plays a vital role in the regulation and control of stress mechanisms in the body and is dysregulated in human and nonhuman primates displaying SIB.17,48,53,63,64,66 In a normally functioning axis, corticotrophin-releasing hormone is synthesized in the hypothalamus and released into the pituitary gland, where the hormone activates the cleavage of proopiomelanocortin, a stress-associated molecule, into the endogenous opioid β-endorphin and ACTH. Typically, β-endorphin is coreleased with ACTH in a highly correlated relationship. In a subgroup of humans exhibiting SIB, plasma levels of β-endorphin are elevated and are dissociated from ACTH after a SIB episode. Elevated β-endorphin concentration with a dampened ACTH level in this population was highly predictive of a positive response to naltrexone.54 A study of longtailed and pigtailed macaques similarly reported that subjects displaying high rates of abnormal behavior including SIB also had elevated levels of β-endorphin. However, ACTH did not vary significantly by abnormal behavior.15
In previous studies with nonhuman primates, drugs affecting the serotonergic system, such as fluoxetine and buspirone, reduced stereotypic and self-biting behaviors.23,30 This finding is not surprising, given that serotonin neurotransmission is involved in the HPA axis and is increased in response to stressful stimuli.27 Recently, investigators described the long-term consequences on the serotonin system in adult rhesus macaques after early adverse experience.4,31,58 Early adverse experiences in rhesus macaques are considered to increase the likelihood of abnormal and self-injurious behavior in adult rhesus macaques.26,36,40 Additional studies investigating dysregulation of the hypothalamic–pituitary–adrenocortical axis and the opioidergic and serotonergic systems may provide insight into their complex relationship in the pathogenesis of abnormal behaviors, including self-injurious behavior.
In this study, we showed that naltrexone is an effective therapeutic intervention for self-biting behavior in rhesus macaques. In addition, our previous and current findings indicate that use of either oral naltrexone or extended-release naltrexone are appropriate options in the treatment of SIB. The advantage of the extended-release formulation is that animals require only once-monthly treatment, thereby greatly increasing the treatment interval. In addition, concerns regarding possible noncompliance by animals are resolved. However, a disadvantage of any opioid antagonist is that narcotic analgesics, such buprenorphine, are rendered much less effective at conventional or prescribed doses. Oral naltrexone has a short half-life, and the disadvantage regarding naltrexone's effects on narcotics might easily be overcome by discontinuing the drug for the period of time that narcotic analgesia is desired. Alternatively, nonnarcotic analgesic strategies might be developed in advance when narcotic antagonists are used to treat SIB in nonhuman primates and implemented as needed. Another point of consideration is that once administered by either route, naltrexone cannot be reversed. Although adverse effects associated with the use of extended-release naltrexone were not evident in the current study, if an animal does experience adverse events, those events cannot be mitigated completely until the drug has been metabolized.
In summary, self-injurious behavior is a serious, aberrant, treatment-refractory problem. Despite treatment challenges, we here have demonstrated that naltrexone is an effective tool in the management of this behavior in rhesus macaques. Our results also indicate that naltrexone may have long-term positive effects, but future studies are required to provide a more comprehensive understanding of potential long-term effects of this drug.
Acknowledgments
We thank the animal care staff, especially Justin Whitney, Shelley Lower, and Yoav Littman, for their support and excellent care for the monkeys. This study was supported by funds from the National Center for Research Resources (R25RR024231, U42 RR016026, U24 RR0181111) to the Tulane National Primate Research Center, a fully AAALAC-accredited facility. The Vivitrol used in this study was provided by Alkermes.
References
- 1.Animal Welfare Regulations. 2008. 9 CFR §3.129. [Google Scholar]
- 2.Anderson JR, Chamove AS. 1980. Self-aggression and social aggression in laboratory-reared macaques. J Abnorm Psychol 89:539–550 [DOI] [PubMed] [Google Scholar]
- 3.Anderson LT, Ernst M. 1994. Self-injury in Lesch–Nyhan disease. J Autism Dev Disord 24:67–81 [DOI] [PubMed] [Google Scholar]
- 4.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. 2004. Rearing condition and rh5-HTTLPR interact to influence limbic–hypothalamic–pituitary–adrenal axis response to stress in infant macaques. Biol Psychiatr 55:733–738 [DOI] [PubMed] [Google Scholar]
- 5.Bayne KA, Dexter S, Suomi S. 1992. A preliminary survey of the incidence of abnormal behavior in rhesus monkeys (Macaca mulatta) relative to housing condition. Lab Anim 21:38–46 [Google Scholar]
- 6.Bayne KA, Haines M, Dexter S, Woodman D, Evans C. 1995. Nonhuman primate wounding prevalence: a retrospective analysis. Lab Anim 24:40–44 [Google Scholar]
- 7.Bentson KL, Crockett CM, Wahl KL, Runeson EP, Bellanca RU, Lee GH, Thom JP, Montgomery HB, Yi MH, McComas JG, Ha JC. 2010. Floating limb behaviors and self-biting are associated in laboratory monkeys. Am J Primatol 72:725–733 [DOI] [PubMed] [Google Scholar]
- 8.Brain KL, Haines J, Williams CL. 1998. The psychophysiology of self-mutilation: evidence of tension reduction. Arch Suicide Res 4:227–242 [Google Scholar]
- 9.Breese GR, Criswell HE, Duncan GE, Mueller RA. 1989. Dopamine deficiency in self-injurious behavior. Psychopharmacol Bull 25:353–357 [PubMed] [Google Scholar]
- 10.Briere J, Gil E. 1998. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatr 68:609–620 [DOI] [PubMed] [Google Scholar]
- 11.Browder DM. 1992. Assessment of individuals with severe disabilities: an applied approach to life skills assessment. Baltimore (MD): Paul H Brookes Publishing. [Google Scholar]
- 12.Buitelaar JK. 1993. Self-injurious behaviour in retarded children: clinical phenomena and biological mechanisms. Acta Paedopsychiatr 56:105–111 [PubMed] [Google Scholar]
- 13.Carlezon WA, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. 2006. Depressive-like effects of the κ-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316:440–447 [DOI] [PubMed] [Google Scholar]
- 14.Cataldo MF, Harris JC. 1982. The biological basis for self-injury in the mentally retarded. Anal Interven Dev Disabil 2:21–39 [Google Scholar]
- 15.Crockett CM, Sackett GP, Sandman CA, Chicz-DeMet A, Bentson KL. 2007. Beta-endorphin levels in longtailed and pigtailed macaques vary by abnormal behavior rating and sex. Peptides 28:1987–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross HA, Harlow HF. 1965. Prolonged and progressive effects of partial isolation on the behavior of macaque monkeys. J Exp Res Pers 1:39–49 [Google Scholar]
- 17.Davenport MD, Lutz CK, Teifenbacher S, Novak MA, Meyer JS. 2008. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biol Pscychiatr 63:990–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean RL. 2005. The preclinical development of medisorb naltrexone, a once-a-month long-acting injection, for the treatment of alcohol dependence. Front Biosci 10:643–655 [DOI] [PubMed] [Google Scholar]
- 19.Dean RL, Todtenkopf MS, Deaver DR, Arastu MF, Dong N, Reitano K, O'Driscoll K, Kriksciukaite K, Gastfriend DR. 2008. Overriding the blockade of antinociceptive actions of opioids in rats treated with extended-release naltrexone. Pharmacol Biochem Behav 89:515–522 [DOI] [PubMed] [Google Scholar]
- 20.Di Nuovo SF, Buono S. 2007. Psychiatric syndromes comorbid with mental retardation: differences in cognitive and adaptive skills. J Psychiatr Res 41:795–800 [DOI] [PubMed] [Google Scholar]
- 21.Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. 2010. Depressive-like effects of the κ-opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 210:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favazza Simeon D, Favazza AR. 2001. Self-injurious behaviors: phenomenology and assessment, p 1–28. In: Simeon D, Hollander E. Self-injurious behaviors: assessment and treatment. Washington (DC): American Psychiatric Publishing. [Google Scholar]
- 23.Fontenot MB, Padgett EE 3rd, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD. 2005. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med 55:67–74 [PubMed] [Google Scholar]
- 24.Goldstein M. 1989. Dopaminergic mechanisms in self-inflicting biting behavior. Psychopharmacol Bull 25:349–352 [PubMed] [Google Scholar]
- 25.Goldstein M, Kuga S, Kusano N, Meller E, Dancis J, Schwarez R. 1986. Dopamine agonist induced self-mutilative biting behavior in monkeys with unilateral ventromedial tegmental lesions of the brainstem: possible pharmacologic model for Lesch–Nyhan syndrome. Brain Res 367:114–120 [DOI] [PubMed] [Google Scholar]
- 26.Harlow HF, Harlow M. 1962. The effect of rearing conditions on behavior. Bull Menninger Clin 26:213–224 [PubMed] [Google Scholar]
- 27.Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O'Rahilly S, Colmers WF, Elmquist JK, Tecott LH. 2007. Serotonin activates hypothalamic–pituitary–adrenal axis via serotonin 2C receptor stimulation. J Neurosci 27:6956–6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellings JA, Warnock JK. 1994. Self-injurious behavior and serotonin in Prader–Willi syndrome. Psychopharmacol Bull 30:245–250 [PubMed] [Google Scholar]
- 29.Herman BH. 1990. A possible role of proopiomelanocortin peptides in self-injurious behavior. Prog Neuropsychopharmacol Biol Psychiatr 14:109–139 [DOI] [PubMed] [Google Scholar]
- 30.Hugo C, Seier J, Mdhlulo C, Daniels W, Harvey BH, Toit DD, Wolfe-Coote SW, Nel D, Dtein DJ. 2003. Fluoxetine decreases stereotypic behavior in primates. Prog Neuropsychopharmacol Biol Psychiatr 27:639–643 [DOI] [PubMed] [Google Scholar]
- 31.Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. 2006. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus macaques. J Neurosci 26:4638–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 33.Jawed SH, Krishnan VR, Cassidy G. 1994. Self-injurious behavior and the serotonin link: 2 case illustrations and theoretical overview. Ir J Psychol Med 11:165–168 [Google Scholar]
- 34.Kemperman I, Russ MJ, Shearin E. 1997. Self-injurious behavior and mood regulation in borderline patients. J Pers Disord 11:146–157 [DOI] [PubMed] [Google Scholar]
- 35.Klonsky ED, Oltmanns TF, Turkkeimer E. 2003. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry 160:1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraemer GW, Schimdt DE, Ebert MH. 1997. The behavioral neurobiology of self-injurious behavior in rhesus monkeys. Current concepts and relations to impulsive behavior in humans. Ann N Y Acad Sci 836:12–38 [DOI] [PubMed] [Google Scholar]
- 37.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. 2008. The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J Neurosci 28:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis M, Baumeister A. 1982. Stereotyped mannerisms in mentally retarded persons: animal models and theoretical analyses. Int Rev Res Ment Retard 11:123–161 [Google Scholar]
- 39.Lewis MH, Gluck JP, Beauchamp AJ, Keresztury MF, Mailman RB. 1990. Long-term effects of early social isolation in Macaca mulatta: changes in dopamine receptor function following apomorphine challenge. Brain Res 513:67–73 [DOI] [PubMed] [Google Scholar]
- 40.Lutz C, Well A, Novak M. 2003. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol 60:1–15 [DOI] [PubMed] [Google Scholar]
- 41.Major CA, Kelly BJ, Novak MA, Davenport MD, Stonemetz KM, Meyer JS. 2009. The anxiogenic drug FG7142 increases self-injurious behavior in male rhesus monkeys (Macaca mulatta). Life Sci 85:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marinus LM, Chase WK, Rasmussen KL, Jorgensen MJ, Novak MA. 1999. Reaction of rhesus macaques with self-injurious behavior to heart rate testing: is biting a coping strategy? Am J Primatol 49:79 [Google Scholar]
- 43.Markowitz PI. 1992. Effect of fluoxetine on self-injurious behavior in the developmentally disabled: a preliminary study. J Clin Psychopharmacol 12:27–31 [DOI] [PubMed] [Google Scholar]
- 44.Matson JL, Minshawi NF, Gonzalez ML, Mayville SB. 2006. The relationship of comorbid behavior problems to social skills in persons with profound mental retardation. Behav Modif 30:496–506 [DOI] [PubMed] [Google Scholar]
- 45.Mitchel G, Raymond E, Ruppenthal G, Harlow H. 1966. Long-term effects of total social isolation upon behavior of rhesus macaques. Psychol Rep 18:567–580 [Google Scholar]
- 46.Mizuno T, Yugari Y. 1975. Prophylactic effect of L-5-hydroxytrypyophan on self-mutilation in the Lesch–Nyhan syndrome. Neuropadiatrie 6:13–23 [Google Scholar]
- 47.New AS, Trestman RL, Mitropoulou V, Benishay DS, Coccaro E, Silverman J, Siever LJ. 1997. Serotonergic function and self-injurious behavior in personality disorder patients. Psychiatr Res 69:17–26 [DOI] [PubMed] [Google Scholar]
- 48.Novak MA. 2003. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol 59:3–19 [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer A, Brantl V, Herz A, Emrich HM. 1986. Psychotomimesis mediated by κ opiate receptors. Science 233:774–776 [DOI] [PubMed] [Google Scholar]
- 50.Ribka E, Baker K. 2004. Naltrexone (naltrexone hydrochloride) as a treatment to decrease incidence and severity of self-injurious behavior in rhesus macaques (Macaca mulatta). Am J Primatol 62:44 [Google Scholar]
- 51.Sandman CA, Hetrick W, Taylor DV, Chicz-DeMet A. 1997. Dissociation of POMC peptides after self-injury predicts responses to centrally acting opiate blockers. Am J Ment Retard 102:182–199 [DOI] [PubMed] [Google Scholar]
- 52.Sandman CA, Hetrick W, Taylor DV, Marion SD, Touchette PE, Barron JL, Martinezzi V, Steinburg RM, Crinella FM. 2000. Long-term effects of naltrexone on self-injurious behavior. Am J Ment Retard 105:103–117 [DOI] [PubMed] [Google Scholar]
- 53.Sandman CA, Kemp AS. 2011. Opioid antagonists may reverse endogenous opiate ‘dependence’ in the treatment of self-injurious behavior. Pharmaceuticals (Basel) 4:366–381 [Google Scholar]
- 54.Sandman CA, Spence MA, Smith M. 1999. Proopiomelanocortin (POMC) dysregulation and response to opiate blockers. Ment Retard Dev Disabil Res Rev 5:314–321 [Google Scholar]
- 55.Sandman CA, Touchette P, Lenjavi M, Marion S, Chicz-DeMet A. 2003. β-Endorphin and ACTH are dissociated after self-injury in adults with developmental disabilities. Am J Ment Retard 108:414–424 [DOI] [PubMed] [Google Scholar]
- 56.Sandman CA, Touchette PE, Marion SD, Chicz-Demet A. 2008. The role of proopiomelanocortin (POMC) in sequentially dependent self-injurious behavior. Dev Psychobiol 50:680–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith MA, McClean JM, Greene JL. 2003. Enhanced sensitivity to the antinociceptive effects of κ opioids in naltrexone-treated rats: dose- and time-dependent effects. Behav Pharmacol 14:641–647 [DOI] [PubMed] [Google Scholar]
- 58.Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, Barr CS, Suomi SJ, Higley JD, Stein EA. 2010. Effects of early-life stress on serotonin1A receptors in juvenile rhesus monkeys measured by positron emission tomography. Biol Psychiatry 67:1146–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanley B, Gameroff M, Michaleson V, Mann J. 2001. Are suicide attempters who self-mutilate a unique population? Am J Psychiatr 158:427–432 [DOI] [PubMed] [Google Scholar]
- 60.Suyemoto KL. 1998. The functions of self-mutilation. Clin Psychol Rev 18:531–554 [DOI] [PubMed] [Google Scholar]
- 61.Symons FJ, Thompson A, Roderiguez MC. 2004. Self-injurious behavior and the efficacy of naltrexone treatment: a quantitative synthesis. Ment Retard Dev Disabil Res Rev 10:193–200 [DOI] [PubMed] [Google Scholar]
- 62.Tenneij NH, Didden R, Stoker JJ, Koot HM. 2009. Markers for aggression in inpatient treatment facilities for adults with mild to borderline intellectual disability. Res Dev Disabil 30:1248–1257 [DOI] [PubMed] [Google Scholar]
- 63.Tiefenbacher S, Davenport MD, Novak MA, Poulit AL, Meyer JS. 2003. Fenfluramine challenge, self-injurious behavior, and aggression in rhesus monkeys. Physiol Behav 80:327–331 [DOI] [PubMed] [Google Scholar]
- 64.Tiefenbacher S, Novak MA, Jorgenson MJ, Meyer JS. 2000. Physiological correlates of self-injurious behavior in captive, socially reared rhesus monkeys. Psychoneuroendocrinology 25:799–817 [DOI] [PubMed] [Google Scholar]
- 65.Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. 2005. The physiology and neurochemistry of self-injurious behavior: a nonhuman primate model. Front Biosci 10:1–11 [DOI] [PubMed] [Google Scholar]
- 66.Tiefenbacher S, Novak MA, Marinus LM, William CK, Miller JA, Meyer JS. 2004. Altered hypothalamic–pituitary–adrenocortical function in rhesus monkeys (Macaca mulatta) with self-injurious behavior. Psychoneuroendocrinology 29:501–515 [DOI] [PubMed] [Google Scholar]
- 67.Turner CA, Lewis MH. 2002. Dopaminergic mechanisms in self-injurious behavior and related disorders p 165–179. In: Schroder SR, Oster-Granite ML, Thompson T. Self-injurious behavior: genes–brain–behavior relationships. Washington (DC): American Psychological Association. [Google Scholar]
- 68.Verebey K, Volavka J, Mule S, Resnick R. 1976. Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther 20:315–328 [DOI] [PubMed] [Google Scholar]
- 69.Wang D, Raehal KM, Bilsky EJ, Sadee W. 2001. Inverse agonists and neutral antagonists at µ opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem 77:1590–1600 [DOI] [PubMed] [Google Scholar]
- 70.Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME. 2008. Differences in δ- and µ-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology 33:653–665 [DOI] [PubMed] [Google Scholar]
- 71.Wentland MP, Lou R, Dehnhardt CM, Duan W, Cohen DJ, Bidlack JM. 2001. 3-Carboxamido analogues of morphine and naltrexone: synthesis and opioid receptor binding properties. Bioorg Med Chem Lett 11:1717–1721 [DOI] [PubMed] [Google Scholar]
- 72.Williams KL, Holden Ko MC, Rice KC, Woods JH. 2003. Effect of opioid antagonists on hypothalamic–pituitary–adrenal activity in rhesus monkeys. Psychoneuroendocrinology 28:513–528 [DOI] [PubMed] [Google Scholar]