Abstract
The goal of this study was to identify objective criteria that would reliably predict spontaneous death in aged inbred mice. We evaluated male and female AKR/J mice, which die at a relatively young age due to the development of lymphoma, as well as male C57BL/6J and BALB/cByJ mice. Mice were implanted subcutaneously with an identification chip that also allowed remote measurement of body temperature. Temperatures and body weights were measured weekly until spontaneous death occurred or until euthanasia was performed for humane reasons. In AKR/J mice, hypothermia and weight loss began about 4 wk prior to death and increased gradually during that antemortem interval. In C57BL/6J and BALB/cByJ mice, these declines began earlier and were more prolonged prior to death. However, C57BL/6J and BALB/cByJ mice developed a relatively precipitous hypothermia during the 2 wk prior to death. For all 3 strains, the derived composite score of temperature × weight, expressed as a percentage of stable values for each mouse, was similarly informative. These changes in individual and composite measures can signal the need for closer observation or euthanasia of individual mice. Validated markers of clinical decline or imminent death can allow the use of endpoints that reduce terminal distress, do not significantly affect longevity or survival data, and permit timely collection of biologic samples.
Abbreviations: T × W, product of temperature and body weight
Experimental endpoints in biomedical research should be determined on the basis of a combination of scientific, ethical, legal, practical, and humane considerations. The use of humane endpoints can be particularly problematic for longevity studies, because the goal of such studies is the determination of lifespan. Similarly, in studies of chronic and ultimately fatal illnesses such as cancer, prolongation of lifespan can be an important measure of the efficacy of new therapeutics. In such studies, premature euthanasia could significantly skew the data and potentially lead to erroneous conclusions. These considerations can create a conflict between collection of necessary data and minimization of animal pain and distress. However, an increasingly recognized concept in aging research is that of health span, as compared with lifespan. Health span refers to the duration of relative health, as compared with symptomatic clinical decline, in aging populations.
In a previous study, we monitored the temperature and body weights of outbred Hsd:ICR mice to determine whether these parameters could provide useful objective markers of imminent death.9 That study indicated that temperature and body weight can provide objective benchmarks to trigger increased observation or euthanasia of individual mice while accurately retaining lifespan data. However, as was emphasized in that study, similar application of those markers to other experimental models requires additional documentation.
To provide such documentation, we undertook the present study, in which we evaluated temperature and body weights across the lifespans of 3 inbred strains of mice: AKR/J, C57BL/6J, and BALB/cByJ. AKR/J mice are notable as the inbred mouse strain with the shortest natural lifespan that is listed in the Mouse Phenome Database (http://phenome.jax.org/db/q?rtn=projects/details&sym=Yuan2). These mice die at a relatively young age due to the development of lymphoma related to the presence of endogenous murine viruses.2,4 C57BL/6J and BALB/cByJ mice are used extensively in biomedical research. Our goal in the present study was to determine whether the markers of temperature and body weight would be informative in predicting clinical deterioration and imminent spontaneous death in these mouse strains.
Materials and Methods
Male and female AKR/J mice (n = 25 per sex) were purchased specifically for this study at 4 wk of age and were housed on arrival in same-sex groups of 5 in 11 in. × 7 in. × 5 in. cages. Male C57BL/6J and BALB/cByJ mice (n = 31 and 13, respectively) were purchased for other approved protocols at our facility but were not needed for their original purpose; these mice were at least 6 mo old when transferred to this study protocol and were maintained in their original groupings (1 to 5 mice per cage). Mouse groupings were not modified for any mice as mice from each cage died. Cages were solid-bottom shoebox-style open-top cages with woodchip bedding (Beta Chip, Northeastern Products, Warrensburg, NY). Mice were maintained by using conventional husbandry practices, with cages changed weekly. Room temperature was maintained at 70 ± 2 °F (21.1 ± 1.0 °C) and relative humidity at 40% to 60%. Food (LabDiet 5001, PMI Nutrition International, St Louis, MO) and tap water were available ad libitum. All mice were free of known infections with common rodent microbial and parasitic agents, as monitored by using monthly testing of sentinel mice housed in the same room. The Laboratory Animal Care and Use Committee at Southern Illinois University School of Medicine approved all animals and experimental procedures used in this study, including experimental endpoints.
Isoflurane-anesthetized mice were subcutaneously implanted with a microchip that allowed remote measurement of body temperature by using a wand-type reader (model IPTT300, BioMedic Data Systems, Seaford, DE). The microchip was implanted by using a 12-gauge needle delivery device, without an incision or wound closure. Individual chips weighed approximately 0.125 g. The chips were not tested for accuracy or otherwise calibrated prior to use but were used according to manufacturer's recommendations. All reported mouse weights were collected after the implantation of the microchip.
The research staff evaluated mice weekly for body weight, temperature, and general signs of illness. These assessments typically occurred during the afternoon hours. Body weights were measured to the nearest gram by using an Ohaus Scout II top-loading balance with an accuracy of ± 0.1 g. The husbandry staff also monitored all mice for signs of illness or deviation from normal at the daily census and health check and in association with changing cages, as is the standard practice of the Division of Laboratory Animal Medicine at our institution. Sick mice were brought immediately to the attention of the veterinarian and the research team, who then evaluated the animal. As warranted by the animal's condition and in consultation with the clinical veterinarian, euthanasia was performed in accordance with endpoints specified in the protocol or the mouse was maintained for continued observation and data collection. Although the goal of the study was to monitor mice until the time of spontaneous death, preemptive euthanasia was performed for humane reasons if mice showed any of the following signs: palpable hypothermia (which, in our experience, generally reflects an abdominal temperature of less than 25 °C), inability or unwillingness to walk, lack of response to manipulation, severe dyspnea or cyanosis, and large, bleeding, or ulcerated tumors. According to these criteria, euthanasia was performed on 1 male AKR/J mouse, 1 female AKR/J mouse, and 1 C57BL/6J mouse. For purposes of data analysis, these mice were considered to have died.
All data are expressed as mean ± SEM. In addition to evaluating raw data averaged across all animals, data were normalized to baseline values for each animal. Baseline values were defined as the average values measured during weeks 12 to 9 prior to death for AKR mice and weeks 16 to 13 prior to death for BALB/cByJ and C57BL/6J mice. Temperatures were normalized as a difference from baseline, whereas body weight and the derived variable temperature × body weight (T × W) were normalized as a proportion of baseline for each individual animal. Paired t tests were used to compare the individualized baseline data to the individually normalized data obtained for each subsequent week prior to death. A P value ≤ 0.05 was considered significant.
Results
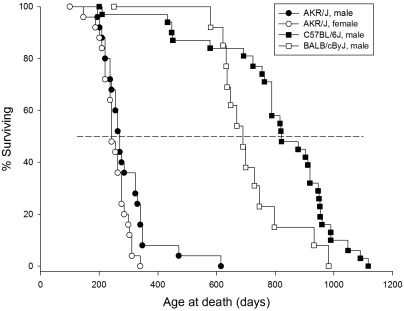
Consistent with data from the Mouse Phenotype Database (http://phenome.jax.org/), female AKR/J mice in our study had a mean survival time of 252 ± 9 d (median, 242 d or 35 wk; Jax median, 254 d), compared with 293 ± 18 d (median, 270 d or 39 wk; Jax median, 288 d) for male AKR/J mice (Figure 1).
Figure 1.
Kaplan–Meier survival plot. The life spans of mice used in this study are depicted. The horizontal dashed line indicates the point at which 50% of the mice remained alive.
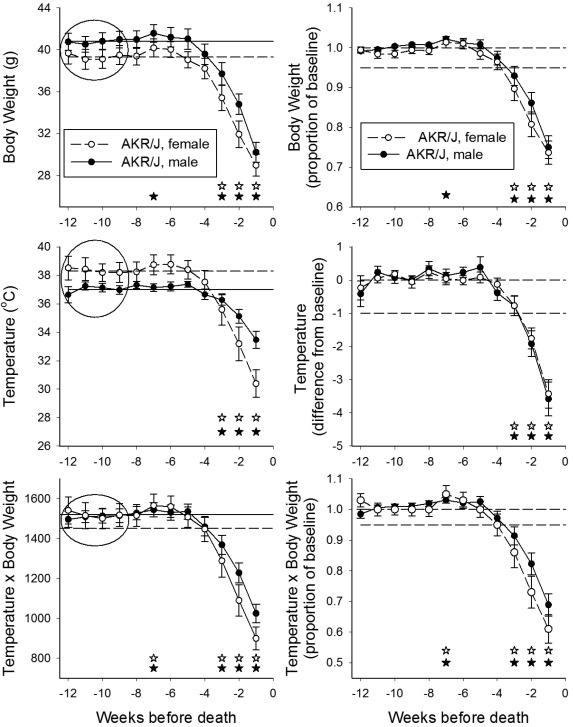
Among AKR/J mice, temperatures and body weight were stable until about 3 wk prior to death (Figure 2). At approximately 3 wk prior to death, both male and female mice began to show significant drops in both body temperature and body weight as compared with weeks 9 through 12. These decreases culminated in an average hypothermia of approximately 4 °C and average weight loss of about 10 g at the last measurement taken prior to spontaneous death (Figure 2, left panels). The derived values of T × W paralleled these responses. For assessment of individual responses, mean baseline values were calculated for each mouse as the average of measures taken during weeks 9 through 12 prior to death. Weekly measures then were expressed as a ratio of the baseline value (body weight and T × W) or as a difference from baseline (temperature; Figure 2, right panels). The pattern of change of these individualized and normalized values paralleled that of the overall group averages, although with less variation. The body weights of 2 of 25 male and 5 of 25 female AKR/J mice did not fall below an arbitrary threshold of 95% of the baseline weight. Although 6 of these 7 mice developed hypothermia of greater than 1 °C, their values for T × W were above 95% of baseline at the time of their deaths.
Figure 2.
Body weight, temperature, and temperature × body weight during the final 12 wk of life in AKR/J mice. Values of temperature and body weight were aligned with respect to the time of collection prior to death or euthanasia and are plotted in order of collection prior to death. In the left panels, data points indicate the mean ± SEM of the actual measured values for male (filled circles) and female (open circles) mice. The horizontal lines represent the average of all values collected during weeks 9 to 12 prior to death for male (solid line) and female (dashed line) mice, as denoted within the ovals. Open and filled stars denote P < 0.05 as compared with the baseline value for female and male mice, respectively. In the right panels, values for each mouse were normalized with respect to that animal's individual baseline values, determined according to the mean of values collected during weeks 9 through 12 prior to death (ovals in the left panels). For body weight and temperature × body weight, data at each time point were converted to a ratio of the baseline value for that mouse; the horizontal solid and dashed lines on those panels respectively indicate ratios of 1.0 and, as a visual benchmark, 0.95. For temperature, data were expressed as a difference from the baseline value for that mouse; the horizontal solid and dashed lines on that panel respectively indicate normothermia and, as a visual benchmark, 1 °C hypothermia.
The mean lifespans of male C57BL/6J and BALB/cByJ mice were 801 ± 39 d (median, 821 d or 117 wk; Jax median, 901 d) and 720 ± 33 d (median, 690 d or 96 wk; Jax median, 707 d), respectively (Figure 1). Jax data were taken from the Mouse Phenotype Database (http://phenome.jax.org/).
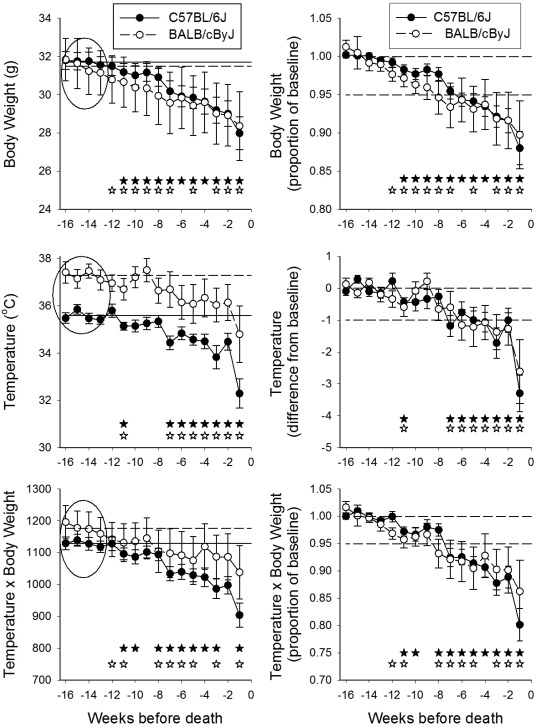
Body weight, temperature, and T × W data from C57BL/6J and BALB/cByJ mice are depicted in terms of both mean measured values (Figure 3, left panels) and individually normalized values (Figure 3, right panels). In comparison with AKR/J and C57BL/6J mice, BALB/cByJ mice showed substantially more variability in these parameters, perhaps reflecting the lower number of BALB/cByJ mice that were evaluated (13 BALB/cByJ compared with 25 each for male and female AKR/J and 31 for C57BL/6J). When group means were calculated in terms of individualized baseline values (weeks 13 to 16), both C57BL/6J and BALB/cByJ mice showed significant weight loss that began at 10 or 11 wk prior to death (P < 0.05) and continued until death occurred (Figure 3, top right panel). Furthermore, both strains developed significant hypothermia beginning at 7 wk prior to death (P < 0.05), with a precipitous fall occurring at the last measurement taken prior to death (Figure 3, middle right panel). However, 8 of 31 C57BL/6J mice and 6 of 13 BALB/cByJ mice had not developed a reduction in body weight to less than an arbitrary benchmark of 95% of the baseline weight by the week prior to death. Furthermore, 6 C57BL/6J mice and 4 BALB/cByJ mice did not show a reduction in temperature of greater than 1 °C in the week prior to death. Of greater concern, some mice developed hypothermia that resolved prior to death and later returned, as is suggested by the variation in temperatures around the −1 °C range (Figure 3, right center panel). Because of this variation, rigid application of a hypothermia benchmark for euthanasia would have resulted in premature euthanasia of some of these mice by several weeks relative to the time of their spontaneous death.
Figure 3.
Body weight, temperature, and temperature × body weight during the final 16 wk of life of C57BL/6J and BALB/cByJ mice. Values of temperature and body weight were aligned with respect to the time of collection prior to death or euthanasia and are plotted in order of collection prior to death. In the left panels, data points indicate the mean ± SEM of the actual measured values for C57BL/6J (filled circles) and BALB/cByJ (open circles) mice. The horizontal lines represent the average of all values collected during weeks 13 to 16 prior to death for C57BL/6J (solid line) and BALB/cByJ (dashed line) mice, as denoted within the ovals. In the right panels, values for each mouse were normalized respect to that animal's individual baseline values, determined according to the mean of values collected during weeks 13 through 16 prior to death (ovals in the left panels). For body weight and temperature × body weight, data at each time point were converted to a ratio of the baseline value for that mouse; the horizontal solid and dashed lines on those panels respectively indicate ratios of 1.0 and, as a visual benchmark, 0.95. For temperature, data were expressed as a difference from the baseline value for that mouse; the horizontal solid and dashed lines on that panel respectively indicate normothermia and, as a visual benchmark, 1 °C hypothermia. Open and filled stars denote P < 0.05 as compared with the baseline value for BALB/cByJ and C57BL/6J mice, respectively.
Discussion
In a previous study,9 we proposed that reductions in temperature and body weight provide reasonably valid benchmarks for performing euthanasia on geriatric mice without markedly influencing experimental outcomes in terms of longevity. Subsequently, we demonstrated that this approach can also be useful for determination of humane endpoints in mice inoculated with a variety of infectious agents.17 Here we test the application of this approach to the assessment of clinical decline and the prediction of imminent death in geriatric mice of 3 inbred strains, including AKR/J mice, which die at a relatively young age as a result of a high incidence of spontaneous lymphoma.2,4 Our findings indicate that statistically significant reductions in temperature, body weight, and their product precede spontaneous death by several weeks in most mice under these conditions. However, although these changes indicate terminal decline and can signal the need for closer monitoring of individual mice, week-to-week changes can be variable among individual mice, particularly for the C57BL/6J and BALB/cByJ strains.10,12 Such variation may preclude use of these markers for prediction of imminent death in these strains, although the progression of changes can be followed to monitor clinical deterioration.
We have suggested 2 approaches by which changes in temperature, body weight, and T × W can be used as signals for preemptive euthanasia in mice. In the first, a threshold for euthanasia can be established for all mice based on deviation from baseline means that are calculated for the entire group of mice. For example, for AKR/J mice, a body weight of less than 34 g or a temperature of below 35 °C would signal that death will occur within 2 to 3 wk and could be a trigger for either euthanasia or more frequent monitoring (Figure 2, left panels). In contrast, the use of these measures for prediction of imminent death in C57BL/6J and BALB/cByJ mice is difficult due to the variable and gradual nature of the changes in these mouse strains (Figure 3, left panels). However, when feasible and validated, the use of a fixed deviation from a predetermined group norm offers an unambiguous criterion that is not dependent on records or calculations. We typically use this approach in our infectious disease studies, in which we immediately euthanize mice whose temperatures fall below an established endpoint.17
The second approach involves calculating individual average baseline values for each mouse (for example, right panels in Figures 2 and 3,17). Euthanasia of each animal would then be determined based on changes relative to that animal's individual norm. Based on this approach, hypothermia of greater than 1 °C would signal the need for more intensive monitoring for individual C57BL/6J and BALB/cByJ mice (Figure 3, right panels), with hypothermia of greater than 3 °C signaling the need for euthanasia. This approach relies on record keeping and assessing each animal against its own records. Although this task may be time-consuming from some perspectives, the value of mice generated during a multiyear study may make the labor costs acceptable.
The selection of either (or neither) of these approaches depends on the goals of the study, including the necessary accuracy associated with the absolute identification of maximal longevity and the loss of data that might be associated with inadvertent spontaneous death of a study subject. The reductions in temperature and body weight that we report here occurred in the context of median lifespans of 35, 39, 117, and 96 wk for female AKR/J and male AKR/J, C57BL/6J and BALB/cByJ mice, respectively, and in lifespans of approximately 24 mo in ICR mice in our previous study.9 Using these benchmarks allows objective quantification of clinical decline and in some models can allow prediction of imminent death. Furthermore, by signaling the need for increased observation or monitoring of specific animals in large colonies, tracking these parameters could perhaps reveal additional markers that would more closely precede death, thereby potentially further refining the approach presented here.
However, caution is warranted in applying these measures, because variation in the patterns of individual mice for individual parameters (weight, temperature, or their product) can be misleading. Although the figures presented here show the periods during which statistically significant deviation from baseline values was present prior to spontaneous death, the relatively early onset, potential spontaneous reversal, and gradual exacerbation of these changes may preclude their use for prediction of imminent death. In that regard, several studies report that mice develop poor thermoregulatory control and maintain lower temperatures during middle age5,12 Furthermore, in our study, some individual mice developed hypothermia of as much as 2 °C that resolved prior to death and later returned. These sporadic and transient changes are reflected in the variation in temperatures around the −1 °C range (Figure 3, right center panel). Because of this variation, rigid application of a hypothermia benchmark for euthanasia would have resulted in premature euthanasia of some of these mice by several weeks relative to the time of their spontaneous death. However, these gradual and intermittent reductions are nonetheless clearly indicative of antemortem clinical decline, given that the mice die within 4 to 6 wk after the onset of these changes. The data reported here for C57BL/6J and BALB/cByJ mice particularly underscore that these (or any) markers should carefully be validated for precision and accuracy before implementing them as markers for imminent death and, on that basis, as signals for euthanasia, in any specific experimental model. However, these measures definitely signal the need for more intensive clinical evaluation.
With regard to the AKR/J mice, the development of humane endpoints for mice used in cancer studies has long been a topic of interest with regard to the welfare of experimental animals. Many publications on this topic recommend the use of condition scoring, tumor burden, and other assessments.1,20 In comparison with these strategies, our approach is highly objective and easily validated. However, the question of whether measurement of temperature and body weight and determination of their product would offer a valid approach to prediction of death in mice with implanted or induced tumors remains to be addressed. Because such models are probably far more commonly used than are spontaneous models, assessment and validation of these markers in those models would be highly valuable, particularly with regard to models of metastatic disease.
As reviewed and emphasized in our earlier study,9 identification of specific benchmarks for euthanasia and validation of those markers in the context of longevity are best determined in the context of specific experimental models and goals. For example, the gradual development of weight loss and hypothermia and its temporal relationship to spontaneous death that we report here for C57BL/6J and BALB/cByJ mice is similar to the pattern we reported for outbred Hsd:ICR mice in our previous study.9 However, in comparison with these strains, AKR/J mice show a more precipitous decline in both measures, even though the development of significant tumor burden in those mice might be expected to mask weight loss. This pattern of decline in AKR/J mice suggests that prediction of imminent death may be even more reliable with regard to specific disease models, as compared with longevity in general. However, benchmarks for both temperature and body weights can vary markedly depending on the mouse strain, housing conditions, and disease model6-8,11,14,16,17-19 and should carefully be identified and validated. In addition, temperatures may vary substantially depending on the measurement site (for example, subcutaneous, abdominal, rectal, or surface) or technique (for example, microchip, telemetry, or rectal). Emphasizing this possibility, an unexpected feature of the current data was the apparent difference in basal body temperatures of C57BL/6J and BALB/cByJ mice (Figure 3). In previous studies, the average daily patterns of temperature of these 2 strains were not significantly different.3,13-16 However, all of the previous studies had used abdominally implanted transmitters and telemetry to measure abdominal temperatures, whereas the current study used subcutaneously implanted microchips. A significant fall in rectal temperature has been reported to occur in C57BL/6J mice they approach death.10
In the current study and our previous work,9 the derived measure of T × W, as compared with the individual measures of temperature and body weight, generally did not provide additional sensitivity or precision for prediction of either imminent death or accelerating clinical decline. However, we nonetheless advise evaluation of this pooled measure in assessment of other disease models, based on the possibility that in some situations relatively modest declines in weight or temperature could have predictive value that might be apparent in the pooled measure but not in the individual measures. In this regard, we again emphasize that specific endpoints markers should be developed and validated in the context of each specific model; our work provides an illustration of the approach, rather than a prescription for values.
An important caveat to our approach is that some mice died without developing either hypothermia or weight loss. Therefore, use of this approach is unlikely to allow detection and prevention of all spontaneous deaths. Nonetheless, applying our strategy can signal the need for increased animal observation, reduce the incidence of spontaneous death, allow the evaluation of pathophysiologic changes in aged mice in the context of end-of-life clinical deterioration, and permit the timely collection of tissues from mice that are near death but not yet agonal. Because the purpose of our study was to validate methods for prediction of imminent death, we purposely invoked only severe clinical endpoints for preemptive euthanasia to test our method thoroughly. However, the information we gained will now be available to guide future similar studies in the selection of earlier and preemptive endpoint markers, both in our studies and hopefully in those of others. In light of our data, we suggest that investigators who conduct similar studies of aging and longevity using other strains of mice validate our approach in their ongoing studies and report their findings to the scientific community.
Acknowledgments
This work was supported in part by NIH grant K26-RR17543 and the Southern Illinois University School of Medicine.
References
- 1.Aldred AJ, Cha MC, Meckling-Gill KA. 2002. Determination of a humane endpoint in the L1210 model of murine leukemia. Contemp Top Lab Anim Sci 41:24–27 [PubMed] [Google Scholar]
- 2.Coffin JM, Stoye JP, Frankel WN. 1989. Genetics of endogenous murine leukemia viruses. Ann N Y Acad Sci 567:39–49 [DOI] [PubMed] [Google Scholar]
- 3.Depino AM, Gross C. 2007. Simultaneous assessment of autonomic function and anxiety-related behavior in BALB/c and C57BL/6 mice. Behav Brain Res 177:254–260 [DOI] [PubMed] [Google Scholar]
- 4.Gilbert DJ, Neumann PE, Taylor BA, Jenkins NA, Copeland NG. 1993. Susceptibility of AKXD recombinant inbred mouse strains to lymphomas. J Virol 67:2083–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzales P, Rikke BA. 2010. Thermoregulation in mice exhibits genetic variability early in senescence. Age (Dordr) 32:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon CJ. 2004. Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68 [PubMed] [Google Scholar]
- 7.Gordon CJ, Fogelson L, Highfill JW. 1990. Hypothermia and hypometabolism: sensitive indices of whole-body toxicity following exposure to metallic salts in the mouse. J Toxicol Environ Health 29:185–200 [DOI] [PubMed] [Google Scholar]
- 8.Paster EV, Villines KA, Dickman DL. 2009. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med 59:234–241 [PMC free article] [PubMed] [Google Scholar]
- 9.Ray MA, Johnston NA, Verhulst SJ, Toth LA. 2010. Determination of humane endpoints in longevity research. J Am Assoc Lab Anim Sci 49:282–288 [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds MA, Ingram DK, Talan M. 1985. Relationship of body temperature stability to mortality in aging mice. Mech Ageing Dev 30:143–152 [DOI] [PubMed] [Google Scholar]
- 11.Soothill JS, Morton DB, Ahmad A. 1992. The HID50 (hypothermia-inducing dose 50): an alternative to the LD50 for measurement of bacterial virulence. Int J Exp Pathol 73:95–98 [PMC free article] [PubMed] [Google Scholar]
- 12.Talan MI, Engel BT. 1986. Temporal decrease of body temperature in middle-aged C57BL/6J mice. J Gerontol 41:8–12 [DOI] [PubMed] [Google Scholar]
- 13.Toth LA. 1996. Strain differences in the somnogenic effects of interferon inducers in mice. J Interferon Cytokine Res 16:1065–1072 [DOI] [PubMed] [Google Scholar]
- 14.Toth LA, Hughes LF. 2006. Sleep and temperature responses of inbred mice with Candida albicans-induced pyelonephritis. Comp Med 56:252–261 [PubMed] [Google Scholar]
- 15.Toth LA, Hughes LF, Rehg JE. 2005. Sleep during concanavalin-A-induced hepatitis and peritonitis in inbred mice. Sleep 28:571–582 [DOI] [PubMed] [Google Scholar]
- 16.Toth LA, Rehg JE, Webster RG. 1995. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J Neuroimmunol 58:89–99 [DOI] [PubMed] [Google Scholar]
- 17.Trammell RA, Toth LA. 2011. Markers for predicting death as an outcome in mice used in infectious disease research. Comp Med. 61:492–498 [PMC free article] [PubMed] [Google Scholar]
- 18.Vlach KD, Boles JW, Stiles BG. 2000. Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal enterotoxic shock. Comp Med 50:160–166 [PubMed] [Google Scholar]
- 19.Warn PA, Brampton MW, Sharp A, Morrissey G, Steel N, Denning DW, Priest T. 2003. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim 37:126–131 [DOI] [PubMed] [Google Scholar]
- 20.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farmingham DA, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA. 2010. Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102:1555–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]