Abstract
Ulcerative dermatitis (UD) is a common syndrome of unknown etiology that results in profound morbidity in C57BL/6 mice and lines on a C57BL/6 background. The lesions are due to severe pruritus-induced self-trauma, progressing from superficial excoriations to deep ulcerations. UD may be behavioral in origin, with ulcerative lesions resulting from self-mutilating behavior in response to unresolved inflammation or compulsion. Alternatively, abnormal oxidative damage may be a mechanism underlying UD. To evaluate whether UD behaves similarly to normal wounds, consistent with a secondary self-inflicted lesion, or is a distinct disorder with abnormal wound response, we evaluated expression levels of genes representing various arms of the oxidative stress response pathway UD-affected and unwounded C57BL/6J mice. No evidence indicated that UD wounds have a defect in the oxidative stress response. Our findings are consistent with an understanding of C57BL/6 UD lesions as typical rather than atypical wounds.
Abbreviations: HO, heme oxygenase; GPX, glutathione peroxidase; Prdx, peroxiredoxin; SOD, superoxide dismutase; UD, ulcerative dermatitis
Ulcerative dermatitis (UD) is a common syndrome of unknown etiology that results in profound morbidity in C57BL/6 mice and strains on a C57BL/6 background.18,26,41 Ulcerations generally present on the dorsal scapulae, although torso, shoulder, and facial lesions are seen also, and may be single or multifocal in distribution.2,18,22 The lesions are due to severe pruritus-induced self-trauma, progressing from superficial excoriations to deep ulcerations.2,18,40 Subsequent inflammation brings heavy concentrations of neutrophils, lymphocytes, macrophages, and mast cells to the lesion site.2 Multifactorial etiologies have been suggested for UD, including age,25,26 sex,2,18,40 diet,6,25,27,31 immune-complex vasculitis,2 and primary follicular dystrophy.42 These proposed etiologies have prompted many treatment options, which have achieved varying degrees of success. Of these, maropitant citrate,45 vitamin E,22 cyclosporine,14 caladryl lotion,9 and toenail trimming24,35 have had the greatest effect on minimization of lesion size. No treatment to date has been curative.
Recent studies have indicated that the ultimate etiology of UD may lie in the behavior patterns of C57BL/6 mice.11,15 These studies suggest that barbering in C57BL/6 mice is a compulsive behavior, showing similar traits to trichotillomania in humans,15 leading to the hypothesis that UD is behavioral and not dermal in origin. In addition, development of UD later in life can be predicted based on increased scratching behavior at a young age.11 These researchers also found that increasing levels of brain serotonin increased hair pulling and induced UD, further implicating UD as a function of behavior. Other recent evidence similarly connects UD with behavioral abnormalities, specifically excessive grooming resulting in oral hair impaction.10,21 The highly significant association found between UD and hair-induced periodontitis suggests that these UD lesions are secondary effects, self-inflicted in response to continual, unresolvable oral inflammation and pain. This theory was supported by a highly significant tendency for facial UD lesions (a less common form of UD) to be ipsilateral to periodontitis (33 of 37, χ2 2-tailed P < 0.0001).10 It is possible that the ultimate cause of the hair-induced periodontitis is due to overgrooming and barbering, again suggesting an ultimate behavioral cause of UD.
Treatment success with maropitant citrate, a tachykinin neurokinin 1 (NK1) receptor antagonist, also adds weight to the theory that UD is behavioral in origin by indicating that substance P, which functions at the NK1 receptor to induce itching and scratching, may play a role in maintaining UD lesions through perpetuation of the itch–scratch–itch cycle.45 Substance P is a neuropeptide with high affinity for the NK1 receptor and has been shown to be a potent inducer of pruritus mediated by various compounds, including histamine from mast cells, in both mice and humans.1,16
In addition, a strain-specific, inappropriate response to oxidative damage may participate in the initiation and maintenance of UD.22 The antioxidant vitamin E has been among the most successful of the numerous, diverse treatments prescribed for UD,22 and unchecked lipid peroxidation is well known to cause pain and pruritus.7 However, if UD were simply a self-inflicted consequence of abnormal behavior, such as scratching or excessive grooming, then it likely would behave similarly to a normal wound, which initiates a robust oxidative stress response to combat oxidative injury.33,34 Therefore, we were prompted to investigate the oxidative stress response of UD to determine whether it is similar to the response exhibited during normal wound-healing.
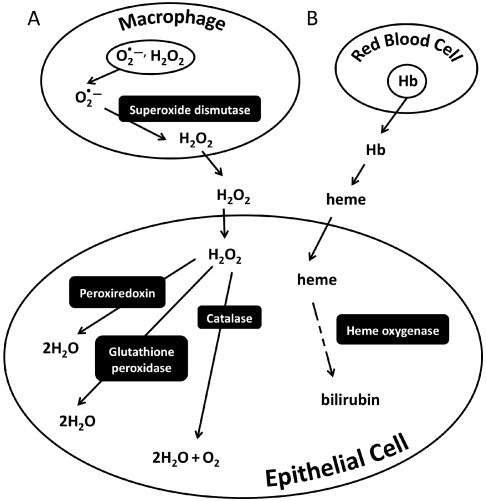
Normal wound healing is a complex interaction of cell types and signaling molecules.23,48 Oxidative free radicals play a critical role in the initiation of wound healing, including cell signaling,32,34,39 and protection of the wound from foreign invaders.33 Reactive oxygen species are generated from respiratory bursts from inflammatory cells, such as neutrophils and macrophages,3,4 and must be countered by internal antioxidant systems within surrounding cells to prevent unnecessary damage.33,50 Several of the most important antioxidant pathways use the enzymes glutathione peroxidase,38 catalase,50 heme oxygenase,5 superoxide dismutases,29 and peroxiredoxins,47 to catalyze important steps in the detoxification of free radicals (Figure 1). The superoxide dismutases act first, within the macrophages and neutrophils themselves, to reduce the superoxide anion to hydrogen peroxide. Superoxide anions are unable to cross cell membranes, but once converted to hydrogen peroxide molecules, they enter the extracellular space and surrounding cells. Hydrogen peroxide can be further detoxified to water by the action of several related enzymes (glutathione peroxidase, catalase, and peroxiredoxin) using slightly different mechanisms. Injury also can result in hemorrhage, leading to the extravasation of RBC, which then lyse and release free hemoglobin into the interstitium for oxidation into free heme. Free heme catalyzes free radical production through Fenton chemistry. In addition, extracellular heme can enter neighboring cells and trigger the antioxidant enzyme heme oxygenase 1 (HO1), which that transforms heme into bilirubin, a potent antioxidant involved in protecting lipids from oxidation.33
Figure 1.
Summary of oxidative stress response pathways. (A) Macrophages and neutrophils are among the initial innate immune cells to respond to an injury. These cells generate respiratory bursts consisting of superoxide anions (O2−) and hydrogen peroxide (H2O2). Superoxide dismutase enzymes convert the superoxide anion to hydrogen peroxide. Hydrogen peroxide can pass out of the cell and into the interstitium, where it can enter neighboring cells and cause oxidative damage. In this example, epithelial cells have been exposed to hydrogen peroxide, resulting in a cellular oxidative stress response. Subsequently several antioxidant pathways are activated, including glutathione peroxidase, catalase, and peroxiredoxins. These enzymes allow the cell to prevent oxidative damage by breaking down the hydrogen peroxide to oxygen and water. (B) If an injury results in inflammation or hemorrhage, RBC are lysed, leading to the release of free hemoglobin (Hb) into the interstitium. Hemoglobin then is oxidized, leading to the release of free heme. Heme catalyzes free radical production through Fenton chemistry. Once heme enters a cell, the inducible enzyme heme oxygenase 1 converts the heme to bilirubin, a potent antioxidant.
The gene expression pattern characteristic of the early oxidative stress response during normal wound healing has been well characterized in various mammalian models33,38,50 but not C57BL/6 mice. Here we evaluated the oxidative stress response in UD-affected C57BL/6J mice at the gene expression level. We postulated that this response would be comparable to that seen in normal wound healing.
Materials and Methods
C57BL/6 ulcerative dermatitis, normal wound, and control groups.
UD group.
Six (3 male, 3 female; age, 1 y) naïve, C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) mice with full-thickness UD nape lesions were selected on the basis of daily health reports. A veterinarian confirmed the diagnosis of full-thickness UD before the mouse was placed on study. All of our mice with UD were reported within approximately 24 h after onset of the lesion and were euthanized immediately by CO2 inhalation. A 200-mg sample of affected skin was excised, any adherent fat removed, and the section quick-frozen in liquid nitrogen and stored at −80 °C.
Normal wound group.
Six (3 male, 3 female; age, 1 y) C57BL/6J mice (The Jackson Laboratory) were anesthetized with isoflurane and shaved in a 2 cm × 2 cm area around the nape of the neck. Two skin biopsies located 1 cm apart were excised by using a 5-mm biopsy punch (Integra LifeSciences, Plainsboro, NJ). The wounds were allowed to heal by second intention for 24 h, at which time mice were euthanized by inhalant CO2 and the wound and a surrounding 1 mm of skin were excised, and flash-frozen as described earlier.
Control group.
Eleven (6 male, 5 female; age, 4 to 12 mo) naïve, C57BL/6J control mice (The Jackson Laboratory) with no dermatitides were euthanized by CO2 inhalation, and a 200-mg sample of nape skin was collected as described earlier. Mice from this group were used for comparison with both the UD and normal wound groups.
BALB/c normal wound and control groups.
Thirteen (7 male, 6 female) BALB/cAnNHsd (BALB/cHsd; Harlan Laboratories, Indianapolis, IN) mice were divided equally by sex into a control group (n = 6) and an experimental group (n = 7), in which wounds were induced by the removal of skin biopsies. Samples were harvested 24 h after wound induction for analysis as described earlier for C57BL/6J mice.
Animal care.
Mice were received at the Division of Laboratory Animal Medicine, at the University of California Los Angeles. All mice were housed in an SPF, AAALAC-accredited facility, where sentinel mice are tested quarterly and remain negative for mouse parvovirus (types 1 and 2 and NS1), minute virus of mice, mouse norovirus, mouse hepatitis virus, Sendai virus, lymphocytic choriomeningitis virus, polyomavirus, K virus, pneumonia virus of mice, mouse adenovirus, epizootic diarrhea of infant mice, mice encephalomyelitis virus, reovirus, ectromelia virus, Mycoplasma pulmonis, and Helicobacter spp. as well as endo- and ectoparasites. After experimental intervention, mice were singly housed in polycarbonate cages (Lab Products, Seaford, DE) on corncob bedding (Bed-O'Cobs, The Andersons, Maumee, OH) and were given enrichment nesting material (Nestlets, Ancare, Bellmore, NY). Mice received rodent diet (PMI Nutrition International, Richmond, IN) ad libitum. The animal room was environmentally controlled, with temperature maintained between 68 to 79 °F (20.0 to 26.1 °C), relative humidity between 30% and 70%, and a 12:12-h light:dark cycle. The IACUC of the University of California—Los Angeles approved all animal use activity in this study.
Quantitative PCR.
Total RNA was isolated by homogenizing tissue in TRIzol reagent (Invitrogen, Carlsbad, CA) and processing according to the manufacturer's instructions. RNA was reverse-transcribed into cDNA with the iSCRIPT kit (BioRad Laboratories, Hercules, CA). Real-time quantitative PCR was performed with the BioRad iCycler (BioRad Laboratories) as previously described.49 Expression levels were normalized to those of the endogenous control gene hypoxanthine–guanine phosphoribosyltransferase (Hprt). The following genes were analyzed by real-time qualitative PCR: glutathione peroxidase 1 (GPX1), heme oxygenase 1 (HO1), peroxiredoxin 1 (Prdx1), peroxiredoxin 6 (Prdx6), catalase (Cat), superoxide dismutase 1 (SOD1), and superoxide dismutase 2 (SOD2; Sigma Aldrich, St Louis, MO). The primers used for these genes are listed in Table 1.
Table 1.
Primer sequences for real-time qualitative PCR analysis
| Forward primer (5′→3′) | Reverse primer (5′→3′) | |
| GPX1 | CGG TTT CCC GTG CAA TCA GTT C | ACT GGG TGT TGG CAA GGC ATT C |
| HO1 | GAA CAT CGA CAG CCC CAC CAA G | CAG CAT CAC CTG CAG CTC CTC A |
| Prdx1 | ACG ACT AGT CCA GGC CTT CC | GGC AGA AAA ATG GTC CAG TG |
| Prdx6 | GGC CCT GAC AAG AAA CTG AA | TCG GAG AGG GTG GGA ACT AC |
| Cat | CCA CCT GAA GGA CGC TCA GCT TT | CTT TTC CCT TCG CAG CCA TGT G |
| SOD1 | AAC CAG TTG TGT TGT CAG GAC | CCA CCA TGT TTC TTA GAG TGA GG |
| SOD2 | CGA AGC CCC TGT TTA TCT GA | CTC ACC GAG GTC ATC TCT GC |
| Hprt | TAT GGC GAC CCG CAG CCC T | CAT CTC GAG CAA GAC GTT CAG |
Statistical analyses.
The gene expression data were prepared for analysis by calculating log2(normalized expression) = log2(experimental gene expression / Hprt expression). Log transformation was performed to improve the normality of the gene expression data. These values were regressed on sex to correct for any potential effect. The residuals from this regression were used for 2-way ANOVA, comparing UD-affected with control animals for the 7 genes described, with the group variables of gene, UD or control status, and their interaction. Because these analyses yielded significant F statistics for the UD–control variable (P = 0.0004) and gene×UD–control interaction (P = 0.0004), one-tailed Student t tests were performed to identify the genes that were activated in the UD samples. Both STATA (StataCorp, College Station, TX) and Excel (Microsoft, Redmond, WA) statistical programs were used. A power calculation was performed as a follow-up to the negative results found for SOD1 and SOD2 in this analysis, by using the G*Power 3 statistical package,13 to determine the effect size (Cohen d) detectable at 80% power for a one-tailed t test with an α value of 0.05. An α value of 0.05 was used for all analyses.
Results
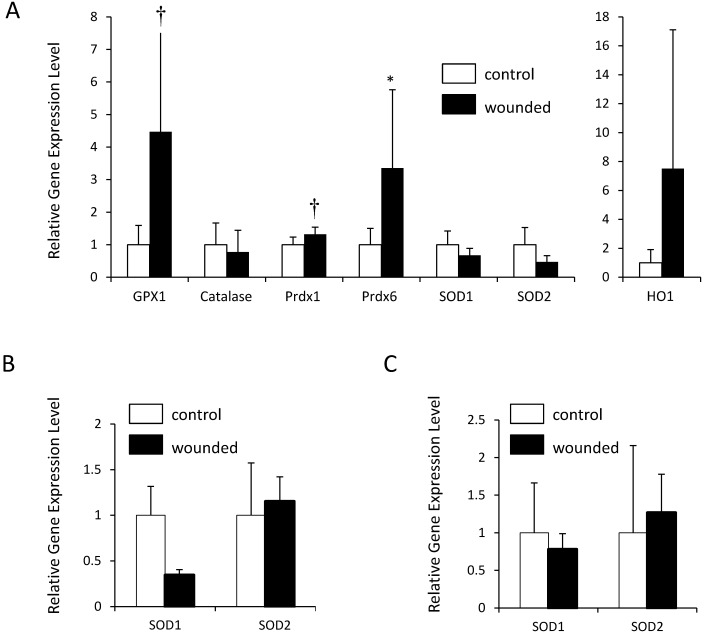
Skin biopsies were collected from C57BL/6J mice with UD and from unaffected C57BL/6J controls of similar age. Samples were obtained approximately 24 h after onset, a time point previously shown to exhibit a peak in the upregulation of oxidative response genes.20,33,38 The samples were evaluated for gene expression levels of a panel of enzymes known to be involved in the normal oxidative stress response, including GPX1, HO1, Prdx1, Prdx6, Cat, SOD1, and SOD2 (Figure 1). Gene expression differed significantly (P = 0.0004) depending on whether the mice had UD or not. We then used pairwise one-tailed t tests to ask which of the genes were upregulated in the UD group (Figure 2 A).
Figure 2.
At the gene expression level, the oxidative stress response of UD wounds resembles that in normal wound healing. mRNA levels for oxidative stress response genes in UD and normally wounded mice were measured by qualitative PCR and normalized to hypoxanthine–guanine phosphoribosyltransferase (Hprt). Expression levels are shown as fold change (mean ± 1 SD) as compared with controls. HO1 is depicted separately, due to the larger scale. (A) C57BL/6 UD-wounded mice compared with C57BL/6 controls (6 wounded compared with 11 control mice for Gpx1, Prdx1, SOD1, and SOD2; 6 wounded compared with 5 control mice for Cat, Prdx6, and HO1). (B) SOD1 and SOD2 expression in C57BL/6 normally wounded animals compared with C57BL/6 controls (6 wounded compared with 6 control mice). (C) SOD1 and SOD2 expression in BALB/cHsd normally wounded animals compared with BALB/cHsd controls (7 wounded compared with 6 control mice). For all comparisons, statistical analyses were performed on log-transformed expression levels (see Methods). *, P < 0.05, †, P < 0.01 compared with levels in corresponding controls.
The gene expression profile of the oxidative stress response in UD-affected mice was largely consistent with previous studies: GPX1 and Prdx6 were robustly and significantly upregulated (P = 0.0046 and P = 0.0433, respectively), but Cat was not (Figure 2 A). Prdx1 was modestly upregulated (P = 0.0077). HO1 demonstrated high variability in the UD group; the data suggest upregulation of HO1 in UD, but this comparison was not statistically significant. SOD1 and SOD2 showed no evidence of upregulation in UD, contrary to previous published evidence that these genes are upregulated in response to a wound.38 A power calculation of sensitivity determined that we had 80% power to detect an effect size of 1.323 or larger (see Methods). However, because no previous studies have specifically assessed the wound healing response in the C57BL/6J strain, we could not rule out the possibility that this nonresponse of the SOD genes simply represents variation between strains.
To evaluate SOD1 and SOD2 in a model of wound healing in C57BL/6J mice, we created full-thickness skin wounds by using a 5-mm biopsy punch as in a previous study38 and collected samples 24 h later. We also performed this same wounding experiment in another commonly used inbred strain, BALB/cHsd, to further investigate the possibility of strain variation. In both strains, neither SOD1 nor SOD2 was upregulated in response to wounding (Figure 2 B and C), although the significant (P < 0.05) upregulation of GPX1, HO1, Prdx6, and Prdx1 indicated that the wounding process had indeed elicited a normal oxidative stress response in these models (data not shown). This absence of response of the SOD genes in normal wounding models suggests that an absence of an SOD response in UD does not represent a defect of oxidative stress.
Discussion
The C57BL/6 strain is uniquely susceptible to UD, a specific type of self-perpetuated wound injury with unknown etiology.18,26,41 We compared the oxidative stress response at the gene expression level in C57BL/6J mice affected by UD with previously published data,38 to look for evidence of a defect in oxidative stress pathways.
Because the antioxidant vitamin E has been among the most successful of the myriad treatments used to alleviate UD, aberrant oxidative injury has been proposed as a possible mechanism for UD lesion development.22 However, a different possibility for the origin of UD has been raised by recent work demonstrating a significant behavioral component involving increased scratching behavior and increased barbering in C57BL/6 mice.11,15 Other studies have associated UD with alopecia,27,42 potentially due to a defect in vitamin A metabolism or to a primary follicular dystrophy resulting in skin inflammation.42 However, the UD lesions in our current study were not preceded by onset of alopecia; therefore, we believe that the UD we describe here represents an etiology that is unique from the one described in these other studies.41,42 If behavioral characteristics are indeed responsible for the onset of UD, then UD lesions likely would not deviate from the normal oxidative stress response activated during wound healing and would not be subject to aberrant oxidative injury.
Many oxidative stress response genes are induced during normal wound healing in mice38 and rats,36 but the C57BL/6 mouse strain has not, to our knowledge, been evaluated in this way. We chose a variety of genes for antioxidant enzymes to represent several different pathways, as well as a spectrum of known responders and nonresponders in wound healing.
One of the most potent responders is GPX1, a selenoenzyme that detoxifies hydrogen peroxide to water.50 Expression of GPX1 is induced within 24 h of full-thickness excisional wounds.38 In our current study, GPX1 was significantly upregulated in UD wounds, consistent with this previous work.
An important response gene in an alternative pathway is HO1, an inducible enzyme that converts free heme through multiple steps to bilirubin, a potent antioxidant.5,44 The HO1 gene is transiently upregulated at day 1 of normal wound healing.17,33 HO1 expression appeared to be increased in UD wounds, although potent within-group variation kept this comparison from reaching statistical significance. The brevity of the window for HO1 upregulation after wounding is likely responsible for the variability in HO1 expression, given that it is difficult to determine the precise time at which the wound was initiated due to the spontaneous nature of the injury.
The Prdx enzymes catalyze the reduction of hydrogen peroxide and other peroxides.47 In studies of normal wound healing in rats and humans, the Prdx6 member of this enzyme family was upregulated in response to skin wounds.19,28 We similarly noted that Prdx6 is significantly upregulated in UD wounds. In addition, another member of the enzyme family, Prdx1, was upregulated. Prdx1 expression in a previous study was not increased in response to skin wounds,47 perhaps because the less sensitive assay used previously could not detect the modest difference that occurred in our current study.
Catalase is another common antioxidant enzyme that detoxifies hydrogen peroxide to oxygen and water.50 However, previous work indicates that this gene is not upregulated in the normal response to a skin wound.38 Our observations were consistent with this finding.
Upstream of the effects of these hydrogen peroxidases are the 2 forms of superoxide dismutase (SOD1, a constitutively expressed cytosolic enzyme, and SOD2, an inducible mitochondrial form), which convert the highly reactive superoxide anion to hydrogen peroxide.37,46 In a previous study, both forms exhibited upregulated gene expression at 24 h after wounding in a substrain of BALB/c mice.38 However, in our current study, neither SOD1 nor SOD2 was upregulated in UD wounds. To evaluate whether this nonresponse was simply a difference between strains (C57BL/6J compared with the substrain of BALB/c used in the previous study), we performed 2 normal wound healing experiments in cohorts of C57BL/6J and BALB/cHsd mice. In both of these experiments, neither SOD1 nor SOD2 was upregulated in response to wound healing, despite a response from the other oxidative stress genes measured that was consistent with the literature and with our findings in UD. Therefore the lack of SOD1 and SOD2 upregulation in UD wounding is not evidence of a UD-specific or even a C57BL/6-specific defect in oxidative stress, given that these genes also are not upregulated in C57BL/6 and BALB/c normal wound healing. Superoxide dismutase may represent an arm of the oxidative stress response pathway whose upregulation in wound healing is specific to that particular substrain of BALB/c mice or that is not activated at this early (24 h) time point in C57BL/6J and BALB/cHsd strains. Importantly, the response of the UD group does not differ from either of the surgically wounded groups in our study, indicating that failure to upregulate the SOD genes likely does not represent impairment of wound healing.
Ultimately, the oxidative stress response pattern that emerges for UD wounds in C57BL/6 mice is remarkably similar to that of a normal wound healing process, as observed in both C57BL/6J and BALB/cHsd mouse strains in our study, as well as in various models from previous studies.33,38 This consistency indicates that the initial oxidative stress response in UD is unimpaired, further suggesting that UD may be related to behavior. Our findings are especially exciting in light of the recently proposed mechanism of UD as a behavioral manifestation, in which the unique trigger lies not in a dysfunction of the skin or wound itself but in an unrelated susceptibility to increased scratching behavior11,15 or overgrooming leading to hair-induced periodontitis.10 Furthermore, that UD is a functionally normal wound could explain why no single type of treatment has been universally successful. Notably, the treatments that have produced the best results for UD cases9,14,22 are remedies that have been used in other mammals to treat similar inflammatory skin conditions.8,12,30,37,43 In light of our findings, the success of these treatments is likely due to their ability to improve all types of wound healing rather than to UD-specific impairment in the oxidative stress response. Even simply halting the mouse's itch–scratch–itch cycle by toenail trimming can be an effective treatment,24,35 reinforcing the self-inflicted nature of the syndrome and the idea of UD as a typical rather than atypical wound.
In conclusion, our results suggest that the etiology of UD does not include a defect in the oxidative stress response. Our findings are consistent with the theory that UD in the C57BL/6 strain may be a secondary result of strain-related behavioral characteristics.
Acknowledgments
This work was supported in part by NIH grants PO1 HL28481 (KR and RMC) and T32 HG002536 (LSC).
References
- 1.Andoh T, Nagasawa T, Satoh M, Kuraishi Y. 1998. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther 286:1140–1145 [PubMed] [Google Scholar]
- 2.Andrews AG, Dysko RC, Spilman SC, Kunkel RG, Brammer DW, Johnson KJ. 1994. Immune complex vasculitis with secondary ulcerative dermatitis in aged C57BL/6NNia mice. Vet Pathol 31:293–300 [DOI] [PubMed] [Google Scholar]
- 3.Babior BM. 2000. Phagocytes and oxidative stress. Am J Med 109:33–44 [DOI] [PubMed] [Google Scholar]
- 4.Babior BM, Lambeth JD, Nauseef W. 2002. The neutrophil NADPH oxidase. Arch Biochem Biophys 397:342–344 [DOI] [PubMed] [Google Scholar]
- 5.Baranano DE, Rao M, Ferris CD, Snyder SH. 2002. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA 99:16093–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackwell BN, Bucci TJ, Hart RW, Turturro A. 1995. Longevity, body weight, and neoplasia in ad-libitum-fed and diet-restricted C57BL6 mice fed NIH31 open-formula diet. Toxicol Pathol 23:570–582 [DOI] [PubMed] [Google Scholar]
- 7.Calabrese V, Scapagnini G, Catalano C, Bates TE, Geraci D, Pennisi G, Giuffrida Stella AM. 2001. Regulation of heat-shock protein synthesis in human skin fibroblasts in response to oxidative stress: role of vitamin E. Int J Tissue React 23:127–135 [PubMed] [Google Scholar]
- 8.Clarke MW, Burnett JR, Croft KD. 2008. Vitamin E in human health and disease. Crit Rev Clin Lab Sci 45:417–450 [DOI] [PubMed] [Google Scholar]
- 9.Crowley M, Delano ML, Kirchain SM. 2008. Successful treatment of C57BL/6 ulcerative dermatitis with caladryl lotion. J Am Assoc Lab Anim Sci 47:109–110 [Google Scholar]
- 10.Duarte-Vogel SM, Lawson GW. 2011. Association between hair-induced oronasal inflammation and ulcerative dermatitis in C57BL/6 mice. Comp Med 61:13–19 [PMC free article] [PubMed] [Google Scholar]
- 11.Dufour BD, Adeola O, Cheng HW, Donkin SS, Klein JD, Pajor EA, Garner JP. 2010. Nutritional upregulation of serotonin paradoxically induces compulsive behavior. Nutr Neurosci 13:256–264 [DOI] [PubMed] [Google Scholar]
- 12.Eschler DC, Klein PA. 2010. An evidence-based review of the efficacy of topical antihistamines in the relief of pruritus. J Drugs Dermatol 9:992–997 [PubMed] [Google Scholar]
- 13.Faul F, Erdfelder E, Lang AG, Buchner A. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191 [DOI] [PubMed] [Google Scholar]
- 14.Feldman S, McVay L, Kessler MJ. 2006. Resolution of ulcerative dermatitis of mice by treatment with topical 0.2% cyclosporine. J Am Assoc Lab Anim Sci 45:92–93 [Google Scholar]
- 15.Garner JP, Weisker SM, Dufour B, Mench JA. 2004. Barbering (fur and whisker trimming) by laboratory mice as a model of human trichotillomania and obsessive–compulsive spectrum disorders. Comp Med 54:216–224 [PubMed] [Google Scholar]
- 16.Hagermark O, Hokfelt T, Pernow B. 1978. Flare and itch induced by substance P in human skin. J Invest Dermatol 71:233–235 [DOI] [PubMed] [Google Scholar]
- 17.Hanselmann C, Mauch C, Werner S. 2001. Heme oxygenase 1: a novel player in cutaneous wound repair and psoriasis? Biochem J 353:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kastenmayer RJ, Fain MA, Perdue KA. 2006. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 45:8–12 [PubMed] [Google Scholar]
- 19.Kumin A, Huber C, Rulicke T, Wolf E, Werner S. 2006. Peroxiredoxin 6 is a potent cytoprotective enzyme in the epidermis. Am J Pathol 169:1194–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumin A, Schafer M, Epp N, Bugnon P, Born-Berclaz C, Oxenius A, Klippel A, Bloch W, Werner S. 2007. Peroxiredoxin 6 is required for blood vessel integrity in wounded skin. J Cell Biol 179:747–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson GW. 2010. Etiopathogenesis of mandibulofacial and maxillofacial abscesses in mice. Comp Med 60:200–204 [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson GW, Sato A, Fairbanks LA, Lawson PT. 2005. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci 44:18–21 [PubMed] [Google Scholar]
- 23.McKay IA, Leigh IM. 1991. Epidermal cytokines and their roles in cutaneous wound healing. Br J Dermatol 124:513–518 [DOI] [PubMed] [Google Scholar]
- 24.Mufford T, Richardon L. 2009. Nail trims versus the previous standard of care for treatment of mice with ulcerative dermatitis. J Am Assoc Lab Anim Sci 48:546 [Google Scholar]
- 25.Myers D. 1996. The effect of weaning age and diets. In: Users of JAX mice. Bar Harbor (ME): The Jackson Laboratory. [Google Scholar]
- 26.Myers D. 1996. Notice to JAX mice users. In: Users of JAX mice. Bar Harbor (ME): The Jackson Laboratory. [Google Scholar]
- 27.Myers D. 1997. C57BL/6J skin lesion problem eliminated. In: Users of JAX mice. Bar Harbor (ME): The Jackson Laboratory. [Google Scholar]
- 28.Novoselov VI, Baryshnikova LM, Yanin VA, Amelina SE, Fesenko EE. 2003. The influence of peroxyredoxin VI on incised-wound healing in rats. Dokl Biochem Biophys 393:326–327 [DOI] [PubMed] [Google Scholar]
- 29.Ojha N, Roy S, He G, Biswas S, Velayutham M, Khanna S, Kuppusamy P, Zweier JL, Sen CK. 2008. Assessment of wound-site redox environment and the significance of Rac2 in cutaneous healing. Free Radic Biol Med 44:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivry T, DeBoer DJ, Favrot C, Jackson HA, Mueller RS, Nuttall T, Prelaud P. 2010. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol 21:233–248 [DOI] [PubMed] [Google Scholar]
- 31.Perkins SN, Hursting SD, Phang JM, Haines DC. 1998. Calorie restriction reduces ulcerative dermatitis and infection-related mortality in p53-deficient and wild-type mice. J Invest Dermatol 111:292–296 [DOI] [PubMed] [Google Scholar]
- 32.Rhee SG. 1999. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31:53–59 [DOI] [PubMed] [Google Scholar]
- 33.Schafer M, Werner S. 2008. Oxidative stress in normal and impaired wound repair. Pharmacol Res 58:165–171 [DOI] [PubMed] [Google Scholar]
- 34.Sen CK, Roy S. 2008. Redox signals in wound healing. Biochim Biophys Acta 1780:1348–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seta S. 2009. A simplified method for the treatment of mouse dermatitis. J Am Assoc Lab Anim Sci 48:548 [Google Scholar]
- 36.Shukla A, Rasik AM, Patnaik GK. 1997. Depletion of reduced glutathione, ascorbic acid, vitamin E, and antioxidant defence enzymes in a healing cutaneous wound. Free Radic Res 26:93–101 [DOI] [PubMed] [Google Scholar]
- 37.Simon D. 2011. Systemic therapy of atopic dermatitis in children and adults. Curr Probl Dermatol 41:156–164 [DOI] [PubMed] [Google Scholar]
- 38.Steiling H, Munz B, Werner S, Brauchle M. 1999. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp Cell Res 247:484–494 [DOI] [PubMed] [Google Scholar]
- 39.Stone JR, Yang S. 2006. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8:243–270 [DOI] [PubMed] [Google Scholar]
- 40.Stowe HD, Wagner JL, Pick JR. 1971. A debilitating fatal murine dermatitis. Lab Anim Sci 21:892–897 [PubMed] [Google Scholar]
- 41.Sundberg JP, Brown K, McMahon W. 1994. Chronic ulcerative dermatitis in black mice, p 485–492. In: Sundberg JP. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. Boca Raton (FL): CRC Press. [Google Scholar]
- 42.Sundberg JP, Taylor D, Lorch G, Miller J, Silva KA, Sundberg BA, Roopenian D, Sperling L, Ong D, King LE, Everts H. 2011. Primary follicular dystrophy with scarring dermatitis in C57BL/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet Pathol 48:513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tani S, Noguchi M, Hosoda Y, Sugibayasi K, Morimoto Y. 1998. Characteristics of spontaneous erythema appeared in hairless rats. Exp Anim 47:253–256 [DOI] [PubMed] [Google Scholar]
- 44.Tenhunen R, Marver HS, Schmid R. 1968. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61:748–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams-Fritze MJ, Carlson Scholz JA, Zeiss C, Deng Y, Wilson SR, Franklin R, Smith PC. 2011. Maropitant citrate for treatment of ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 50:221–226 [PMC free article] [PubMed] [Google Scholar]
- 46.Wlaschek M, Scharffetter-Kochanek K. 2005. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen 13:452–461 [DOI] [PubMed] [Google Scholar]
- 47.Wood ZA, Schroder E, Robin Harris J, Poole LB. 2003. Structure, mechanism, and regulation of peroxiredoxins. Trends Biochem Sci 28:32–40 [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi Y, Yoshikawa K. 2001. Cutaneous wound healing: an update. J Dermatol 28:521–534 [DOI] [PubMed] [Google Scholar]
- 49.Zhang P, O'Loughlin L, Brindley DN, Reue K. 2008. Regulation of lipin 1 gene expression by glucocorticoids during adipogenesis. J Lipid Res 49:1519–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao W, Diz DI, Robbins ME. 2007. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol 80 Spec No 1:S23–S31 [DOI] [PubMed] [Google Scholar]