Abstract
The aim of this study was to evaluate the protective effects of Ginkgo biloba (GB) against testicular damage and oxidative stress as well as caudal sperm indices in a cisplatin- (CIS-) induced rodent model. Adult male Wistar rats were given vehicle, single i.p. dose of CIS alone (10 mg/kg), GB alone (200 mg g/kg every day for five days), or single dose of CIS followed by GB (50, 100, or 200 mg/kg every day for five days). On day 6, after the first drug treatment oxidative and apoptotic testicular toxicity was evaluated. CIS-treated rats displayed decreased weights of testes and epididymis as well as caudal sperm count and motility. This reproductive toxicity was accompanied with increased germ-cell degeneration in seminiferous tubules and increased germ-cell apoptosis, increased testicular MDA levels and MPO activity, and decreased SOD and CAT activities in testes. Intensive expressions of COX-2, iNOS, and NF-κB p65 in testicular tissues were detected in CIS-treated group. Oral GB administrations at all doses to CIS-treated rats effectively alleviated all of the CIS-induced toxicity in reproductive system. The present results provide further insights into the mechanisms of protection against CIS-induced reproductive toxicity and confirm the essential antioxidant potential of a GB extract.
1. Introduction
Chemotherapy has emerged as an efficient mode of treatment for various carcinogenesis. Effective systemic drugs are increasingly used to treat cancer patients. Cisplatin (cis-diamminedichloroplatinum-II, CIS), one of the most effective and widely prescribed anticancer drugs, is still used in the treatment of many types of solid tumors including testicular cancer [1, 2]. It has been proven highly effective in curing testicular cancer in combination with other drugs even at an advanced stage of the disease [3]. CIS kills cancer cells by forming covalent adducts with the cellular DNA molecules and thereby terminating the vital processes like replication and transcription and inducing apoptosis [4].
In spite of its high efficiency in the treatment of testicular cancer, CIS has severe adverse effects on spermatogenesis and even leads to a condition of azoospermia [5, 6]. Spermatogenesis is a complex process which is highly influenced by hormone molecules and temperature and involves an array of testicular cells such as germ cells, Sertoli cells, Leydig cells, and peritubular cells [7, 8]. Acute exposure to antineoplastic agents like CIS has shown an increase in the frequency of germ-cell apoptosis [9, 10] in experimental animals. Moreover, it can also lead to decreased reproductive organ weights, azoospermia, and degenerated spermatogenic cells [11, 12]. The molecular mechanism by which CIS causes reproductive toxicity and germ-cell apoptosis remains to be elucidated. However, pathogenesis of testicular damage followed by CIS exposure is generally ascribed to oxidative stress mediated by increased free radical generation and depletion of antioxidants. Free radicals have been reported to mediate reactions responsible for a wide range of CIS-induced side effects [9, 11]. Consequently, antioxidants have been shown to protect nonmalignant cells and organs against damage by CIS [9, 11, 13].
Ginkgo biloba (GB) has been used in traditional Chinese medicine for about 5000 years, and it is one of the herbal drugs that is used widely according to its antioxidant properties and ability to modify vasomotor function, affect ion channels to inhibit activation of platelets and smooth muscle cells [14], stimulate neurotransmitters [15], decrease adhesion of blood cells to endothelium, and modify signal transduction [14]. In addition, GB has been used in the treatment of Alzheimer's disease and cognitive impairment. The major GB components are flavonoglycosides and terpene lactones. GB extract was also reported for many decades to increase peripheral and cerebral blood flow as well as for the treatment of dementia [16]. Furthermore, extract of GB leaves has been shown to have a strong antioxidant that directly scavenges ROS [15].
Inducible nitric oxide synthase (iNOS) is responsible for the formation of high levels of nitric oxide (NO) under oxidative stress resulting in autocytotoxicity [17]. Previous studies have shown that NO along with ROS triggers cell death and the oxidation products of NO can induce lipid peroxidation [18–20]. Transcription factors like NF-kB stimulated under oxidative stress can also induce iNOS expression [21]. Inhibiting NF-kB and thereby iNOS using antioxidants has already proved to be effective in attenuating the CIS-induced testicular injury [20]. Cyclooxygenase- (COX)-2 is an inducible form of COX which plays a physiological role in inflammation and tumor proliferation [22]. COX-2 selective inhibitors have been found to be effective in ameliorating CIS-induced nephrotoxicity in rats [23].
To determine whether or not standardized GB extract could attenuate toxicity and oxidative stress in testicular tissues, this study was designed to assess the preventive role of GB extract on the biochemistry and pathology of CIS-induced testicular abnormalities in rats. The modulating roles of NF-kB, iNOS, and COX-2 in CIS-induced oxidative testicular injury induced were also evaluated in the present work.
2. Materials and Methods
2.1. Animals
Sixty adult male Wistar rats (8 weeks old weighing 120–240 g) were obtained from the Animal House, United Arab Emirates University,UAE. They were kept in polycarbonate cages and supplied with standard pellet diet and tap water under a 12 h light/dark cycle and room temperature of 22–24°C. This study was approved by the Animal Ethics Committee, UAE University,UAE.
2.2. Chemicals and Plant
CIS was purchased from Hospira UK Limited, Warwickshire, UK. GB-leaf extract was obtained from General Nutrition Corporation, Pittsburgh, USA. Thiobarbituric acid, 1,1,3,3-tetramethoxy-propan, phosphoric acid, sulfuric acid and hydrogen peroxide were obtained from Sigma Chemical (St. Louis, MO, USA). The GB extract is standardized to Ginkgo flavonoglycosides (24%) and terpene lactones (6%) which represent the major active contents of GB.
2.3. Experimental Protocol
Rats were divided into six groups (n = 10). The groups were treated as follows: control or normal (N) received water (5 mL/kg body weight) for five days and a single intraperitoneal injection (i.p.) of saline on the first day (5mL/kg body weight). Same dose volume (5 mL) was used for all other groups; GB alone (GB) received only 200 mg/kg body weight of GB water extract; CIS alone (CIS) where rats were given CIS as single dose (10 mg/kg; i.p.) on the first day of treatment, a dose that induced testicular toxicity in rats [24]. Rats of the protective groups (CIS+L: 50 mg/kg GB; CIS+M; 100 mg/kg GB; CIS+H: 200 mg/kg GB) received GB for five days 1 h after a single dose of CIS on the first day of treatment. GB was dissolved in water and administrated orally at three-dose levels 50 (low dose; L), 100 (medium dose; M), and 200 (high dose; H) mg/kg body weight. Doses of GB were selected based on previously reported pharmacological properties of this plant [19]. Five days after the administration of CIS or GB, the rats were sacrificed after being anesthetized with diethyl ether. Rats were weighed in regularly, and their testes and epididymis were dissected out and weighed.
2.4. Sperm Motility and Count
Total sperm number was determined by using a Neubauer hemocytometer. Cauda epididymis was dissected out, weighed, immediately minced in 5 mls of physiological saline, and then incubated at 37°C for 30 minutes to allow sperms to leave the epididymal tubules. The percentage of motile sperm was recorded from left cauda epididymis using a phase contrast microscope at 400x magnification. The total number of sperm per gram of cauda (of the right side) was then calculated.
2.5. Biochemistry
Testes were homogenized separately in ice-cold Tris-KCl buffer (150 mmol/L). The w/v ratio of the tissue to the homogenization buffer was (1 : 10 w/v). Aliquots were prepared and used for determination of different biochemical markers.
Supernatants were collected, and assays for lipid peroxidation and CAT were performed. Determination of MDA in testicular homogenate is based on its reaction with thiobarbituric acid (TBA) to form a pink complex with an absorption maximum at 535 nm. CAT activity was determined by measuring the exponential disappearance of H2O2 at 240 nm and was expressed in units/mg of protein as described by Aebi [25], and total protein was estimated by Lowry's method. Superoxide dismutase (SOD) activity in testicular tissues was determined according to the method described in [26]. This method is based on the ability of SOD to inhibit the auto-oxidation of pyrogallol at alkaline pH. Myeloperoxidase (MPO) activity in testicular tissues was determined as described in [27]. One unit of MPO activity is defined as that which degrades 1 μmol H2O2/min at 25°C.
2.6. Histology
For the histological examinations, small pieces of testis were fixed in 10% neutral phosphate-buffered formalin, and the hydrated 5 μm thick sections were stained with hematoxylin and eosin. Sections were examined under a Leica DMRB/E light microscope (Heerbrugg, Switzerland).
2.7. Immunohistochemistry
Terminal deoxynucleotidyl transferase-mediated triphosphate nick-end labeling (TUNEL) technique was used to determine apoptosis. Deparaffinized and gradually hydrated, 4 μm thick sections of testes have been used to assess apoptosis, and TUNEL was performed using the ApopTag Plus Peroxidase in situ Apoptosis Detection Kit (Serologicals Corporation, Norcross, GA, USA). The principle of the method is based on the catalytic activity of terminal deoxynucleotidyl transferase which adds digoxigenin nucleotides to the terminal 3′-OH of DNA molecule and thereby detecting the DNA fragmentation associated with apoptosis. Further immunohistochemical analysis was done using sequential mounted sections of the specimen. Initially the antigens were revealed by incubating the sections in a heated water bath for 15 minutes followed by the blocking of the endogenous peroxidase activity with 0.3% H2O2 in methanol. Anti-rat primary antibodies (1 : 100 dilution) for COX-2 (Clone SP-21), iNOS (Ab-1) and NF-kB p65 (Rel A, Ab-1) from rabbit were obtained from Thermo Fisher Scientific (Anatomical Pathology, Fremont, USA). The sections were first incubated in primary antibodies overnight at 4°C. After overnight incubation, the slides were washed with PBS, and the sections were incubated with polyvalent biotin-labeled goat anti-rabbit secondary antibody (1 : 200 dilution) for 10 minutes at room temperature. The sections were then stained using Universal LSAB plus kit and a DAB plus substrate kit as the chromogen followed by a light counterstaining with hematoxylin. Tissue images were captured by optical microscopy (Olympus DP71).
2.8. Statistical Analysis
Data are expressed as group mean ± SE. The statistical analysis was carried out using ANOVA, with SPSS version 10 (SPSS, Chicago, IL, USA). ANOVA was carried out to detect the differences between all the various groups. When significant differences were detected, analysis of a difference between the means of the treated and control groups was carried out using Dunnett's t-test.
3. Results
3.1. Histopathological Effects
Testicular tissues in the control group showed normal arrangement of germinal and Sertoli cells without any histopathological lesions. CIS-treated groups showed moderate to severe testicular atrophy with severe cellular disorganization and degeneration in seminiferous tubules (Figures 1 and 2) and interstitium. CIS treatment also induced depletion of Leydig cells between the tubules. Degenerated Sertoli cells were also observed in the lumen. Animals pretreated with GB showed normal testicular morphology with irregular arrangement of germ cells and slight degeneration of seminiferous epithelium and shedding of germ cells in some tubules.
Figure 1.
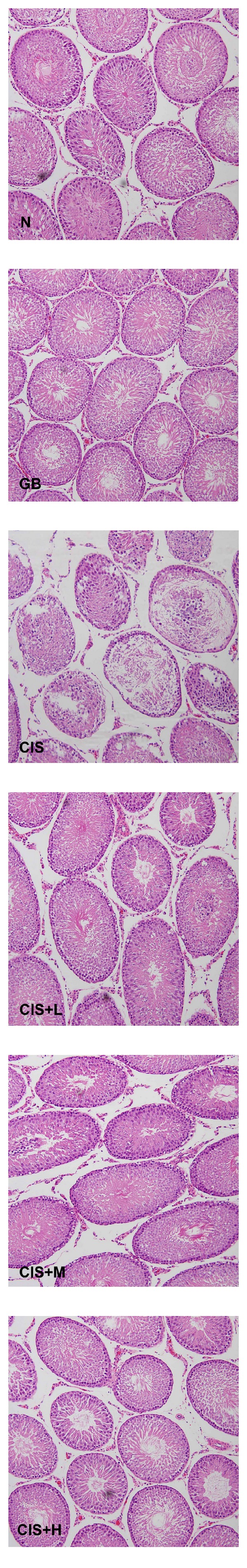
Photomicrograph of cross-sections of the testes of control (N), GB alone (GB), CIS alone (CIS), and the three GB-protected rats (CIS+L: 50 mg/kg GB; CIS+M: 100 mg/kg GB; CIS+H: 200 mg/kg GB). Testes of control and GB alone treated groups show normal arrangement of germ cells and Sertoli cells. However, CIS-treated testes show severely damaged seminiferous tubules. Rats protected with GB were less affected by CIS (H & E 200x).
Figure 2.
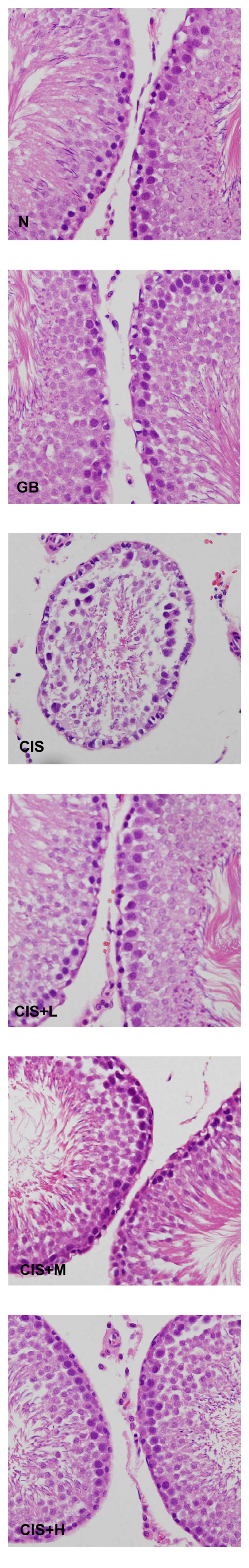
Photomicrograph of cross-sections of the testes of control (N), GB alone (GB), CIS alone (CIS), and the three GB-protected rats (CIS+L: 50 mg/kg GB; CIS+M: 100 mg/kg GB; CIS+H: 200 mg/kg GB). Testes of control and GB alone treated groups show normal arrangement of germ cells and Sertoli cells. However, CIS-treated testes show extensive atrophy of seminiferous tubules and degenerations of germ cells. Rats protected with different GB doses clearly show less degeneration of some tubules and irregular derangement of some germ cells (H & E 400x).
3.2. Effects on Weights of Testes and Epididymis
The weights of testes and epididymis in rats after CIS administration were found to be significantly decreased, compared with the control group (Table 1). No significant changes in the weights of testes and epididymis were found in rats treated with GB alone. However, GB caused significant improvements in the weights of testes (P < 0.05 and P < 0.001) and showed some recovery of epididymal weights of rats of all protected groups (Table 1). The administration of CIS alone or with prior GB administrations at all three tested doses did not alter the body weight of the animals (data not shown).
Table 1.
The effect of the GB extracts on testicular epididymal weights and on epididymal sperm count, motility, and abnormality in CIS-treated rats.
| Parameters | N | GB | CIS | CIS+L | CIS+M | CIS+H |
|---|---|---|---|---|---|---|
| Testes weight (gm) | 3.51 ± 0.06 | 3.59 ± 0.11 | 2.99 ± 0.12* | 3.49 ± 0.08# | 3.52 ± 0.18# | 3.65 ± 0.13### |
| Epididymis weight (gm) | 1.64 ± 0.06 | 1.53 ± 0.06 | 1.30 ± 0.04** | 1.38 ± 0.05** | 1.46 ± 0.08 | 1.44 ± 0.05 |
| Sperm (count.106)/gm of cauda | 131.0 ± 7.73 | 134.9 ± 11.64 | 83.94 ± 8.03* | 94.50 ± 20.25 | 94.67 ± 7.77 | 118.78 ± 8.78 |
| Sperm motility (%) | 79.125 ± 3.34 | 68.12 ± 2.64 | 28.125 ± 1.52*** | 54.0 ± 5.59∗∗∗### | 49.0 ± 3.83∗∗∗### | 50.0 ± 4.2∗∗∗### |
*P < 0.05 versus control, #P < 0.05 versus CIS, **P < 0.01 versus control, ###P < 0.001 versus CIS, ***P < 0.001 versus control.
3.3. Effects on Sperm Motility and Count
After CIS was administered, the caudal sperm count (P < 0.05) and motility decreased significantly (P < 0.001). Administration of GB attenuated the depletion of sperm counts where there was no significant difference between preventive groups and the control. However, all doses of GB significantly (P < 0.001) increased the CIS-induced decreases in sperm motility. Administration of GB attenuated the CIS-induced decrease of sperm count. Effect of GB extract on caudal sperm count and motility is given in Table 1.
3.4. Effects on Oxidative Stress on Testicular Tissues
CIS-intoxicated rats, testicular MDA levels, and MPO activity were significantly (P < 0.001) increased, and SOD and CAT activities were significantly (P < 0.001) decreased indicating the potent oxidative action of CIS on testicular tissues. Coadministration of GB with CIS neutralized these abnormalities in levels of MDA, SOD, CAT, and MPO reflecting the effect of GB against CIS-induced oxidative stress in testes (Table 2).
Table 2.
The effect of the GB extracts on oxidative stress parameters associated with CIS treatment in testicular tissues of rats.
| Parameters | N | GB | CIS | CIS+L | CIS+M | CIS+H |
|---|---|---|---|---|---|---|
| MDA (nmol/mg protein) | 0.97 ± 0.03 | 0.86 ± 0.08 | 1.2 ± 0.08*** | 1.0 ± 0.07c | 0.84 ± 0.06a | 0.98 ± 0.05c |
| MPO (mu/mg protein) | 16.92 ± 0.34 | 17.13 ± 0.31 | 24.65 ± 2.2*** | 18.88 ± 0.93b | 19.69 ± 1.54c | 16.93 ± 0.59c |
| CAT (u/mg protein) | 146.79 ± 2.9 | 144.07 ± 2.03 | 84.89 ± 3.24*** | 127.9 ± 3.9∗∗∗a | 118.0 ± 3.64∗∗∗a | 134.4 ± 1.88∗a |
| SOD (u/mg protein) | 3.37 ± 0.02 | 3.37 ± 0.01 | 3.62 ± 0.05*** | 3.26 ± 0.08a | 3.16 ± 0.09a | 3.25 ± 0.06a |
Values are expressed as mean ± SEM of eight rats per group. Concentration is expressed as nmol/mg protein for MDA. Activity is expressed as unit/mg protein for CAT and SOD. Activity is expressed as m unit/mg protein for MPO. Significance was determined by one-way analysis of variance followed by Dennett's t-test: *P < 0.05; ***P < 0.001 versus control. cP < 0.05; bP < 0.01; aP < 0.001 versus CIS group.
3.5. Immunohistochemistry
Apoptotic cell death in seminiferous tubules was assessed using TUNEL technique. TUNEL-positive nuclei were observed in brown color in the seminiferous tubules of control and CIS-treated rats (Figure 3). However, testicular tissues exposed to CIS contained high frequency of TUNEL-positive germ cells in contrast to those treated with GB.
Figure 3.
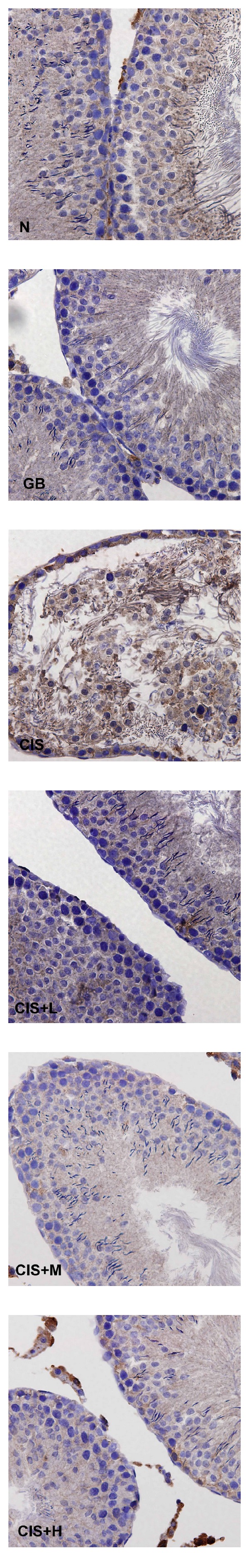
Terminal deoxynucleotidyl transferase-mediated triphosphate nick-end labeling-(TUNEL-) positive cells in seminiferous tubules of rats treated with vehicle (N), GB alone (GB), CIS alone (CIS), and the three GB-protected rats (CIS+L: 50 mg/kg GB; CIS+M: 100 mg/kg GB; CIS+H: 200 mg/kg GB). Photomicrographs show variable levels of apoptosis in different experimental groups Brown staining indicates TUNEL-positive cells. Tissues were counterstained with hematoxylin, 400x.
Immunohistochemical findings showed an increase in the expression of COX-2 (Figure 4), iNOS (Figure 5), and NF-kB (Figure 6) after CIS administration. Coadministration with GB extract markedly reduced the CIS-induced overexpression of COX-2, iNOS, and NF-kB which shows no significant variation when compared to the control.
Figure 4.
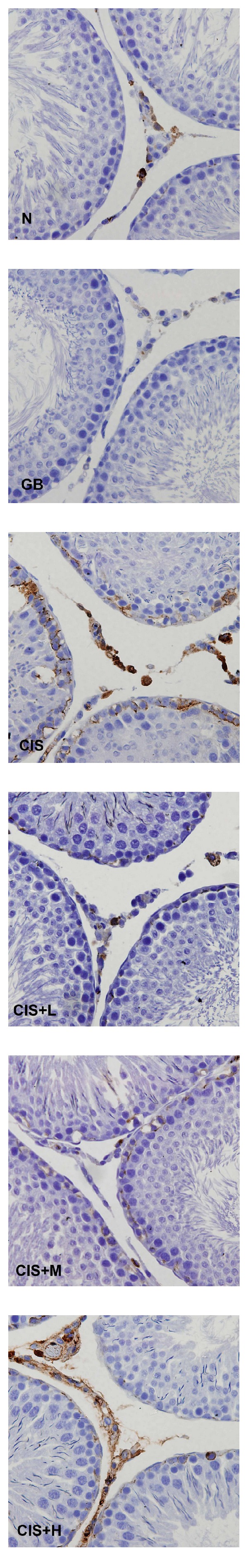
Immunohistochemical staining showing COX-2 expression in control (N), GB alone (GB), CIS alone (CIS), and the three GB-protected rats (CIS+L: 50 mg/kg GB; CIS+M: 100 mg/kg GB; CIS+H: 200 mg/kg GB). Photomicrographs show variable levels of COX-2 expression in different experimental groups. Brown staining indicates COX-2 expression. CIS group show increased levels of COX-2 expression compared to N and all three GB-protected groups. Tissues were counterstained with hematoxylin, 400x.
Figure 5.
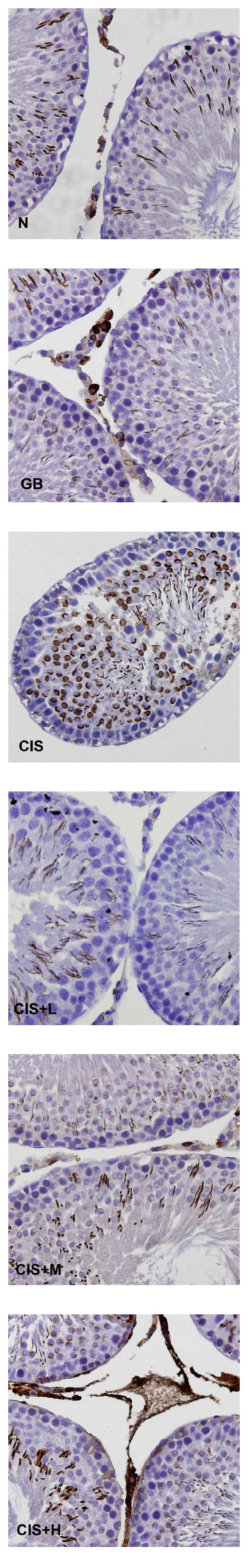
Immunohistochemical staining showing iNOS expression in control (N), GB alone (GB), CIS alone (CIS), and the three GB-protected rats (CIS+L: 50 mg/kg GB; CIS+M: 100 mg/kg GB; CIS+H: 200 mg/kg GB). Photomicrographs show variable levels of iNOS expression in different experimental groups. Brown staining indicates iNOS expression. CIS group shows increased levels of iNOS expression compared to N and the GB-protected groups. Tissues were counterstained with hematoxylin, 400x.
Figure 6.
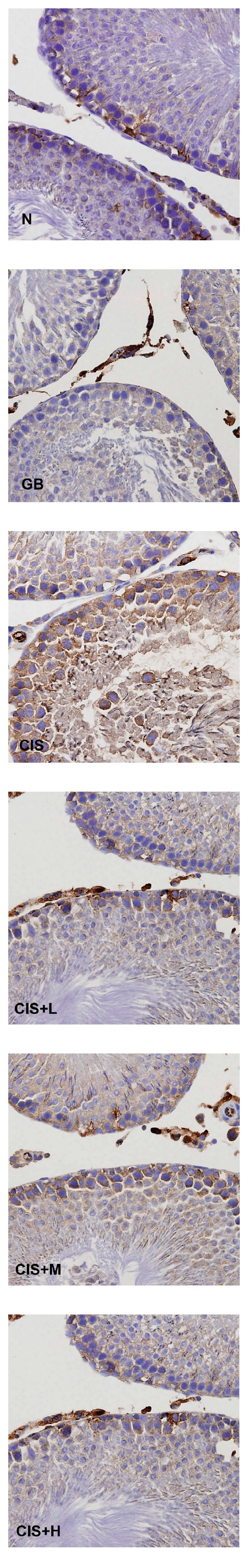
Immunohistochemical staining showing NF-kB expression in control (N), GB alone (GB), CIS alone (CIS), and the three GB-protected rats (CIS+L: 50 mg/kg GB; CIS+M: 100 mg/kg GB; CIS+H: 200 mg/kg GB). Photomicrographs show variable levels of NF-kB expression in different experimental groups. Brown staining indicates NF-kB expression. CIS-treated rats show increased NF-kB levels compared to N and all other GB-protected groups. Tissues were counterstained with hematoxylin, 400x.
4. Discussion
CIS is one of the leading anticancer drugs in the chemotherapy treatment of variety of cancer types; it induces a testicular damage, sperm dysfunction, germ-cell apoptosis, and abnormalities in Leydig cells in rats [28–30]. Our previous study has shown that CIS impaired rat testicular structure through inflicting oxidative stress and inducing cell apoptosis [9, 24]. Current data shows that pretreatment with GB extract (24% Ginkgo biloba flavonoglycoside, 6% terpene lactones) offers protection against the histopathological lesions induced by CIS. Also the increase in apoptotic changes induced by CIS has been found to be decreased in those rats which were treated with different doses of GB extract.
These testicular protective effects of GB were accompanied with restoration of the normal level of CAT, SOD, and MPO in CIS-treated animals. Normal physiological levels of NF-kB, iNOS, and COX-2 expression have also been maintained by the Coadministration of GB extract.
Recently studies have shown that herbal plants extracts with protective effects against CIS-induced reproductive damages are due to the presence of antioxidant agents [31]. The present investigation illustrates that the administration of GB extract restores the control values of oxidative stress markers. This study provides evidence that the antioxidative properties of GB may contribute to its ability to restore the level of SOD and CAT enzyme and to reduce the MDA content as well as MPO levels in the testicular tissues. The antioxidant activity of GB could be attributed to its active components, namely, flavonoglycoside and terpene lactones.
The current study also showed an elevated level of NF-kB, iNOS, and COX-2 as a result of CIS treatment. Oxidative stress and subsequent activation of signaling kinases are known to stimulate transcription factors, like NF-kB [32–34]. NF-kB function as a link between oxidative damage and inflammation. This factor transduces oxidative stimuli to nucleus to modulate the expression of many genes involved in inflammatory responses [35, 36]. One such gene is that for iNOS, it is generally thought that excessive NO production due to elevated iNOS can cause cytotoxic effects and has the potential to induce germ-cell apoptosis [20, 37, 38]. According to the data obtained, GB extract reduces the formation of NF-kB and iNOS to the normal level as in untreated rats. Rise in COX-2 levels induced by CIS, observed during the immunohistochemical analysis, seems to be efficiently reducing in the GB-treated animals.
In conclusion, this study provides evidence that CIS adversely damages testicular tissue and significantly reduces sperm production through increasing oxidative stress and inducing apoptosis and upregulations of NF-kB, iNOS, and COX-2, while GB treatment effectively attenuated these oxidative and apoptosis actions of CIS in testes.
Acknowledgments
This paper was funded by the Faculty of Science Dean's Grant and partially by UAEU Grant no. 1170-02-02-10 for A. Amin.
References
- 1.Elwell KE, Hall C, Tharkar S, et al. A fluorine containing bipyridine cisplatin analog is more effective than cisplatin at inducing apoptosis in cancer cell lines. Bioorganic and Medicinal Chemistry. 2006;14(24):8692–8700. doi: 10.1016/j.bmc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed EA, Omar HM, Elghaffar SKA, Ragb SMM, Nasser AY. The antioxidant activity of Vitamin C, DPPD and l-cysteine against Cisplatin-induced testicular oxidative damage in rats. Food and Chemical Toxicology. 2011;49(5):1115–1121. doi: 10.1016/j.fct.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Peckham MJ, Barrett A, Liew KH. The treatment of metastatic germ-cell testicular tumours with bleomycin, etoposide and cis-platin (BEP) British Journal of Cancer. 1983;47(5):613–619. doi: 10.1038/bjc.1983.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson SW, Ferry KV, Hamilton TC. Recent insights into platinum drug resistance in cancer. Drug Resistance Updates. 1998;1(4):243–254. doi: 10.1016/s1368-7646(98)80005-8. [DOI] [PubMed] [Google Scholar]
- 5.Schilsky RL, Lewis BJ, Sherins RJ, Young RC. Gonadal dysfunction in patients receiving chemotherapy for cancer. Annals of Internal Medicine. 1980;93(1):109–114. doi: 10.7326/0003-4819-93-1-109. [DOI] [PubMed] [Google Scholar]
- 6.Loehrer PJ, Einhorn LH. Cisplatin. Annals of Internal Medicine. 1984;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- 7.Huleihel M, Lunenfeld E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian Journal of Andrology. 2004;6(3):259–268. [PubMed] [Google Scholar]
- 8.Yamaguchi K, Ishikawa T, Kondo Y, Fujisawa M. Cisplatin regulates Sertoli cell expression of transferrin and interleukins. Molecular and Cellular Endocrinology. 2008;283(1-2):68–75. doi: 10.1016/j.mce.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Amin A, Hamza AA, Kambal A, Daoud S. Herbal extracts counteract cisplatin-mediated cell death in rat testis. Asian Journal of Andrology. 2008;10(2):291–297. doi: 10.1111/j.1745-7262.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 10.Lirdi LC, Stumpp T, Sasso-Cerri E, Miraglia SM. Amifostine protective effect on cisplatin-treated rat testis. Anatomical Record. 2008;291(7):797–808. doi: 10.1002/ar.20693. [DOI] [PubMed] [Google Scholar]
- 11.Atessahin A, Sahna E, Turk G, et al. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. Journal of Pineal Research. 2006;41:21–27. doi: 10.1111/j.1600-079X.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 12.Turk G, Atessahin A, Sonmez M, Çeribas AO, Yuce A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertility and Sterility. 2008;89:1474–1481. doi: 10.1016/j.fertnstert.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 13.Atessahin A, Karahan I, Turk G, Gur S, Yilmaz S, Ceribasi AO. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reproductive Toxicology. 2006;21:42–47. doi: 10.1016/j.reprotox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovascular Drug Reviews. 2004;22(4):309–319. doi: 10.1111/j.1527-3466.2004.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 15.Trompezinski S, Bonneville M, Pernet I, Denis A, Schmitt D, Viac J. Gingko biloba extract reduces VEGF and CXCL-8/IL-8 levels in keratinocytes with cumulative effect with epigallocatechin-3-gallate. Archives of Dermatological Research. 2010;302(3):183–189. doi: 10.1007/s00403-009-0979-x. [DOI] [PubMed] [Google Scholar]
- 16.Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cellular and Molecular Life Sciences. 2003;60(9):1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogdan C. The multiplex function of nitric oxide in (auto) immunity. Journal of Experimental Medicine. 1998;187(9):1361–1365. doi: 10.1084/jem.187.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaninotto F, La Camera S, Polverari A, Delledonne M. Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiology. 2006;141(2):379–383. doi: 10.1104/pp.106.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi-Hai G, Qin W, Xie-Nan H, An-Sheng S, Jing N, Jing-Shan S. Protective effect of Ginkgo biloba leaf extract on learning and memory deficit induced by aluminum in model rats. Chinese Journal of Integrative Medicine. 2006;12(1):37–41. doi: 10.1007/BF02857428. [DOI] [PubMed] [Google Scholar]
- 20.Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertility and Sterility. 2009;92(3):1124–1132. doi: 10.1016/j.fertnstert.2008.07.1758. [DOI] [PubMed] [Google Scholar]
- 21.Gilad E, Wong HR, Zingarelli B, et al. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: role of inhibition of NFκB activation. The FASEB Journal. 1998;12(9):685–693. doi: 10.1096/fasebj.12.9.685. [DOI] [PubMed] [Google Scholar]
- 22.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends in Pharmacological Sciences. 2003;24(2):96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 23.Yamamato E, Izawa T, Sawamoto O, Juniantito V, Kuwamura M, Yamate J. Amelioration of cisplatin-induced rat renal lesions by a cyclooxygenase (COX)-2 selective inhibitor. doi: 10.1016/j.etp.2010.12.005. Experimental and Toxicologic Pathology. In press. [DOI] [PubMed] [Google Scholar]
- 24.Amin A, Hamza AEA. Effects of Roselle and Ginger on cisplatin-induced reproductive toxicity in rats. Asian Journal of Andrology. 2006;8(5):607–612. doi: 10.1111/j.1745-7262.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 25.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 26.Nandi A, Chatterjee IB. Assay of superoxide dismutase activity in animal tissues. Journal of Biosciences. 1988;13(3):305–315. [Google Scholar]
- 27.Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. Journal of Pharmacological Methods. 1990;24(4):285–295. doi: 10.1016/0160-5402(90)90013-b. [DOI] [PubMed] [Google Scholar]
- 28.Meistrich ML, Finch M, Da Cunha MF, Hacker U, Au WW. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Research. 1982;42(1):122–131. [PubMed] [Google Scholar]
- 29.Boekelheide K. Mechanisms of toxic damage to spermatogenesis. Journal of the National Cancer Institute. Monographs. 2005;(34):6–8. doi: 10.1093/jncimonographs/lgi006. [DOI] [PubMed] [Google Scholar]
- 30.Cherry SM, Hunt PA, Hassold TJ. Cisplatin disrupts mammalian spermatogenesis, but does not affect recombination or chromosome segregation. Mutation Research. 2004;564(2):115–128. doi: 10.1016/j.mrgentox.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Azu OO, Duru FIO, Osinubi AA, et al. Long-term treatment with Kigelia africana fruit extract ameliorates the testicular toxicity following cisplatin administration in male Sprague-Dawley rats. Journal of Medicinal Plants Research. 2011;5(3):388–397. [Google Scholar]
- 32.Bowie A, O’Neill LAJ. Oxidative stress and nuclear factor-κB activation: a reassessment of the evidence in the light of recent discoveries. Biochemical Pharmacology. 2000;59(1):13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 33.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological Reviews. 2001;81(2):807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 34.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-κB: molecular basis and biological significance. Oncogene. 2006;25(51):6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 35.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. Journal of Biological Chemistry. 1994;269(7):4705–4708. [PubMed] [Google Scholar]
- 36.Kleniert H, Pautz A, Linke K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. European Journal of Pharmacology. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 37.Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annual Review of Pharmacology and Toxicology. 2001;41:203–236. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- 38.Lue Y, Sinha Hikim AP, Wang C, Leung A, Swerdloff RS. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: evidence from null mutant mice. Endocrinology. 2003;144(7):3092–3100. doi: 10.1210/en.2002-0142. [DOI] [PubMed] [Google Scholar]


