Abstract
The genus Hypericum L. (St. John’s Wort, Hypericaceae) includes, at the most recent count, 469 species that are either naturally occurring on, or which have been introduced to, every continent in the world, except Antarctica. These species occur as herbs, shrubs, and infrequently trees, and are found in a variety of habitats in temperate regions and in high mountains in the tropics, avoiding only zones of extreme aridity, temperature and/or salinity. Monographic work on the genus has resulted in the recognition and description of 36 taxonomic sections, delineated by specific combinations of morphological characteristics and biogeographic distribution ranges. Hypericum perforatum L. (Common St. John’s wort, section Hypericum), one of the best-known members of the genus, is an important medicinal herb of which extracts are taken for their reported activity against mild to moderate depression. Many other species have been incorporated in traditional medicine systems in countries around the world, or are sold as ornamentals. Several classes of interesting bioactive secondary metabolites, including naphthodianthrones (e.g. hypericin and pseudohypericin), flavonol glycosides (e.g. isoquercitrin and hyperoside), biflavonoids (e.g. amentoflavone), phloroglucinol derivatives (e.g. hyperforin and adhyperforin) and xanthones have been identified from members of the genus. A general overview of the taxonomy of the genus and the distribution of relevant secondary metabolites is presented.
Keywords: antidepressant, hyperforin, Hypericaceae, hypericin, secondary metabolite chemistry, systematics
INTRODUCTION
From the time of Linnaeus, the genus Hypericum has been treated as a natural unit by most taxonomists, although the discussion whether to treat this genus and its nearest relatives as a separate family (i.e. Hypericaceae) or as part of subfamily Hypericoideae within Guttiferae sensu lato has been contentious (Robson 1977, 1981; Stevens 2001, 2007). Recent molecular phylogenetic analyses of the tremendously diverse flowering plant order Malpighiales, which encompasses more than 16,000 species, support the hypotheses that a) Hypericaceae, including Hypericum, is a distinct family apart from other members of Guttiferae s.l.; b) the sister family to the Hypericaceae is Podostemaceae, whose representatives are almost exclusively thalloid aquatics; c) the clade of Hypericaceae-Podostemaceae is sister to a taxon (now referred to as Calophyllaceae) formerly subfamily Kielmeyeroideae of Guttiferae s.l.; d) and that a clade containing the remaining members of Guttiferae s.l. (Clusiaceae sensu stricto) and Bonnetiaceae forms the base of the broader clade (Korotkova et al. 2009; Wurdack and Davis 2009).
Nine genera have been taxonomically assigned to Hypericaceae: Cratoxylum Blume, Eliea Cambess., Harungana Lamarck, Hypericum L., Lianthus N.Robson, Santomasia N.Robson, Thornea Breedlove & McClintock, Triadenum Rafinesque, and Vismia Vand. Approximately 80% of the diversity of the family is within Hypericum. The majority of the species belonging to this genus and its nearest relatives (Lianthus and Santomasia) have capsular (rarely baccate or tricoccoid) fruits and yellow to orange petals (in very rare cases red or white). Species of Hypericum (in nearly all species), Lianthus and Santomasia differ from other genera of Hypericaceae by their conspicuous lack of interstaminal fasciclodes, which are present in other members of Hypericoideae (Thornea and Triadenum) as well as members of the subfamilies Cratoxyloideae (Eliea, Cratoxylum) and Vismioideae (Vismia and Harungana) (Robson 1977, 1981).
Detailed morphological descriptions of 457 species of Hypericum have been given in parts 3-8 of the monograph by Robson (1985 onwards), and an additional 12 species are described in part 9 (Robson in prep.). Species of the genus have been classified into 36 taxonomic sections (Table 1). A general overview of Hypericum botany has been included in a volume of Medicinal and Aromatic Plants – Industrial Profiles, with a particular focus on H. perforatum (Robson 2003).
Table 1.
(modified from Nürk and Blattner (submitted) with permission). Classification of the genus Hypericum L.A detailing sections, subsection and series (sensu Robson 1981 onwards), number of species per section, general distribution and specific citation for the systematic treatment. E = east, S = south, W = west, N = north.
| Classification Section / Subsect. / Series |
Number of species |
Distribution | Systematic treatment | |||
|---|---|---|---|---|---|---|
| 1. | Campylosporus (Spach) R. Keller | 10 | Tropical & SE Africa + adjacent islands, SW Iran | Robson, 1985: 178 | ||
| 2. | Psorophytum (Spach) Nyman | 1 | Spain (Balearic Islands) | Robson, 1985: 202 | ||
| 3. | Ascyreia Choisy | 43 | SE Europe, W to SE Asia, S China | Robson, 1985: 206; 2001: 49 | ||
| 4. | Takasagoya (Y. Kimura) N. Robson | 5 | Japan (Ryuku Island), Taiwan, Philippines | Robson, 1985: 288 | ||
| 5. | Androsaemum (Duhamel) Gordon | 4 | Macaronesia, W & S Europe to Iran, Saudi Arabia & Yemen | Robson, 1985: 297 | ||
| 6. | Inodora Stef. | 1 | NE Turkey, Georgia | Robson, 1985: 314 | ||
| 6a. | Umbraculoides N. Robson | 1 | Mexico (Oaxaca) | Robson, 1985: 317 | ||
| 7. | Roscyna (Spach) R. Keller | 2 | Central to E Asia, NE America | Robson, 2001: 52 | ||
| 8. | Bupleuroides Stef. | 1 | NE Turkey, Georgia | Robson, 2001: 49 | ||
| 9. | Hypericum L. | 42 | Europe, NW Africa, Asia, NW America; introduced (H. perforatum) into many other parts of the world |
Robson, 2002: 66 | ||
| 1. | Hypericum | 19 | Robson, 2002: 66 | |||
| 1. | Hypericum | 12 | Robson, 2002: 66 | |||
| 2. | Senanensia N. Robson | 7 | Robson, 2006: 28 | |||
| 2. | Erecta N. Robson | 23 | Robson, 2006: 42 | |||
| 9a. | Concinna N. Robson | 1 | USA (N California) | Robson, 2001: 61 | ||
| 9b. | Graveolentia N. Robson | 9 | SE Canada, eastern USA to Guatemala | Robson, 2006: 79 | ||
| 9c. | Sampsonia N. Robson | 2 | NE India to S Japan | Robson, 2001: 63 | ||
| 9d. | Elodeoida N. Robson | 5 | E & SE Asia (China to Kashmir) | Robson, 2001: 66 | ||
| 9e. | Monanthema N. Robson | 7 | E & SE Asia (China to Sri Lanka) | Robson, 2001: 75 | ||
| 10. | Olympia (Spach) Nyman | 4 | S Balkan peninsula, W Turkey, Aegean Islands | Robson, 2010a in press. | ||
| 11. | Campylopus Boiss. | 1 | S Bulgaria, NE Greece, NW Turkey | Robson, 2010a in press. | ||
| 12. | Origanifolia Stef. | 13 | Turkey, Georgia, Syria | Robson, 2010a in press. | ||
| 13. | Drosocarpium Spach | 11 | Madeira, Mediterranean to W Caucasus | Robson, 2010a in press. | ||
| 14. | Oligostema (Boiss.) Stef. | 6 | Europe, Macaronesia, Mediterranean | Robson, 2010a in press. | ||
| 15. | Thasia Boiss.B | 1 | Greece, Bulgaria, Turkey | Robson, 2010a in press. | ||
| 16. | Crossophyllum SpachB | 3 | N Aegean region, Turkey, Caucasus | Robson, 2010a in press. | ||
| 17. | Hirtella Stef. | 30 | W Mediterranean & S Europe to Altai | Robson, 2010b in press. | ||
| 1. | Stenadenum N. Robson | 12 | ||||
| 2. | Platyadenum N. Robson | 18 | ||||
| 1. | Lydia Sennikov | 5 | ||||
| 2. | Scabra N. Robson | 3 | ||||
| 3. | Abbreviata Semikov | 10 | ||||
| 18. | Taeniocarpium Jaub. & Spach | 28 | Europe, Mediterranean to Iran & Mongolia | Robson, 2010b in press. | ||
| 19. | Coridium Spach | 6 | Mediterranean, Alps, Caucasus | Robson, 2010b in press. | ||
| 20. | Myriandra (Spach) R. Keller | 29 | E & central North America to Honduras, Bermuda & Caribbean Islands; introduced (?) into the Azores |
Robson, 1996: 92 | ||
| 1. | Centrosperma R. Keller | 14 | Robson, 1996: 94 | |||
| 2. | Pseudobrathydium R. Keller | 1 | Robson, 1996: 112 | |||
| 3. | Suturosperma R. Keller | 7 | Robson, 1996: 113 | |||
| 4. | Brathydium (Spach) R. Keller | 2 | Robson, 1996: 122 | |||
| 5. | Ascyrum (L.) N. Robson | 5 | Robson, 1996: 124 | |||
| 21. | Webbia (Spach) R. Keller | 1 | Canary Islands, Madeira | Robson, 1996: 133 | ||
| 22. | Arthrophyllum Jaub. & Spach | 5 | S Turkey, Syria, Lebanon | Robson, 1996: 137 | ||
| 23. | Triadenioides Jaub. & Spach | 5 | S Turkey, Syria, Lebanon, Socotra | Robson, 1996: 141 | ||
| 24. | Heterophylla N. Robson | 1 | Turkey (NW & W-central Anatolia) | Robson, 1996: 146 | ||
| 25. | Adenotrias (Jaub. & Spach) R. Keller | 3 | S Morocco to Mediterranean | Robson, 1996: 147 | ||
| 26. | Humifusoideum R. Keller | 12 | Tropical & S Africa, Madagascar, SE to E Asia | Robson, 1996: 153 | ||
| 27. | Adenosepalum Spach | 25 | Canary Islands, Madeira, Europe, Africa, SW Asia | Robson, 1996: 170 | ||
| 1. | Aethiopica N. Robson | 7 | Robson, 1996: 172 | |||
| 2. | Pubescentes N. Robson | 6 | Robson, 1996: 181 | |||
| 3. | Caprifolia N. Robson | 3 | Robson, 1996: 189 | |||
| 4. | Adenosepalum | 9 | Robson, 1996: 193 | |||
| 28. | Elodes (Adans.) W. Koch | 1 | Azores & W Europe | Robson, 1996: 208 | ||
| 29. | Brathys (Mutis ex L. F.) Choisy | 87 | Central & South America, Caribbean Islands, SE Canada & eastern USA (S to Florida) |
Robson, 1987: 12; 1990: 12 | ||
| 1. | Styphelioides N. Robson | 2 | Robson, 1990: 16 | |||
| 2. | Phellotes N. Robson | 32 | Robson, 1990: 16 | |||
| 3. | Brathys | 39 | Robson, 1990: 27 | |||
| 4. | Spachium R. Keller | 14 | Robson, 1990: 29 | |||
| 30. | Trigynobrathys (Y. Kimura) N. Robson | 52 | South America to S Canada, E to SE Asia, the Hawaiian Islands, Australia, New Zealand, Africa; introduced into Europe |
Robson, 1990: 47 | ||
| 1. | Connatum (R. Keller) N. Robson | 27 | Robson, 1990: 51 | |||
| 2. | Knifa (Adans.) N. Robson | 25 | Robson, 1990: 95 | |||
Up to now, 457 species in 36 sections have been described in the monograph (Robson 1981 onwards). However, 9 species have been described additionally by several authors: H. dogonbadanicum Assadi (section Campylosporus, Iran), Iran. Journ. Bot. 2, 89 (1984); H. fosteri N. Robson (section Ascyreia, China), Acta Phytotax. Sin. 43, 271 (2005); H. wardianum N. Robson (section Ascyreia, China), Acta Phytotax. Sin. 43, 273 (2005); H. enshiense L.H. Wu & F.S. Wang (section Hypericum, China), Acta Phytotax. Sin. 42, 76 (2004); H. chejuense S.-J. Park & K.-J. Kim (section Hypericum subsection Erecta, Korea), Novon 15, 258 (2005); H. jeongjocksanense S.-J. Park & K.-J. Kim (section Hypericum subsection Erecta, Korea), Novon 15, 260 (2005); H. hubeiense L.H. Wu & D.P. Yang (section Elodeoida, China), Acta Phytotax. Sin. 42, 74 (2004); H. austroyunnanicum L.H. Wu & D.P. Yang (section Elodeoida, China), Acta Phytotax. Sin. 40, 77 (2002); H. haplophylloides Halácsy & Bald. (section “24a.” Haplophylloides N. Robson [in prep.: Hypericum monograph part 9], Albania), Verh. Zool.-Bot. Ges. Wien 42, 576 (1893). The following species were omitted from the monograph in error: Hypericum huber-morathii N. Robson (section Adenosepalum, Turkey), Notes Roy. Bot. Gard. Edinburgh 27, 197 (1967); H. minutum Davis & Poulter (section Adenosepalum, Turkey), Notes Roy. Bot. Gard. Edinburgh 21, 182 (1954); H formosissimum Takht. (section Adenosepalum, Turkey, Armenia, Iran), Not. Syst. Bot. Tiflis = Zametki po Sistematike i Geografii Rasteniĭ 9 (1940).
Sections 15 (Thasia) and 16 (Crossophyllum) have been recently merged (Robson 2010a, in press).
Of the currently recognized species of Hypericum, H. perforatum L. (Common St. John’s wort) is the best-known. This perennial herb is found in its native range distributed throughout Eurasia, but has been introduced to all other continents, except Antarctica (Robson 2002). Other species of Hypericum have been incorporated in traditional medicine systems in countries around the world, or are sold as ornamentals (Huxley et al. 1992; Moerman 1998). The flowering tops of H. perforatum are usually prepared as a decoction or infusion and taken internally for sedative or tonic purposes, or applied externally as a poultice or prepared as an oil-infusion to treat sciatica, neuralgia and speed wound-healing. Extracts of the inflorescences and upper stem leaves have been prescribed for many years in Europe, and are available as dietary supplements in the United States, to treat mild to moderate depression (Müller 2005). Hypericum perforatum was among the top ten best-selling herbal dietary supplements sold in the United States in 2008, with sales estimated at ca. 8.2 million USD (ABC 2008), and it represented nearly 13% of all European herbal product sales in 2004, valued at more than €70 million in Germany alone (Bäcker et al. 2006). The economic value of this plant to the herbal industry is one among several factors that have stimulated research into phytochemical diversity of H. perforatum in particular and of other members of the genus in general.
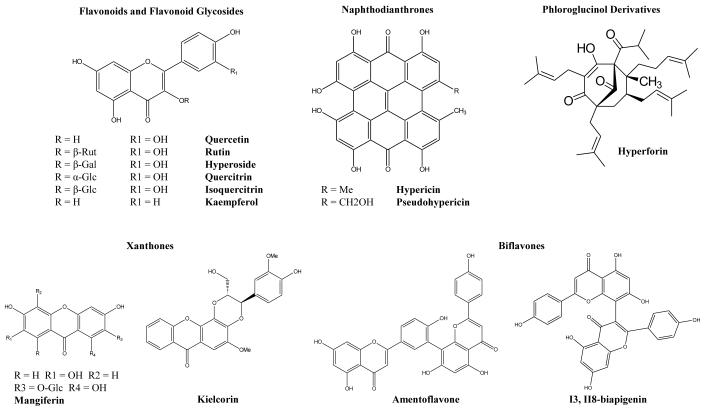
Several classes of bioactive secondary metabolites have been identified from extracts of H. perforatum, including naphthodianthrones (e.g. hypericin and pseudohypericin), flavonol glycosides (e.g. isoquercitrin and hyperoside), biflavonoids (e.g. amentoflavone), phloroglucinol derivatives (e.g. hyperforin and adhyperforin) and xanthones (see Fig. 1), and the distribution of these metabolites in related species of Hypericum is of considerable interest (Hölzl and Petersen 2003; Avato 2005). The primary focus of research has been on the anti-depressant properties of isolated substances from H. perforatum, although many compounds have been isolated from this and other species that additionally display interesting anti-inflammatory, anti-microbial and anti-proliferative activities (Avato 2005; Cuesta-Rubio and Picinnelli 2005; Dell’Aica et al. 2007). Numerous reviews of the botanical, phytochemical and pharmacological characteristics of H. perforatum have been published during the past 10 years (see particularly Nahrstedt and Butterweck 1997; Ernst and Izzo 2003; Müller 2005; Wurglics and Schubert-Zsilavecz 2006), and readers are directed toward these specific references (and citations therein) for more detailed information.
Fig. 1. Some bioactive secondary metabolites in Hypericum.
The taxonomy of the genus Hypericum, as currently treated, is largely based on morphology. A working hypothesis proposing relationships among species of Hypericum, formed on the basis of information from numerous original studies in morphology, distribution, floral vasculature and (to a limited extent) cytology, as well as from relevant literature, was initially proposed by Robson (1977) in the first part of his monograph of the genus. Earlier studies of the genus and nearest relatives by Choisy (1821, 1824) and Spach (1836a/b) provided a taxonomic foundation for subsequent studies that focused specifically on Hypericum (e.g. Keller 1893, 1925). A phylogenetic network among Hypericum species was constructed and elaborated by Robson (1977 onwards) through the analysis of morphological similarities for a broad range of characters across the genus, followed by a closer examination of differences within perceived groups (Table 2). In this way, both stable and variable characters were analyzed, and trends for variable characters were identified. Two important assumptions were made in the construction of this hypothesis: a) character trends identified for currently existing taxa reflect evolutionary trends within the genus and b) a direction for each trend was stated (see overview in Table 2). The phylogenetic network thus formed is a result of the correlation of trends rather than of individual characters, and displays some similarity to a Wagner tree (see Wagner 1969), except that the numerical basis of character assessment is lacking. Due to the extremely time-consuming nature of numerical analysis with a large number of species in the time prior to availability of high-speed computation, this methodology provided an invaluable way to elucidate cladistic relationships among taxa of Hypericum (see discussion in Robson, 1981, pp 65-73).
Table 2.
Character trends used for classification (according to Robson 1977)
| Character | Trend | |
|---|---|---|
| Habit | trees → shrubs → perennial herbs → annual herbs | |
| Indumentum | absent → present | |
| Glands | pale → dark | |
| pale channels → pale dots | ||
| dark dots → dark lines or streaks | ||
| fewer dark glands → more dark glands (concentration and number) | ||
| Stem | 4-lined → 2-lined → terete | |
| Leaves | sessile | → shortly petiolate |
| → amplexicaul → perfoliate | ||
| deciduous → persistent | ||
| opposite → 3-whorled → 4-whorled | ||
| parallel venation → reticulate venation | ||
| Perianth | 5-merous → 4-merous → 3-merous | |
| Sepals | persistent → deciduous | |
| unequal → nearly equal | ||
| free → united | ||
| margin entire → dentate → ciliate → fimbriate | ||
| Petals | persistent → deciduous → persistent | |
| asymmetric → symmetric | ||
| Stamen fascicles | persistent → deciduous → persistent | |
| 5 → 4 | ||
| free → variously united | ||
| Styles and placentae | 5 → 4 → 3 → 2 | |
| Placentation | loosely axile | → definitely axile |
| → parietal | ||
| Ovules per placenta | ∞ → 2 | |
| Seeds | narrowly winged → carinate → cylindrical | |
| Basic chromosome numbers | 12 | → 7 (6, dihaploids of 12?) |
| → 14 | ||
The hypothesis has evolved as additional parts of the Hypericum monograph have been published (Robson 1981 to present). Members of three sections of Hypericum (sections 1, 3 and 7, see Table 1) possess character states which are treated as basal, while those sections radiating outward from these three basal sections display character states considered more advanced (sections 2, 3 and 20-30 from section 1; sections 4-6 and 10-19 from section 3; and sections 8-9 from section 7, respectively). Readers are referred to the individual monograph parts for detailed discussions of relationships among members within and between sections as well as character trend analysis for morphological features.
A numerical cladistic analysis of the species of Hypericum has recently been performed using 89 morphological characters (originally described in Robson 1981 onwards) that were identified and considered to be phylogenetically informative (Nürk and Blattner submitted) and readers are referred to the original paper for further detailed discussions of sectional relationships proposed on the basis of this analysis. Currently, additional research on taxonomy of the genus Hypericum is being performed by several groups in Europe and North America, applying molecular tools to further elucidate phylogenetic relationships within the genus. A final analysis of all available evidence awaits the completion of these studies.
MORPHOLOGICAL CHARACTERS AND VARIATION
Habit
Within the genus, trees, shrubs and perennial and annual herbs occur. True trees, in the sense of having a single stem, are rare within the genus, most woody members having multiple stems arising near the base. However, certain members of section 1 (e.g. H. bequaertii) in Africa attain more than 10 m of height and can possess a single woody trunk. Shrubs and dwarf shrubs may have erect or spreading stems, but do not root from nodes that come in contact with the ground. Perennial herbs, however, display a marked tendency to root from horizontal nodes, particularly among species occurring in wet habitats (e.g. members of section 30 in bogs, marshes and moist páramo habitats), unlike annual herbs, which generally have tap roots and a highly developed system of secondary hair roots.
Indumentum
Many species of Hypericum are entirely glabrous, including those in sections 1-10 and 13-16. An exception is H. setiferum in section 13, which in contrast to other species belonging to this section has scattered appressed hairs on the underside of the leaf. Other species in sections 11-12, 17-18 and 27 have simple uniseriate hairs that can be described with such terms as scabrid to hirsute, depending on their length. Some species of section 27 as well as H. elodes in section 28 have long fine hairs (Robson 1981). Stellate hairs have been observed in particular members of the subfamily Vismioideae, but within subfamily Hypericoideae only simple hairs are found.
Glands
Two distinct types of glands have been identified in Hypericum, the so-called “dark” and “pale” glands. The first type is characterized by clusters of specialized cells with a black to reddish coloration indicative of their naphthodianthrone (i.e. hypericin and/or pseudohypericin, Fig. 1) content (Mathis and Ourisson 1963; Ciccarelli et al. 2001a). These glands have been observed in members of ca. 2/3 of the taxonomic sections and are often limited to particular organs (Robson 2003). The size and number of these glands correlates positively with the content of naphthodianthrones (Zobayed et al. 2006). When tissues containing these glands are crushed between the fingers, the released naphthodianthrones give a red stain, which imbued the plant with magical protective powers, according to folkloric tradition. The name St. John’s wort has to do with the belief that Hypericum (ύπέρεικov or hyper eikon – above the image) possessed the power to ward off evil spirits. In the early 16th century, Paracelsus described this red sap as “Johannes-blut,” suggesting that it symbolized the blood of the martyred St. John, thus contributing to the German and English common names used today (Robson 1977; Müller 2005).
The second type of gland (“pale” glands), clear to amber in color, is actually a schizogenous intercellular space lined by flattened cells that secrete essential oil components and phloroglucinol derivates, such as hyperforin (Fig. 1) (Ciccarelli et al. 2001a; Adam et al. 2002). These glands appear as light points or streaks when the leaves are held up to the sun. Numerous studies have examined the anatomy and chemical constituents of these individual gland types in particular species (Ciccarelli et al. 2001a, 2001b; Piovan et al. 2004; Soeberg et al. 2007). Also of interest is the overall distribution of the glands, particularly on leaves, sepals or petals, either intramarginal or laminar.
In the Hypericaceae, pale glands (varying in form, from streaks to dots) have been observed in all three subfamilies, while dark glands occur most prevalently in members of the Vismioideae and Hypericoideae, but are also present in Cratoxylum (Cratoxyloideae) (Robson 1974, 1981). The naphthodianthrone hypericin has only, thus far, been reported for flowering plants from species of Hypericum. Interestingly, Kusari et al. (2008) isolated an endophytic fungus from the stems of H. perforatum, cultures of which were shown to produce both hypericin and the supposed precursor, emodin, although this has not yet been proved to be the source of these compounds in the host tissues. Anthraquinones have frequently been isolated from Cratoxylum (Boonnak et al. 2006) and from members of Vismioideae (Bilia et al. 2000; Noungoue et al. 2008), while related simple quinones have been identified in other representatives of the Guttiferae sensu lato (i.e. Clusiaceae, Calophyllaceae) and Bonnetiaceae (Permana et al. 2003; Ee et al. 2004). Dark glands have been observed in Marila and Mammea (Calophyllaceae), and it has been speculated that these contain hypericin, but isolation studies have not yet confirmed this (Stevens 2007).
The distribution of such hypericin-containing glands is of particular interest due to the antifeedant activity of this molecule on generalist herbivores. Studies have shown that when generalist insects feed upon H. perforatum plants, a chemical defense mechanism is triggered that boosts the production of naphthodianthrones in the affected tissues by 30-100%, and generalist feeders are consequently repelled. In contrast, specialist insects such as the beetle Chrysolina quadrigemina that is used to control weedy populations of H. perforatum in the United States, can feed upon these tissues without difficulty, simply allowing the compound to pass through their digestive systems unchanged. In addition, the feeding activity of specialist insects upon the plant did not trigger a similar chemical defense cascade to that seen with generalist feeders (Duffey and Pasteels 1993; Sirvent et al. 2003). For this reason, such specialist insects are considered serious pests by those cultivating of H. perforatum. Such studies support the construction of the hypothesis that evolutionary selective pressures may have influenced the distribution and frequency of dark glands among species of Hypericum, and within a species, on particular organs. The trend toward an increase in dark secretory tissue in more advanced members of the genus was initially proposed by Robson (1977, 1981). According to the phylogenetic networks proposed by Robson (1981 onwards) and Nürk and Blattner (submitted), the ability to biosynthesize naphthodianthrones in these tissues seems to have arisen several times independently within Hypericum.
Stem
The presence of 4 thin ridges of tissue along the stems is closely associated with the opposite-decussate nature of the leaves in Hypericum. These lines of tissue may be minor, resulting in their being called “ridges” or more prominent, thus becoming “wings.” 2-lined, terete and occasionally 6-lined (in section 20) conditions occur throughout the genus. Internodes along the branches of most species with a tree and shrub habit generally become terete with age, although some evidence of stem lines can often be detected even in mature plants. The number of lines along the stem is considered an important field character in the distinction between H. perforatum (2-lined, most frequently tetraploid) and H. maculatum (4-lined, diploid), with which it is most likely to be confused. However, experimental crosses have shown that this is an incompletely dominant character (Noack 1939), and hybrids between these species (which occur in nature in regions where the distributions overlap, e.g. H. x desetangsii) shown reduced or incomplete lateral ridges, leading to problems with identification. Both pale and dark glands have been observed on the stems of various species of Hypericum, but species with eglandular stems are present in various parts of the genus. In section 9, such glands are confined to the stem lines, while in other sections such as 12 and 17 they may be dispersed over the surface (Robson 1981).
Leaves
Leaf arrangement in Hypericum is nearly always opposite and decussate, although whorls of 3-4 leaves occur exceptionally throughout the genus and in all species of section 19. The leaves lack stipules and may be either sessile or shortly petiolate (longer petioles are seen in species of sections 9 and 27). A basal articulation may be present (in which cases, the leaves are generally deciduous above or at the articulation) or absent (leaves generally persistent). Several species belonging to sections 1 and 29 have a reflexed leaf-base (auricle-like), while true auricles are observed only in sections 13 and 15/16. The laminar venation can span the full range from truly dichotomous to pinnate to densely reticulate. Leaf shape can vary from ovoid to elongate to linear (“ericoid”). Leaves are generally shorter than the internodes, but a tendency towards elongation of the latter can be observed in taxa considered as advanced. As previously noted, pale or dark glands are variously distributed within or at the leaf margin, or on the main laminar surface.
Sepals
Sepal number is 4-5, or rarely 3 in section 20, and individual sepals that are quincuncial when 5 and opposite, and decussate when 4, are either nearly equal (as in section 17) or unequal (as in sections 5, 7 and 14) in size and shape. Species with flowers tending toward tetramery are present in sections 9e (H. monanthemum subsp. filicaule) and 20 (e.g. H. hypericoides, H. microsepalum). Sepals may be united near the base (obvious in members of sections 17, 18 and 22), and free margins may display a variety of elaborations, having protruding marginal glands, (gland-dotted) teeth, or fine hairs. As previously mentioned, the distribution of dark (i.e. potentially hypericin-containing) glands has been considered taxonomically useful. Sepals of sections 2-5, 7, 20, 24-25 and 29-30 lack dark glands, while in sections 9-19, they are consistently present (see Fig. 2).
Fig. 2. Relationships among sections of Hypericum adapted from Robson (2003).
Sections denoted in blue lack naphthodianthrones; sections in pink produce naphthodianthrones in fewer than 2 organs; sections in red produce naphthodianthrones in 2 or more organs.
Petals
Hypericum petals are almost uniformly yellow, although there are gradations of this color from pale lemon to a deep buttery-orange. White to pinkish petals are seen in H. albiflorum var. albiflorum (section 12) and sometimes in H. geminiflorum (section 4), while some species have petals that may become suffused, streaked or otherwise tinged with red (particularly on the outer surface, visible when the flower is in bud). The petals of H. capitatum var. capitatum (section 17) are deep crimson. As with the sepals, petal lengths may be unequal or equal. The petals are ± asymmetrical in all species except those belonging to sections 25 and 28, and marginal elaborations (glands, fine teeth or cilia) occur. In members of sections 25 and 28, sterile bodies have developed between the stamen fascicles (fasciclodes) that act as lodicules by helping to spread the petals of the pseudo-tubular flower. These species herefore display a specialized insect pollination syndrome.
Glands on petals are present in nearly all species, at least at the lamina. Laminar glands are absent in sections 25 and 30, and only section 25 possesses entirely eglandular petals. Marginal glands are characteristic for sections 10-17 and occur in some species of sections 9, 18 and 27. In sections 17-19, marginal glands frequently occur on cilia, and in sections 10-16, they are generally sessile. It has been proposed that the hypericin content of the glands may be inferred from the intensity of their red color, but it is important to consider that other pigments (such as bisanthranone compounds known as skyrin derivatives) can denote a red color (see Wirz et al. 2000). For example, both H. xylosteifolium (section 6) and sometimes H. bupleuroides (section 8) have dark (red-colored) glands on the petal margin, although the presence of hypericin and pseudohypericin has not yet been confirmed in these species.
Stamen fascicles and stamens
The stamens, which can range in number from 5 to more than 200, are found in bundles termed fascicles. Hypericum flowers have 4-5 stamen fascicles, which may be free from one another (as in sections 1, 3-7) or fused in a variety of combinations (mostly in a way described as 2+2+1 resulting in 3 apparent fascicles). In sections 20, 29 and 30 (in part), the stamens form a continuous ring. Stamens are typically persistent but sometimes deciduous, and possess an anther gland on the connective tissue that varies in color from amber to black. The latter type of gland color (i.e. potentially hypericin-containing) occurs most frequently in sections 12-15, and occasionally in sections 23, 26 and 27. Ten types of pollen have been recognized by Clarke (in Robson 1981). It is predominantly of type X (sections 9-11, 13-19), although type IV is also common (sections 2-3, 5, 8-9, 12, 22-23). Type I pollen is found only in section 1; type VII in 20; type V in 25; type IX in 28 and type VIII in 29-30.
Styles and placentae
The ovary in Hypericum is (2-)3-5-merous, with a corresponding number of styles (which may be variously free or sometimes united). Fusion of the styles is partial in sections 1, 3 and 7 (all in part), complete in sections 1 (in part) and 4 and styles are free in sections 5 and 26. The developing seeds are borne on axile (entirely in sections 17-19) or parietal (in sections 28-30) placentae (number of ovules per placenta is ∞-2, depending on the species) and some sections (i.e. 20 and 26) show a transition between these two states.
Fruit
Hypericum fruits, unlike those of some other members of Hypericaceae, are capsular and dehisce from the apex. When mature, the capsule may be dry or remain (as in some species of sections 3, 5 and 26) fleshy, and have particular elongate or punctate glands on the outer surface in a wide variety of shapes and elaborations (termed vittae when narrow and linear and vesicles when short and globose). These are generally pale amber in color, although reddish-black vesicles have been observed for some species of section 13, but the contents of these vesicles have only rarely been studied. Extractions of the outer surfaces of the fruits of particular species resulted in the isolation of phloroglucinol and other terpenoid derivatives, which may indicate a biosynthetic congruence between these glands and the pale glands of the vegetative tissue (Gronquist et al. 2001; Crockett et al. 2008).
Seeds
Seeds of Hypericum are small (0.3-1.5 mm long), yellowish-brown to dark purple-brown, cylindric to ellipsoid, and may be narrowly winged. In some cases, a basal thickening or ridge may be observed, or rarely an apical caruncle (in section 25), which acts as an ant attractant to improve seed dispersal. Sculpturing of the seed testa varies from reticulate via foveolate to scalariform or papillose, and linear-reticulate testa sculpturing appears to be the plesiomorphic character state for Hypericum. However, evolution towards scalariform or papillose testa sculpturing appears to be homoplastic within the genus.
Some species of Hypericum seem to require highly specific conditions for germination and survival of the seedling past the 6-leaf stage. For example, H. lloydii (section 20) is native to habitats of degraded granite and, as a seedling, is particularly susceptible to fungal infection when conditions are too moist, while other species germinate and, at least during early stages, grow under water (e.g. H. lissophloeus and H. chapmanii, section 20) (Crockett, pers. obs.). For most species of Hypericum, germination requirements are poorly known, and this would be an interesting subject for future study.
Basic chromosome number
Summaries of chromosome numbers in Hypericum appear in Robson and Adams (1968) and Robson (1981). Basic numbers (n) in Hypericum are proposed to form a descending series from 12 – 7 and counts of n = 6 have been made for H. setosum, H. cumulicola (apparently dihaploids) and H. gentianoides (if the last is tetraploid), all in section 30. Counts of n = 9 and 10 are most frequently reported for species with a shrubby habit, while n = 7 and 8 is most frequent for herbs (Robson 1981; da Cruz et al. 1990). The ploidy level is generally diploid, but tetraploids (on base numbers n = 8, 9, 10) have been reported from several sections and hexaploids have been reported from sections 3 (most frequently) and 9.
PHYTOCHEMICAL CHARACTERS AND VARIATION
A diverse array of secondary metabolites is encountered in nature, each produced through a series of metabolic actions within the cell. In higher plants, specific products of primary metabolism are fed into the acetate, shikimate, mevalonate and deoxyxylulose phosphate pathways leading to the production of secondary compounds. Products of the acetate pathway, starting with the intermediate building block of acetyl-CoA, include fatty acids and aromatic polyketides (including simple phenols and anthraquinones). The shikimate pathway, fed by primary metabolites from glycolysis and the pentose phosphate pathway, leads to aromatic amino acids (often further involved in the biosynthesis of alkaloids), benzoic and cinnamic acids, lignans, phenylpropanes and coumarins. Combinations of the two aforementioned pathways result in the production of flavonoids, stilbenes, flavonolignans and isoflavonids. Both the mevalonate pathway, based upon the acetyl-CoA building block, and the deoxyxylulose phosphate pathway, fed by two intermediates from glycolysis, are responsible for the biosynthesis of terpenoids and steroids (Dewick 2002).
Considerations of bioactivity
Secondary metabolites with demonstrated bioactivity can serve as biomarkers (i.e. compounds of pharmaceutical interest, considered specific to a particular taxon), and this type of marker identification and tracking is of particular use at the level of species, population and individual (Crockett and Khan 2003). Much research been conducted on the presence and amount of biomarkers in H. perforatum, and a complex of naphthodianthrones (hypericin and pseudohypericin), acylphloroglucinol derivatives (hyperforin), biflavones (I3, II8-biapigenin, amentoflavone) and flavonoid glycosides (rutin, hyperoside, isoquercitrin, quercitrin, quercetin) is generally considered characteristic for this species (Nahrstedt and Butterweck 1997; Hölzl and Petersen 2003 and citations therein). Additional studies have examined the effects of morphological (i.e. organ dependant) and diurnal variability on the qualitative and quantitative aspects of biomarker production (Ayan et al. 2006; Kaçar et al. 2008). These compounds, as a combined set, have been used to ascertain plant identity, and the presence of rutin has been cited as particularly important for chemical authentication of plant samples as true H. perforatum. Certain populations of plants in Italy and Austria have been recognized, however, which are morphologically identified as H. perforatum, but lack rutin (Umek et al. 1999; Mártonfi et al. 2001). Additional studies have shown that although many other species of Hypericum contain naphthodianthrones, acylphloroglucinol derivatives and flavonoids, few contain the specific complex of 9-10 biomarker compounds that have been considered typical for H. perforatum (Crockett et al. 2005; Smecerovic et al. 2008; Verma et al. 2008).
Due to the extremely large number of compounds from different chemical classes that have been reported from species of Hypericum, this article makes no attempt to review the phytochemical literature exhaustively. Readers are referred to reviews by Avato (2005), Nahrstedt and Butterweck (1997), Hölzl and Petersen (2003) and Kitanov and Blinova (1987). Instead, Table 3 provides a general overview of the distribution of particular biomarker compounds (i.e. those with perceived relevance to the pharmaceutical industry) by section within the genus, with reference to selected examples of extant phytochemical literature. During the literature review, isolated reports of naphthodianthrones in species of sections 2 and 21 were found (Salgues 1961; Kartnig et al. 1996), however these reports must be treated with care due to the lack of morphological support (i.e. lack of dark glands in these sections) and absence of confirmation from a second source. Interestingly, three taxonomic sections other than section Hypericum (9) that produce 9-10 of the compounds used as biomarkers for H. perforatum have been identified: sections 13 (10), 18 (9) and 27 (10). Due to the morphological distinctness and limited geographic distribution (in their native ranges) of most species belonging to these sections as opposed to H. perforatum, however, the likelihood of adulteration or misidentification is predicted to be quite low.
Table 3.
Distribution of Biomarker Compounds Hypericum
| Section | quercetin | rutin | hyperoside | quercitrin | isoquercitrin | amentoflavone | I3, II8-biapigenin | hypericin | pseudohypericin | hyperforin | Selected References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Campylosporus | X | X | X | X | Cardona and Seone 1983; Kitanov 2001 | ||||||
| 2 | Psorophytum | X | X | X | X | X | Alberto et al. 1981; Mathis and Ourisson 1963 | |||||
| 3 | Ascyreia | X | X | X | X | X | Doğanca and Öksüz 1993; Seabra and Alves 1990 | |||||
| 4 | Takasagoya | X | X | Chen et al. 1989 | ||||||||
| 5 | Androsaemum | X | X | X | X | X | X | Crockett 2005, Bonkanka 2008 | ||||
| 6 | Inodora | X | X | X | X | X | Makovetska 1999a; Zapesochnaya et al. 1967 | |||||
| 7 | Roscyna | X | X | X | X | X | Komissarenko et al. 1992; Park et al. 2000 | |||||
| 8 | Bupleuroides | X | X | X | X | X | X | Makovetska 1999a, Kitanov 2001; Ayan et al. 2009 | ||||
| 9 | Hypericum | X | X | X | X | X | X | X | X | X | X | Makovetska 1999b, Kitanov 2001; Butterweck 2007 |
| 9a | Concinna | X | Mathis and Ourisson 1963 | |||||||||
| 9b | Graveolentia | X | X | X | X | X | X | X | X | Makovetska 2000a; Mathis and Ourisson 1963; Crockett 2005 | ||
| 9c | Sampsonia | X | Mathis and Ourisson 1963 | |||||||||
| 9d | Elodeoida | X | Mathis and Ourisson 1963 | |||||||||
| 10 | Olympia | X | X | X | X | X | X | Akhtardzhiev et al. 1973; Kitanov 1987; Mathis and Ourisson 1963 | ||||
| 11 | Campylopus | X | X | Makovetska 1999a; Crockett 2005 | ||||||||
| 12 | Organifolia | X | X | X | Mathis and Ourisson 1963; Çirak et al. 2008 | |||||||
| 13 | Drosocarpium | X | X | X | X | X | X | X | X | X | X | Crockett 2005; Çirak and Radušienė 2007 |
| 14 | Oligostema | X | X | X | X | X | X | X | X | Kitanov et al. 1979; Kitanov 1988a; Seabra and Alves 1990; Mathis and Ourisson 1963 | ||
| 15 | Thasia | X | X | Mathis and Ourisson 1963 | ||||||||
| 16 | Crossophyllum | X | X | X | X | X | X | X | X | Doğanca and Öksüz 1989; Crockett 2005, Çirak et al. 2009 | ||
| 17 | Hirtella | X | X | X | X | X | X |
Mathis and Ourisson 1963; Zaichikova and Barbanov 1980; Makovetska 2000b; Ayan et al. 2009 |
||||
| 18 | Taeniocarpium | X | X | X | X | X | X | X | X | X | Kitanov 1988b; Mathis and Ourisson 1963; Shatunova 1978 | |
| 19 | Coridium | X | X | X | X | X | X | Mathis and Ourisson 1963; Crockett 2005; Alali et al. 2009 | ||||
| 20 | Myriandra | X | X | X | X | X | X | X | Alyukina 1970; Crockett 2005 | |||
| 21 | Webbia | X | X | X | X | Cardona et al. 1989; Makovetska 2001a; Mathis and Ourisson 1963 | ||||||
| 22 | Arthrophyllum | X | X | X | Makovetska 2001a; Crockett 2005 | |||||||
| 23 | Triadenioides | X | X | X | X | X | Makovetska 2001a; Mathis and Ourisson 1963 | |||||
| 24 | Heterophylla | X | X | X | X | X | Makovetska 2001a | |||||
| 25 | Adenotrias | X | Makovetska 2001a; Crockett 2005 | |||||||||
| 26 | Humifusoideum | X | X | X | X | X | X | Makovetska 2001a; Mathis and Ourisson 1963 | ||||
| 27 | Adenosepalum | X | X | X | X | X | X | X | X | X | X | Umek et al. 1999; Crockett 2005, Alali et al. 2009 |
| 28 | Elodes | X | X | X | X | X | X | X | Seabra and Alves 1990; Mathis and Ourisson 1963 | |||
| 29 | Brathys | X | X | X | X | X | Makovetska 2001b | |||||
| 30 | Trigynobrathys | X | X | X | X | X | Makovetska 2001b; Rocha et al. 1995 |
Considerations of chemotaxonomy
The presence or absence of compounds belonging to a specific class of secondary metabolites, either on the level of plant family, genus, species, within a species (i.e. population analysis), or even within a single plant (i.e. metabolic characterization) is of particular interest in the field of pharmacognosy. These compounds may serve as chemotaxonomic markers at higher taxonomic levels (i.e. family to species), indicating that particular biosynthetic pathways have been conserved within a taxon, or alternatively, have arisen two or more times within a taxon through evolutionary convergence.
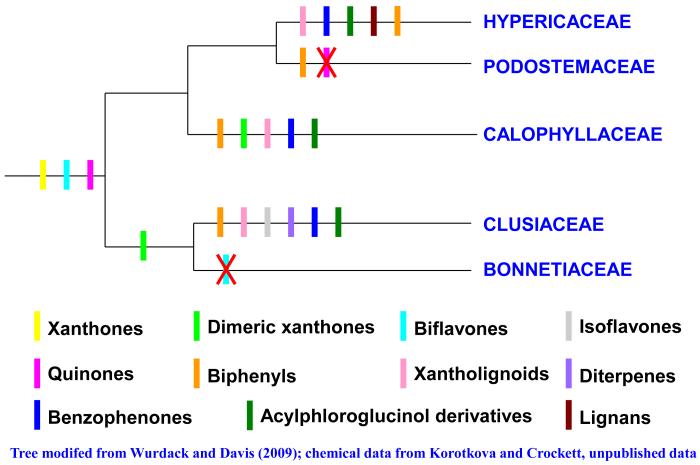
The clade supported by molecular data (Wurdack and Davis 2009) as containing Guttiferae s.l. (Hypericaceae, Clusiaceae s.s. and Calophyllaceae), Bonnetiaceae and Podostemaceae is phytochemically unique within the Malpighiales due to the shared possession of xanthones, compounds related to flavonoids with elements derived from both acetate and shikimate pathways, by its members (Kubitzki et al. 1978; Bennett and Lee 1989; Burkhardt et al. 1992) (see Fig. 1). Excellent reviews of the occurrence of xanthones among members of the Guttiferae s.l. (including Hypericaceae) and within Hypericum may be found in Bennett and Lee (1989) and Demirkiran (2007), respectively. Biflavones and quinones have also been reported from all families of this clade, but are not restricted to this clade within Malpighiales (Korotkova and Crockett, unpublished data). Dimeric xanthones have been isolated from both Bonnetiaceae and Clusiaceae sensu stricto (Bennett et al. 1990; Sordat-Diserens et al. 1992) as well as from the nearest sister taxon, Calophyllaceae (Cortez et al. 1998). Further phytochemical studies of species from Bonnetiaceae are needed to provide additional chemotaxonomic support for the link with Clusiaceae s.s. The latter taxon shares the possession of xantholignoids, acylphloroglucinol derivatives, benzophenones and biphenyls with both Calophyllaceae and the more derived Hypericaceae (Pinto and Sousa 2003; Baggett et al. 2005). Biphenyls have also been isolated from Podostemaceae, but as with Bonnetiaceae, too few phytochemical studies have been published to speculate further on shared chemical characters between this family and Hypericaceae (Cardona et al. 1990; Burkhardt et al. 1992; Cortez et al. 1998; Seo et al. 1999). An overview of the distribution of these compound classes in this clade is shown in Fig. 3.
Fig. 3. Distribution of relevant compound classes in Guttiferae s.l., Podostemaceae and Bonnetiaceae.
A colored bar at the base of a clade indicates the presence of this class of compounds in all families in the clade; a colored bar along the tree branch indicates the presence of this class of compounds in that specific family; a red “X” indicates the absence of the indicated class of compounds in the specified family. Tree modifed from Wurdack and Davis (2009); chemical data from Korotkova and Crockett, unpublished data.
Of the 9 genera of Hypericaceae, four (Eliea, Harungana, Lianthus and Santomasia) are monotypic, Thornea has only 2 species and Triadenum, fewer than 10. It is perhaps not surprising, therefore, that the phytochemistry of these plants is poorly known. Harungana madagascariensis is an exception due to its use as a traditional medicinal plant against bacterial, viral and fungal infections, and has been the subject of several chemical investigations that have revealed the presence of triterpenes, prenylated anthrones, anthraquinones, flavonoids, xanthones and benzophenones (Iinuma et al. 1995, 1996; Kouam et al. 2005). Compounds belonging to these classes of secondary metabolites have also been isolated from species of Cratoxylum and Vismia, emphasizing the evolutionary ties and shared biosynthetic capabilities of these taxa (Bennett et al. 1993; Seo et al. 2002; Cuesta-Rubio et al. 2005; Pattanaprateeb et al. 2005; Boonnak et al. 2009; Noungoue et al. 2009). Xanthones have more frequently isolated from Cratoxylum, and anthraquinones and benzophenones, from Vismia. Both of these genera, however, display an interesting tendency to biosynthesize dimeric and/or conjugated secondary metabolites such as anthraquinobenzophenones (Seo et al. 2002), bisxanthones (Laphookhieo et al. 2006), and xanthonolignoids (Delle Monarche et al. 1993; Iinuma et al. 1996), which have not yet been isolated from Harungana.
Of all genera in Hypericaceae, Hypericum has been the subject of the highest number of studies but, although phytochemical investigations have been conducted on at least one or more representatives belonging to 34 of the 36 taxonomic sections, the secondary chemistry of an estimated 60% of the species is still largely unknown. Representatives of all secondary metabolite classes that have been identified from members of Cratoxyloideae and Vismioideae also occur in Hypericum, making subfamilial chemotaxonomic distinctions challenging. It is, however, relevant to note that the highly prenylated anthrones, anthraquinones and xanthones frequently found in Vismia and Cratoxylum (Bilia et al. 2000; Boonnak et al. 2006) are uncommon in Hypericum, although certain specialized bianthrones such as hypericin have been isolated exclusively from the latter. Simple benzophenone derivatives, in some cases glycosylated or oxidized, have been isolated from some species of Cratoxylum (Seo et al. 2002; Yu et al. 2009), but compounds with elaborate prenylation patterns upon phloroglucinol base structures (acylphloroglucinols or prenylated benzophenones) have been more frequently isolated from Hypericum (Winkelmann et al. 2001; Baggett et al. 2005; Hashida et al. 2008) as compared to other members of Hypericaceae.
Although numerous benzophenones and acylphloroglucinols that vary considerably according to their acylation, prenylation, methylation, oxidation and cyclization patterns have been isolated from genera within Guttiferae s.l., representatives of less than a third of the taxonomic sections of Hypericum have been surveyed for these compounds. Thus, the examination of structural diversity for these compounds across the genus has not been thoroughly investigated enough to draw any firm conclusions regarding their utility as chemotaxonomic markers. However, it is encouraging to note that such compounds as uliginosin B and japonicin A have been each independently isolated from two species of section 30 (uliginosin B: Ferraz 2002; Taylor and Brooker 1969; japonicin A: Gu et al. 1984; Rocha et al. 1995), and that chinensin II has been isolated from two species of section 3 (Decosterd et al. 1991; Nagai and Tada 1987), indicating that these compounds may have some chemotaxonomic utility at the sectional or subsectional level.
Researchers have suggested that the oxygenation and prenylation patterns of xanthones have potential chemotaxonomic value due to their variability (Bennett and Lee 1989; Demirkiran 2007). More than 100 xanthones have been isolated and identified from Hypericum, many of which differ according to patterns of hydroxyl, methoxy, prenyl, butenyl and glycoside substitutions on the base structure. Two of the most common xanthones isolated from Hypericum (mangiferin and isomangiferin), however, belong to the group of 1,3,6,7-tetrahydroxyxanthones. A study by Kitanov and Blinova (1987) found xanthones with this specific pattern of oxygenation in Hypericum species representing 17 taxonomic sections of the genus, and they have been isolated from the most basal member of the family, Cratoxylum (Kitanov et al. 1988), indicating that they represent a conserved chemical character. The distribution of xanthones with a 1,3,5,6-tetrahydroxylation pattern seems to be more limited within Hypericum (sections 1, 3, 5 and 30), although they have been reported additionally from two species of Cratoxylum (Sia et al. 1995; Boonnak et al. 2006). Xanthonolignoids have been to date reported from only a few species of Hypericum belonging to sections 3, 21 and 27. Although many papers detailing the isolation of xanthones from Hypericum in recent years have appeared, many species remain to be investigated. The chemotaxonomic utility of these compounds, therefore, can not be properly assessed until their distributions and chemical characteristics among more members of the genus have been evaluated.
CONCLUDING REMARKS
Despite preliminary findings that indicate that certain classes of secondary metabolites might have chemotaxonomic utility at lower taxonomic levels, a cautionary note must be added. Most phytochemical investigations of these species were conducted using material collected from their native habitats, although a small subset of species were either collected from cultivation in a botanical garden or micropropagated. Results of such studies are, for various reasons, notoriously difficult to repeat and verify. Advantages to using cultivated material include the facts that native populations are not damaged by collection of the large amounts of material generally needed for phytochemical investigation; environmental conditions can be recorded and, in some cases, controlled; studies can be planned so that an adequate amount of material exists for the isolation of minor components; and seasonal and/or temporal changes in chemical composition can be more easily tracked. Many herbacous Hypericum species can be grown from seed (see Faron et al. 2004), while woody species are readily grown from cuttings (Crockett unpublished data), and researchers who plan to perform intensive phyotchemical investigations of particular Hypericum species – particularly endemic taxa – may obtain better results using cultivated material, rather than relying upon material collected from wild populations.
For those researchers who wish to continue working with material collected from the wild, a careful consideration of the multitude of available analytical tools, many of which allow the detection and identification of secondary metabolites starting with extremely small amounts of plant material, is valuable. Metabolic characterization studies of various parts of a single plant and for single cells within a particular tissue type have recently been conducted, primarily with H. perforatum. In these studies, the significant effects of ontogenetic, diurnal and seasonal variation on the production of secondary metabolites, particularly the naphthodianthrones, has been described (Southwell and Burke 2001; Seidler-Łożykovska 2003; Ayan et al. 2006; Coucerio et al. 2006). Hyperforin and hypericin accumulation has been measured through microcapillary sampling of single glands in H. perforatum (Soelberg et al. 2007). A recent study combining the tools of laser microdissection with laser desorption/ionization mass spectrometry allowed the single-cell localization and identification of naphthodianthrones and biflavones in this species (Hölscher et al. 2009). The increased sensitivity of detection of secondary metabolites provided by the use of these techniques, as well as the broader availability of such instruments, will allow much more detailed and efficient studies of the phytochemistry of Hypericum species in the future.
ACKNOWLEDGMENT
The contribution of Nicolai Nürk to Table 1 is gratefully acknowledged.
REFERENCES
- Adam P, Arigoni D, Bacher A, Eisenreich W. Biosynthesis of hyperforin in Hypericum perforatum. Journal of Medicinal Chemistry. 2002;45:4786–4793. doi: 10.1021/jm0209782. [DOI] [PubMed] [Google Scholar]
- Akhtardzhiev K, Nakov N, Tsendov I. Polyphenol compounds in Hypericum species growing in Bulgaria. II. Flavonoids in Hypericum olympicum. Farmatsiya (Sofia) 1973;23:37–40. [Google Scholar]
- Alali FQ, Tawaha K, Gharaibeh M. LC-MS and LC-PDA analysis of Hypericum empetrifolium and Hypericum sinaicum. Zeitschrift für Naturforschung C. 2009;64:476–482. doi: 10.1515/znc-2009-7-802. [DOI] [PubMed] [Google Scholar]
- Alberto MJ, Villar E, Seoane E. Xanthones and quercetin from Hypericum balearicum L. Anales de Quimica Serie C - Quimica Organica y Bioquimica. 1981;77:355–356. [Google Scholar]
- Alyukina LS. Content of flavonoids in some types of St. John’s Worts. Trudy Botanicheskogo Instituta Akademii Nauk SSSR. 1970;28:161–169. [Google Scholar]
- American Botanical Council (ABC) Herbal supplement sales experience slight increase in 2008. HerbalGram. 2008;82:58–61. [Google Scholar]
- Avato P. A survey of the Hypericum genus: secondary metabolites and bioactivity. Studies in Natural Product Chemistry. 2005;30:603–634. [Google Scholar]
- Ayan AK, Çirak C, Yanar O. Variations in total phenolics during ontogenetic, morphogenetic and diurnal cycles in Hypericum species from Turkey. Journal of Plant Biology. 2006;49:432–439. [Google Scholar]
- Ayan AK, Radušienė J, Çirak C, Ivanauskas L, Janulis V. Secondary metabolites of Hypericum scabrum and Hypericum bupleuroides. Pharmaceutical Biology. 2009;47:847–853. [Google Scholar]
- Bäcker W, Bart H-J, Bischoff F, Grabley S, Goedecke R, Johannisbauer W, Jordan V, Stockfleth R, Strube J, Wiesmet V. Phytoextrakte - Produkte und Prozesse. 2006 Available online: http://www.dechema.de/dechema_media/Downloads/Extraktion/Phytoextrakte.pdf.
- Baggett S, Mazzola EP, Kennelly EJ. The benzophenones: isolation, structural elucidation and biological activities. Studies in Natural Product Chemistry. 2005;32:721–771. [Google Scholar]
- Bennett GJ, Lee H-H. Xanthones from Guttiferae. Phytochemistry. 1989;28:967–998. [Google Scholar]
- Bennett GJ, Lee H-H, Lowrey TK. Novel metabolites from Ploiarium alternifolium: a bixanthone and two anthraquinonylxanthones. Tetrahedron Letters. 1990;31:751–754. [Google Scholar]
- Bennett GJ, Harrison LJ, Sia GL, Sim KY. Triterpenoids, tocotrienols and xanthones from the bark of Cratoxylum cochinchinense. Phytochemistry. 1993;32:1245–1251. [Google Scholar]
- Bilia AR, Yusuf AW, Braca A, Keita A, Morelli I. New prenylated anthraquinones and xanthones from Vismia guineensis. Journal of Natural Products. 2000;63:16–21. doi: 10.1021/np990226j. [DOI] [PubMed] [Google Scholar]
- Bonkanka CX, Smelcerovic A, Zuehlke S, Robanal RM, Spiteller M, Sánchez-Mateo C. HPLC-MS analysis and anti-oedematogenic activity of Hypericum grandifolium Choisy (Hypericaceae) Planta Medica. 2008;74:719–725. doi: 10.1055/s-2008-1074526. [DOI] [PubMed] [Google Scholar]
- Boonnak N, Karalai C, Chantrapromma S, Ponglimanont C, Fun H-K, Kanjana-Opas A, Laphookhieo S. Bioactive prenylated xanthones and anthraquinones from Cratoxylum formosum ssp. pruniflorum. Tetrahedron. 2006;62:8850–8859. [Google Scholar]
- Boonnak N, Karalai C, Chantrapromma S, Ponglimanont C, Fun H-K, Kanjana-Opas A, Chantrapromma K, Kato S. Anti-Pseudomonas aeruginosa xanthones from the resin and green fruits of Cratoxylum cochinchinense. Tetrahedron. 2009;65:3003–3013. [Google Scholar]
- Burkhardt G, Schild W, Becker H, Grubert M. Biphenyls and xanthones in the Podostemaceae. Phytochemistry. 1992;31:543–548. [Google Scholar]
- Cardona ML, Seoane E. Flavonoids and xanthones of Hypericum ericoides L. Anales de Quimica Serie C - Quimica Organica y Bioquimica. 1983;79:144–148. [Google Scholar]
- Cardona ML, Fernández I, Pedro JR, Serrano A. A new pyranoxanthone and flavonoids from Hypericum canariensis. Heterocycles. 1989;29:2297–2300. [Google Scholar]
- Cardona ML, Fernández I, Pedro JR, Serrano A. Xanthones from Hypericum reflexum. Phytochemistry. 1990;29:3003–3006. [Google Scholar]
- Chen MT, Kuoh YP, Wang CH, Chen CM, Kuoh CS. Additional constituents of Hypericum subalatum. Journal of the Chinese Chemical Society -- Taipei. 1989;36:165–168. [Google Scholar]
- Choisy JD. Prodromus d’une monographie de la familie des Hypéricinées. Geneva and Paris: 1821. [Google Scholar]
- Choisy JD. Hypericineae. In: De Candolle AP, editor. Prodromus Systematis Naturalis Regni Vegetabilis. Vol. 1. Paris: 1824. pp. 541–556. [Google Scholar]
- Ciccarelli D, Andreucci AC, Pagni AM. The “black nodules” of Hypericum perforatum L. subsp. perforatum: morphological, anatomical, and histochemical studies during the course of ontogenesis. Israel Journal of Plant Sciences. 2001a;49:33–40. [Google Scholar]
- Ciccarelli D, Andreucci AC, Pagni AM. Translucent glands and secretory canals in Hypericum perforatum L. (Hyperiacaceae): morphological, anatomical and histochemical studies during the course of ontogenesis. Annals of Botany. 2001b;88:637–644. [Google Scholar]
- Çirak C, Radušienė J. Variation of hyperforin in Hypericum montbretii during its phonological cycle. Natural Product Research. 2007;21:1151–1156. doi: 10.1080/14786410701589758. [DOI] [PubMed] [Google Scholar]
- Çirak C, Radušienė J, Çamas N. Pseudohypericin and hyperforin in two Turkish Hypericum species: Variation among plant parts and phenological stages. Biochemical Systematics and Ecology. 2008;36:377–382. [Google Scholar]
- Çirak C, Ivanauskas L, Janulis V, Radušienė J. Chemical constituents of Hypericum adenotrichum Apach, an endemic Turkish species. Natural Product Research, Part A: Structure and Synthesis. 2009;23:1189–1195. doi: 10.1080/14786410802393209. [DOI] [PubMed] [Google Scholar]
- Cortez DAG, Young MCM, Marston A, Wolfender JL, Hostettmann K. Xanthones, triterpenes and a biphenyl from Kielmeyera coriacea. Phytochemistry. 1998;47:1367–1374. [Google Scholar]
- Coucerio MA, Afreen F, Zobayed SMA, Kozai T. Variation in concentrations of major bioactive compounds of St. John’s wort: effects of harvesting time, temperature and germplasm. Plant Science. 2006;170:128–134. [Google Scholar]
- Crockett SL, Khan IA. Challenges to standardization: marker compounds in plant species related and unrelated to top-selling herbs. Journal of Herbs, Spices and Medicinal Plants. 2003;10:13–24. [Google Scholar]
- Crockett SL, Schaneberg B, Khan IA. Phytochemical profiling of new and old world Hypericum species. Phytochemical Analysis. 2005;16:479–485. doi: 10.1002/pca.875. [DOI] [PubMed] [Google Scholar]
- Crockett SL, Wenzig E-V, Kunert O, Bauer R. Antiinflammatory phloroglucinol derivatives from Hypericum empetrifolium. Phytochemistry Letters. 2008;1:37–43. doi: 10.1016/j.phytol.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta-Rubio O, Piccinelli AL, Rastrelli L. Chemistry and biological activity of polyisoprenylated benzophenone derivatives. Studies in Natural Product Chemistry. 2005;32:671–720. [Google Scholar]
- da Cruz ND, Boaventura YMS, Sellito YM. Cytological studies of some species of the genus Clusia L. (Guttiferae) Revista Brasileria de Genetica. 1990;13:335–345. [Google Scholar]
- Decosterd LA, Hoffmann E, Kyburz R, Bray D, Hostettmann K. A new phloroglucinol derivative from Hypericum calycinum with antifungal and in vitro antimalarial activity. Planta Medica. 1991;57:548–551. doi: 10.1055/s-2006-960203. [DOI] [PubMed] [Google Scholar]
- Dell’Aica I, Garbisa S, Caniato R. The renaissance of Hypericum perforatum: bio- medical research catches up with folk medicine. Current Bioactive Compounds. 2007;3:109–119. [Google Scholar]
- Delle Monarche F, Mac-Quhae MM, Delle Monarche G, Bettolo GBM, de Lima RA. Chemistry of the Vismia genus. Part 9. Xanthones, xanthonolignoids, and other constituents of the roots of V. guaramirangae. Phytochemistry. 1983;22:227–232. [Google Scholar]
- Demirkiran O. Xanthones in Hypericum: synthesis and biological activities. Topics in Heterocyclic Chemistry. 2007;9:139–178. [Google Scholar]
- Dewick PM. Medicinal Natural Products. A Biosynthetic Approach. Wiley and Sons; New York, USA: 2002. total. [Google Scholar]
- Doğanca S, Öksüz S. Constituents of Hypericum adenotrichum. Fitoterapia. 1989;60:93. [Google Scholar]
- Doğanca S, Tüzün S. Flavonoids of Hypericum calycinum. Fitoterapia. 1993;64:92. [Google Scholar]
- Duffey SS, Pastells JM. Transient uptake of hypericin by chrysomelids is regulated by feeding behavior. Physiological Entomology. 1993;18:119–129. [Google Scholar]
- Ee GCL, Ng KN. Larvicidal anthraquinones and triterpenes from Ploiarium alternifolium (Theaceae) Asian Journal of Chemistry. 2004;16:429–433. [Google Scholar]
- Ernst E, Izzo AA. The clinical pharmacology of Hypericum perforatum. In: Ernst E, editor. Hypericum: The Genus Hypericum. Taylor and Francis; New York, USA: 2003. pp. 155–172. [Google Scholar]
- Faron MLB, Perecin MB, Do Lago AA, Bovi OA, Maia NB. Light, temperature and potassium nitrate in the germination of Hypericum perforatum L and H. brasiliense Choisy seeds. Bragantia. 2004;63:193–199. [Google Scholar]
- Ferraz ABF. Uliginosin B from Hypericum myrianthum. Biochemical Systematics and Ecology. 2002;30:989–991. [Google Scholar]
- Gronquist M, Bezzerides A, Attygale A, Meinwald J, Eisner M, Eisner T. Attractive and defensive functions of the ultraviolet pigments of a flower (Hypericum calycinum) Proceedings of the National Academy of Sciences USA. 2001;98:13745–13750. doi: 10.1073/pnas.231471698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Feng S, Wang X. Studies on the active principles of Di er Cao - the isolation and structure of japonicine A. Kexue Tongbao (Foreign Language Edition) 1984;29:548–549. [Google Scholar]
- Hashida W, Tanaka N, Kashiwada Y, Sekiya M, Ikeshiro Y, Takaishi Y. Tomoeones A-H, cytotoxic phloroglucinol derivatives from Hypericum ascyron. Phytochemistry. 2008;69:2225–2230. doi: 10.1016/j.phytochem.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Hölscher D, Shroff R, Knop K, Gottschaldt M, Crecelius A, Schneider B, Heckel DG, Schubert US, Svatos A. Matrix-free UV-laser desorption/ionization (LDI) mass spectrometric imaging at the single-cell level: distribution of secondary metabolites of Arabidopsis thaliana and Hypericum species. Plant Journal. 2009;60:907–918. doi: 10.1111/j.1365-313X.2009.04012.x. [DOI] [PubMed] [Google Scholar]
- Hölzl J, Petersen M. Chemical constituents of Hypericum. In: Ernst E, editor. Hypericum: The Genus Hypericum. Taylor and Francis; New York, USA: 2003. pp. 77–93. [Google Scholar]
- Huxley A, Griffiths M, Levy M, editors. The new Royal Horticultural Society Dictionary of Gardening. Vol. 2. Stockton Press; New York, USA: 1992. total. [Google Scholar]
- Iinuma M, Tosa H, Ito T, Tanaka T, Aqil M. Two prenylated anthrones in Harungana madagascariensis. Phytochemistry. 1995;40:267–270. [Google Scholar]
- Iinuma M, Tosa H, Tanaka T, Ito T, Yonemori S, Chelladurai V, Aquil M, Takahasi Y, Naganawa H. Occurrence of xanthonolignoids in guttiferaeous plants. Heterocycles. 1996;43:1521–1527. [Google Scholar]
- Kaçar O, Göksu E, Azkan N. Effects of morphogenetic and diurnal variability on the hypericin content in St. John’s wort (Hypericum perforatum L.) African Journal of Biotechnology. 2008;7:2163–2168. [Google Scholar]
- Kartnig T, Göbel I, Heydel B. Production of hypericin, pseudohypericin and flavonoids in cell cultures of various Hypericum species and their chemotypes. Planta Medica. 1996;62:51–53. doi: 10.1055/s-2006-957796. [DOI] [PubMed] [Google Scholar]
- Keller R. Hypericum. In: Engler A, Prantl K, editors. Die Naturliche Pflanzenfamilien. 3b. 1893. pp. 208–215. [Google Scholar]
- Keller R. Hypericum. In: Engler A, Prantl K, editors. Die Naturliche Pflanzenfamilien. 2nd edn Vol. 21. 1925. pp. 175–183. [Google Scholar]
- Kitanov GM. Phytochemical study and analysis of Hypericum L. species growing in Bulgaria. IV. Quantitative determination of the flavonoids. Farmatsiya (Sofia) 1987;37:35–40. [Google Scholar]
- Kitanov GM. A biflavone and flavanol and xanthone glycosides from Hypericum aucheri. Khimiya Prirodnykh Soedinenii. 1988a;3:454–455. [Google Scholar]
- Kitanov GM. Miquelianin and other polyphenols from Hypericum hirsutum. Khimiya Prirodnykh Soedinenii. 1988b;1:132–134. [Google Scholar]
- Kitanov GM. Hypericin and pseudohypericin in some Hypericum species. Biochemical Systematics and Ecology. 2001;29:171–178. doi: 10.1016/s0305-1978(00)00032-6. [DOI] [PubMed] [Google Scholar]
- Kitanov GM, Assenov I, The VD. Flavonols and xanthones from Cratoxylum pruniflorum Kurz. (Guttiferae) Pharmazie. 1988;43:879–880. [Google Scholar]
- Kitanov GM, Blinova KF. Current state of chemical investigation in the genus Hypericum. Khimiya Prirodnykh Soedinenii. 1987;2:185–203. [Google Scholar]
- Kitanov GM, Blinova KF, Akhtardzhiev K, Rumenin V. Flavonoids of Hypericum aucheri. Khimiya Prirodnykh Soedinenii. 1979;6:854–855. [Google Scholar]
- Komissarenko NF, Levashova IG, Zdanova VP. Flavonoids of Hypericum ascyron. Khimiya Prirodnykh Soedinenii. 1992;5:580–581. [Google Scholar]
- Korotkova N, Schneider JV, Ouandt D, Worberg A, Zizka G, Borsch T. Phylogeny of the eudicot order Malpighiales: analysis of a recalcitrant clade with sequences of the petD group II intron. Plant Systematics and Evolution. 2009 in press. [Google Scholar]
- Kouam SF, Ngadjui BT, Krohn K, Wafo P, Ajaz A, Choudhary MI. Prenylated anthronoid antioxidants from the stem bark of Harungana madagascariensis. Phytochemistry. 2005;66:1174–1179. doi: 10.1016/j.phytochem.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Kubitzki K, Mesquita AL, Gottlieb OR. Plant chemosystematics and phylogeny. Part VIII. Chemosystematic implications of xanthones in Bonnetia and Archytaea. Biochemical Systematics and Ecology. 1978;6:185–187. [Google Scholar]
- Kusari S, Lamshöft M, Zühlke S, Spiteller M. An endophytic fungus from Hypericum perforatum that produces hypericin. Journal of Natural Products. 2008;71:159–162. doi: 10.1021/np070669k. [DOI] [PubMed] [Google Scholar]
- Kusari S, Kuehlke S, Borsch T, Spiteller M. Positive correlations between hypericin and putative precursors detected in the quantitative secondary metabolite spectrum of Hypericum. Phytochemistry. 2009;70:1222–1232. doi: 10.1016/j.phytochem.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Laphookhieo S, Syers JK, Kiattansakul R, Chantrapromma K. Cytotoxic and antimalarial prenylated xanthones from Cratoxylum cochinchinense. Chemical and Pharmaceutical Bulletin. 2006;54:745–747. doi: 10.1248/cpb.54.745. [DOI] [PubMed] [Google Scholar]
- Lin Y-L, Wu YS. Polyprenylated phloroglucinol derivatives from Hypericum sampsonii. Helvetica Chimica Acta. 2003;86:2156–2163. [Google Scholar]
- Makovetska OY. Research of biologically active substances of Hypericum L. species. Report II. Farmatsevtichnii Zhurnal (Kiev) 1999a;1:47–52. [Google Scholar]
- Makovetska OY. Research of biologically active substances of Hypericum L. species. Report III. Farmatsevtichnii Zhurnal (Kiev) 1999b;2:24–29. [Google Scholar]
- Makovetska OY. Flavonoids of certain species of Hypericum L. Chemistry of Natural Compounds. 2000a;35:582–583. Author’s name published as Makovetskaya EY. [Google Scholar]
- Makovetska OY. Research of biologically active substances of Hypericum L. species. Farmatsevtichnii Zhurnal (Kiev) 2000b;5:40–47. [Google Scholar]
- Makovetska OY. Research on biologically active substances of Hypericum L. species. Farmatsevtichnii Zhurnal (Kiev) 2001a;1:75–80. Author’s name published as Makovetskaya EY. [Google Scholar]
- Makovetska OY. Research of biologically active substances of Hypericum L. species. Report XII. Farmatsevtichnii Zhurnal (Kiev) 2001b;5:46–53. [Google Scholar]
- Mártonfi P, Repčák M, Ciccarelli D, Garbari F. Hypericum perforatum L. - chemotype without rutin from Italy. Biochemical Systematics and Ecology. 2001;29:659–661. doi: 10.1016/s0305-1978(00)00094-6. [DOI] [PubMed] [Google Scholar]
- Mathis C, Ourisson G. Chemotaxonomic study of the genus Hypericum. I. Distribution of hypericin. Phytochemistry. 1963;2(2):157–171. [Google Scholar]
- Moerman DE. Native American Ethnobotany. Timber Press; Portland, Oregon, USA: 1998. total. [Google Scholar]
- Müller WE, editor. St. John’s Wort and its Active Principles in Depression and Anxiety. Birkhäuser Verlag; Basel, Switzerland: total. [Google Scholar]
- Nagai M, Tada M. Antimicrobial compounds, chinensin I and II from Hypericum chinense L. Chemical Letters. 1987;7:1337–1340. [Google Scholar]
- Nahrstedt A, Butterweck V. Biologically active and other chemical constituents of the herb Hypericum perforatum L. Pharmacopsychiatry. 1997;30:129–134. doi: 10.1055/s-2007-979533. [DOI] [PubMed] [Google Scholar]
- Noack KL. Über Hypericum Kreuzungen. VI. Fortpflanzungsverhältnisse und Bastarde von H. perforatum L. Zeitschrift für induktive Abstammungs- und Vererbungslehre. 1939;76:569–601. [Google Scholar]
- Noungoue DT, Antheaume C, Chaabi M, Lenta Ndjakou B, Ngouela S, Lobstein A, Tsamo E. Anthraquinones from the fruits of Vismia laurentii. Phytochemistry. 2008;69:1024–1028. doi: 10.1016/j.phytochem.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Noungoue DT, Chaabi M, Ngouela S, Antheaume C, Boyom FF, Gut J, Rosenthal PJ, Lobstein A, Tsamo E. Antimalarial compounds from the stem bark of Vismia laurentii. Zeitschrift für Naturforschung C. 2009;64:210–214. doi: 10.1515/znc-2009-3-410. [DOI] [PubMed] [Google Scholar]
- Nürk NM, Blatter FR. Cladistic analysis of morphological characters in Hypericum (Hypericaceae) Taxon: submitted. [Google Scholar]
- Park H-J, Kwon S-H, Yun S-Y, Lee KT. Isolation of steroids and flavonoids from the herbs of Hypericum ascyron L. Saengyak Hakhoech. 2000;31:39–44. [Google Scholar]
- Pattanaprateeb P, Ruangrungsi N, Cordell GA. Cytotoxic constituents from Cratoxylum arborescens. Planta Medica. 2005;71:181–183. doi: 10.1055/s-2005-837788. [DOI] [PubMed] [Google Scholar]
- Permana D, Lajis NH, Shaari K, Ali AM, Mackeen MM, Kitajima M, Takayama H, Aimi N. A new prenylated hydroquinone from the roots of Garcinia atroviridis Griff ex. T. Anders (Guttiferae) Zeitschrift für Naturforschung B. 2003;58:332–335. [Google Scholar]
- Pinto MMM, Sousa EP. Natural and synthetic xanthonolignoids: chemistry and biological activities. Current Medicinal Chemistry. 2003;10:1–12. doi: 10.2174/0929867033368574. [DOI] [PubMed] [Google Scholar]
- Piovan A, Filippini R, Caniato R, Borsarini A, Maleci LB, Cappelletti EM. Detection of hypericins in the “red glands” of Hypericum elodes by ESI-MS/MS. Phytochemistry. 2004;65:411–414. doi: 10.1016/j.phytochem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Rocha L, Marston A, Potterat O, Auxiliadora M, Kaplan C, Stoeckli-Evans H, Hostettmann K. Antibacterial phloroglucinols and flavonoids from Hypericum brasiliense. Phytochemistry. 1995;40:1447–1452. doi: 10.1016/0031-9422(95)00507-4. [DOI] [PubMed] [Google Scholar]
- Robson NKB, Adams WP. Chromosome numbers in Hypericum and related genera. Brittonia. 1968;20:95–106. [Google Scholar]
- Robson NKB. Hypericaceae. In: Van Steenis CGGJ, editor. Flora Malesianan. Series 1, Vol 8. Cyclopaedia of Collectors. Supplement 2. Noordhoff, Leiden: 1974. pp. 1–29. Part 1. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 1. Infrageneric classification. Bulletin of the British Museum of Natural History (Botany) 1977;5:291–355. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 2. Characters of the genus. Bulletin of the British Museum of Natural History (Botany) 1981;8:55–236. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 3. Sections 1. Campylosporus to 6a. Umbraculoides. Bulletin of the British Museum of Natural History (Botany) 1985;12:163–325. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 8. Sections 29. Brathys (part 2) and 30. Trigynobrathys. Bulletin of the British Museum of Natural History (Botany) 1990;20:1–151. [Google Scholar]
- Robson NKB. Guttiferae. In: Heywood VH, editor. Flowering Plants of the World. 2nd Edn Oxford University Press; New York, USA: 1993. pp. 85–87. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 6. Sections 20. Myriandra to Elodes. Bulletin of the British Museum of Natural History (Botany) 1996;26(2):75–217. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 4(1). Sections 7. Roscyna to 9. Hypericum sensu lato (part 1) Bulletin of the British Museum of Natural History (Botany) 2001;31(2):37–88. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 4(2). Section 9. Hypericum sensu lato (part 2): subsection 1. Hypericum series 1. Hypericum. Bulletin of the British Museum of Natural History (Botany) 2002;32(2):61–123. [Google Scholar]
- Robson NKB. Hypericum botany. In: Ernst E, editor. Hypericum: The Genus Hypericum. Taylor and Francis; New York, USA: 2003. pp. 1–22. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Clusiaceae). Section 9. Hypericum sensu lato (part 3): subsection 1. Hypericum series 2. Senanensia, subsection 2. Erecta and section 9b. Graveolentia. Systematics and Biodiversity. 2006;4:19–98. [Google Scholar]
- Robson NKB. Phytotaxa. 2010a. Studies in the genus Hypericum L. (Clusiaceae). 5(1). Sections 10. Olympia to 15/16. Crossophyllum. submitted. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Hypericaceae). 5(2). Sections 17. Hirtella to 19. Coridium. Phytotaxa. 2010b submitted. [Google Scholar]
- Salgues R. Recherches chimiques et toxicologiques nouvelles sur le genus Hypericum L. (Tourn.) Plant Foods for Human Nutrition. 1961;8:38–64. [Google Scholar]
- Seabra RM, Alves AC. Flavonoids from Hypericum species. Fitoterapia. 1990a;61:146–147. [Google Scholar]
- Seidler-Łożykovska K. Secondary metabolites content of Hypericum sp. in different stages and plant parts. In: Ernst E, editor. Hypericum: The Genus Hypericum. Taylor and Francis; New York, USA: 2003. pp. 100–105. [Google Scholar]
- Seo E-K, Huang L, Wall ME, Wani MC, Navarro H, Mukherjee R, Farnsworth NR, Kinghorn AD. New biphenyl compounds with DNA strand-scission activity from Clusia paralicola. Journal of Natural Products. 1999;62:1484–1487. doi: 10.1021/np9900775. [DOI] [PubMed] [Google Scholar]
- Seo E-K, Kim N-C, Wani MC, all ME, Navarro HA, Burgess JP, Kawanishi K, Kardono LBS, Riswan S, Rose WC, Fairchild CR, Farnsworth NR, Kinghorn AD. Cytotoxic prenylated xanthones and the unusual compounds anthraquinobenzophenones from Cratoxylum sumatranum. Journal of Natural Products. 2002;65:299–305. doi: 10.1021/np010395f. [DOI] [PubMed] [Google Scholar]
- Shatunova LV. Flavonoids from Hypericum hirsutum. Khimiya Prirodnykh Soedinenii. 1978;4:520. [Google Scholar]
- Sia G-L, Bennett GJ, Harrison LJ, Sim K-Y. Minor xanthones from the bark of Cratoxylum cochinchinense. Phytochemistry. 1995;38:152–158. [Google Scholar]
- Sirvent TM, Krasnoff SB, Gibson DM. Induction of hypericins and hyperforins in Hypericum perforatum in response to damage by herbivores. Journal of Chemical Ecology. 2003;29:2667–2681. doi: 10.1023/b:joec.0000008011.77213.64. [DOI] [PubMed] [Google Scholar]
- Smecerovic A, Zuehlke S, Spiteller M, Raabe N, Őzen T. Phenolic constituents of 17 Hypericum species from Turkey. Biochemical Systematics and Ecology. 2008;36:316–319. [Google Scholar]
- Soeberg J, Jørgensen LB, Jäger AK. Hyperforin accumulates in the translucent glands of Hypericum perforatum. Annals of Botany. 2007;99:1097–1100. doi: 10.1093/aob/mcm057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordat-Diserens I, Hamburger M, Rogers C, Hostettmann K. Dimeric xanthones from Garcinia livingstonei. Phytochemistry. 1992;31:3589–3593. [Google Scholar]
- Southwell IA, Bourke CA. Seasonal variation in hypericin content of Hypericum perforatum L. (St. John’s Wort) Phytochemistry. 2001;56:437–441. doi: 10.1016/s0031-9422(00)00411-8. [DOI] [PubMed] [Google Scholar]
- Spach E. Hypericacearum monographiae fragmenta. Annales des Sciences naturelles (Botanique) II. 1836a;5:157–76. [Google Scholar]
- Spach E. Conspectus monographiae Hypericaceaerum. Annales des Sciences naturelles (Botanique) II. 1836b;5:349–69. t. 6. [Google Scholar]
- Stevens PF. Angiosperm Phylogeny Website. 2008 Jun; 2001 onwards. Version 9. Available online: http://www.mobot.org/MOBOT/research/APweb.
- Stevens PF. Clusiaceae-Guttiferae. In: Kubitzki K, editor. The Families and Genera of Vascular Plants (Vol IX) Springer; Berlin, Germany: 2007. pp. 48–66. [Google Scholar]
- Taylor HL, Brooker RM. Isolation of uliginosin A and uliginosin B from Hypericum uliginosum. Lloydia. 1969;32:217–219. [PubMed] [Google Scholar]
- Umek A, Kreft S, Kartnig T, Heydel B. Quantitative phytochemical analysis of six Hypericum species growing in Slovenia. Planta Medica. 1999;65:388–390. doi: 10.1055/s-2006-960798. [DOI] [PubMed] [Google Scholar]
- Verma V, Smelcerovic A, Zuehlke S, Hussain MA, Ahmad SM, Ziebach T, Oazi GN, Spiteller M. Phenolic constituents and genetic profile of Hypericum perforatum L. from India. Biochemical Systematics and Ecology. 2008;36:201–206. [Google Scholar]
- Wagner WH. The construction of a classification. In: Sibley CG, editor. Systematic Biology. National Academy of Sciences; Washington D.C., USA: 1969. pp. 67–103. [Google Scholar]
- Winkelmann K, Heilmann J, Zerbe O, Rali T, Sticher O. New prenylated bi- and tricyclic phloroglucinol derivatives from Hypericum papuanum. Journal of Natural Products. 2001;64:701–706. doi: 10.1021/np0006364. [DOI] [PubMed] [Google Scholar]
- Wirz A, Simmen U, Heilmann J, Calis I, Meier B, Sticher O. Bisanthraquinone glycosides of Hypericum perforatum with binding inhibition to CRH-1 receptors. Phytochemistry. 2000;55:941–947. doi: 10.1016/s0031-9422(00)00305-8. [DOI] [PubMed] [Google Scholar]
- Wurdack KJ, Davis CC. Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. American Journal of Botany. 2009;96:1551–1570. doi: 10.3732/ajb.0800207. [DOI] [PubMed] [Google Scholar]
- Wurglics M, Schubert-Zsilavecz M. Hypericum perforatum: a ‘modern’ herbal antidepressant, pharmacokinetics of active ingredients. Clinical Pharmacokinetics. 2006;45:449–468. doi: 10.2165/00003088-200645050-00002. [DOI] [PubMed] [Google Scholar]
- Yu HY, Jin SL, Zhang X, Liu Y, Ou YF, Wang NL, Yao XS. Two new benzophenone glycosides from Cratoxylum cochinchinensis. Chinese Chemical Letters. 2009;20:459–461. [Google Scholar]
- Zaichikova SG, Barabanov EI. Flavonoids of Hypericum scabrum. Khimiya Prirodnykh Soedinenii. 1980;5:718–719. [Google Scholar]
- Zapesochnaya GG, Kuptsova LP, Kyshtymova TV, Ban’kovskii AI, Mel’nikova TM. Flavonoids of Hypericum maculatum and H. inodorum. Khimiya Prirodnykh Soedinenii. 1967;3:279–280. [Google Scholar]
- Zobayed SMA, Afreen F, Goto E, Kozai T. Plant-environment interactions: accumulation of hypericin in dark glands of Hypericum perforatum. Annals of Botany. 2006;98:793–804. doi: 10.1093/aob/mcl169. [DOI] [PMC free article] [PubMed] [Google Scholar]