Abstract
Many prion diseases are peripherally acquired (eg. orally or via lesions to skin or mucous membranes). After peripheral exposure prions replicate first upon follicular dendritic cells (FDC) in the draining lymphoid tissue before infecting the brain. However, after replication upon FDC within the draining lymphoid tissue, prions are subsequently propagated to most non-draining secondary lymphoid organs (SLO) including the spleen by a previously underdetermined mechanism. The germinal centres in which FDC are situated produce a population of B cells which can recirculate between SLO. We therefore reasoned that B cells were ideal candidates by which prion dissemination between SLO may occur. Sphingosine 1-phosphate receptor 1 (S1PR1) stimulation controls the egress of T and B cells from SLO. S1PR1 signalling-blockade sequesters lymphocytes within SLO resulting in lymphopenia in the blood and lymph. We show that in mice treated with the S1PR modulator FTY720, or with S1PR1-deficiency restricted to B cells, the dissemination of prions from the draining lymph node to non-draining SLO is blocked. These data suggest that B cells interacting with and acquiring surface proteins from FDC, and recirculating between SLO via the blood and lymph, mediate the initial propagation of prions from the draining lymphoid tissue to peripheral tissues.
Keywords: Processes, neuroimmunology, cell trafficking, Tissues, spleen and lymph nodes, Infections, viral, Cells, B cells
Introduction
Prion diseases (transmissible spongiform encephalopathies) are sub-acute neurodegenerative diseases that affect both humans and animals. Many prion diseases, including natural sheep scrapie, bovine spongiform encephalopathy (BSE), chronic wasting disease in cervids, and variant Creutzfeldt-Jakob disease in humans (vCJD), are acquired peripherally such as by oral exposure. After exposure, prions first replicate upon follicular dendritic cells (FDC) as they make their journey from the site of infection to the CNS (a process termed, neuroinvasion) (1-5). FDC are a unique subset of stromal cells resident within primary B cell follicles and germinal centres of lymphoid tissues (6). Prion accumulation and replication upon FDC is critical for efficient neuroinvasion (1-3, 7). During prion disease aggregations of PrPSc, an abnormally folded isoform of the cellular prion protein (PrPC), accumulate in affected tissues. Prion infectivity co-purifies with PrPSc and is considered to constitute the major, if not sole, component of infectious agent (8-9). Although low levels of prion infectivity are present in the blood stream of infected animals (10), prions invade the CNS by spreading from lymphoid tissue via the peripheral nervous system as the depletion of sympathetic nerves impedes neuroinvasion (11).
Dietary exposure to BSE-contaminated meat products is considered the most likely source of vCJD in humans (12). However, in the UK four cases of vCJD have been reported in recipients of blood or blood products derived from vCJD-infected donors (13-16). Initial concern that vCJD might have the potential to contaminate the blood-stream came from data from animal studies which demonstrated that many prion strains accumulate in lymphoid tissues prior to neuroinvasion (for review see (17)). Subsequent findings that PrPSc could likewise be detected in the lymphoid tissues of vCJD patients further raised this concern (18-19). Data from studies of experimental mice show that following peripheral exposure (eg: orally or via skin lesions) prions first replicate on FDC within the draining lymphoid tissue (eg: Peyer’s patch or regional [draining] lymph node) and subsequently spread to most other non-draining secondary lymphoid organs (SLO) including the spleen. A similar situation also appears to occur in patients with vCJD as PrPSc accumulation in lymphoid tissues is restricted during the pre-clinical phase (14) but widespread at the clinical stage of disease (18-19). How the propagation of prions between SLO occurs is uncertain.
The germinal centres in which FDC are situated produce a population of recirculating antigen-specific memory B cells (20). These germinal centre B cells preferentially migrate towards the B cell follicle-specific chemokine CXCL13, allowing B cells from one germinal centre to seed other germinal centres via the blood-stream (20). B cells have been shown to recirculate between lymphoid tissues for several weeks (21), and can transfer antigen reactivity from the draining lymph node to non-draining lymph nodes within a few days of immunisation (22). Several pathogens appear to exploit these characteristics to aid transmission. For example, migrating B cells play a key role in carrying retrovirus infection from lymph nodes to peripheral tissues (23). Naïve B cells have been shown to often acquire FDC surface proteins during cognate antigen capture (24). Coupled with their capacity to migrate between lymphoid tissues (25), these data suggest B cells are ideal candidates by which prion dissemination between SLO may occur. Thus it is plausible that soon after exposure B cells become contaminated with prions within the draining lymphoid tissue and disseminate the agent via the blood-stream and lymph between SLO as they circulate around the host. Indeed, in the peripheral blood of scrapie-affected sheep prion infectivity is associated with the lymphocyte-containing buffy coat fraction (10). Studies also show that B cells within the peripheral blood of deer infected with chronic wasting disease are likewise associated with prion infectivity (26).
In the current study the hypothesis was tested that recirculating B cells disseminate prions between SLO. To do so early prion pathogenesis was studied in mouse models in which lymphocyte egress from SLO was blocked. The sphingosine 1-phosphate receptor 1 (S1PR1) helps to control the egress of newly formed T cells from the thymus and the exit of mature T and B cells from SLO (27-29). S1PR1 is a G protein-coupled receptor which binds the lysophospholipid sphingosine 1-phosphate (S1P). Although ubiquitously synthesized the concentration of S1P in the blood and lymph is higher when compared to SLO. This concentration gradient is considered to promote lymphocyte egress from SLO into the blood and lymph via stimulation of lymphocyte S1PR1. The production of S1P by lymphatic endothelial cells likewise appears to provide an important source of S1P for the egress of lymphocytes from lymph nodes and Peyer’s patches (30). In the absence of S1PR1 stimulation, lymphocytes are sequestrated in SLO causing lymphopenia in the blood-stream and lymph (27). We show here that in mice treated with the S1PR modulator FTY720 or with S1PR1-deficiency restricted to B cells the dissemination of prions from the draining lymph node to non-draining lymph nodes and the spleen is blocked. These data suggest that B cells recirculating between lymphoid tissues via the blood and lymph play an important role in the initial transfer of prions between the draining lymph node and non-draining SLO.
Materials and Methods
Mice
The CD19cre S1PR1loxP/loxP mice (31) and tga20 mice (32) over-expressing PrPc were generated as described previously. All mice were bred on a C57Bl/6 background and were maintained under SPF conditions. All studies using experimental mice and regulatory licences were approved by both The Roslin Institute’s and University of Edinburgh’s Protocols and Ethics Committees. All animal experiments were carried out under the authority of a UK Home Office Project Licence within the terms and conditions of the strict regulations of the UK Home Office ‘Animals (scientific procedures) Act 1986’. Where necessary, anaesthesia appropriate for the procedure was administered, and all efforts were made to minimize harm and suffering. Mice were humanely culled using by a UK Home Office Schedule One method. Prior to their use in experiments, the genotype of each CD19cre S1PR1loxP/loxP mouse was confirmed by PCR analysis of tail DNA for the presence of Cre and S1pr1 by PCR as described (33).
Treatment with FTY720
Chronic S1PR1-blockade was achieved through treatment of mice with FTY720 (Novartis) via drinking water (2 mg/L). A parallel group of mice were provided with regular drinking water as a control.
Prion exposure and disease monitoring
Mice were exposed to ME7 scrapie prions by skin scarification of the medial surface of the left thigh as previously described (34-36). Briefly, approximately 1 cm2 area of hair covering the site to be scarified was trimmed using curved scissors and then removed completely with an electric razor. Twenty-four hours later a 23-gauge needle was used to create a 5 mm long abrasion in the epidermal layers of the skin at the scarification site. Then using a 26-gauge needle one droplet (~6 μl) of 1.0% (wt/vol) brain homogenate prepared from mice terminally-affected with ME7 scrapie prions was applied to the abrasion and worked into the site using sweeping strokes. Every effort was made to ensure the scarification did not cause bleeding. The scarification site was then sealed with OpSite (Smith & Nephew Medical Ltd., Hull, UK) and allowed to dry before the animals were returned to their final holding cages. Following exposure, mice were coded and assessed blindly for the signs of clinical prion disease and culled at a standard clinical endpoint (37). Scrapie diagnosis was confirmed blindly on coded sections by histopathological assessment of vacuolation in the brain. For the construction of lesion profiles, vacuolar changes were scored in nine grey-matter areas of the brain as described (38). Where indicated, some mice were culled at the times indicated post injection with prions and tissues taken for further analysis.
For bioassay of prion infectivity, individual half spleens were prepared as 10% (wt/vol) homogenates in physiological saline. Groups of four tga20 indicator mice were injected i.c. with 20 μl of each homogenate. The scrapie titre in each sample was determined from the mean incubation period in the indicator mice, by reference to a dose/incubation period response curve for ME7 scrapie prions-infected spleen tissue serially titrated in tga20 mice using the relationship: y = 9.4533 – 0.0595x (y, = log ID50 U/20 μl of homogenate; x, incubation period; R2 = 0.9562). As the expression level of cellular PrPc controls the prion disease incubation period, tga20 mice over-expressing PrPc are extremely useful as indicator mice in prion infectivity bioassays as they succumb to disease with much shorter incubation times than conventional mouse strains (32).
Flow cytometric analysis
Peripheral blood samples were prepared at 4 °C in FACS buffer [PBS pH 7.4 containing 5% FCS]. Red blood cells were removed using red blood cell lysing buffer (Sigma). After washing in FACS buffer, non-specific antibody binding to Fc-receptors was blocked using SeroBlock FcR (AbD Serotec). B cells were identified using FITC-conjugated rat anti-mouse CD19 mAb (clone 6D5, Invitrogen) and T cells were detected using RPE-conjugated rat anti-mouse CD4 mAb (clone YTS191.1, AbD Serotec) and analysed on a FACSCalibur flow cytometer (BD Biosciences). Viable cells were gated by forward and side light scatter.
IHC and immunofluorescent analyses
Lymph nodes and spleens were removed and snap-frozen at the temperature of liquid nitrogen. Serial frozen sections (10 μm in thickness) were cut on a cryostat and immunostained with the following antibodies: FDC were visualized by staining with mAb 8C12 to detect CD35 (BD Biosciences PharMingen); cellular PrPC was detected using rabbit PrP-specific 1B3 polyclonal antibody (pAb) (39); B cells were detected using rat anti-mouse B220 mAb (clone RA3-RB2, Caltag, Towcester, UK); T cells were detected using rat anti-mouse CD4 mAb (clone RM4-5, Invitrogen); marginal zone B cells were detected using mAb 1B1 to detect CD1d (BD Biosciences PharMingen); marginal zone sinus-lining cells were detected using mAb MECA-367 (BD Biosciences PharMingen) specific for mucosal vascular addressin cell-adhesion molecule 1 (MADCAM1); S1PR1 was detected using S1PR1-specific rabbit polyclonal antibody H-60 (Santa Cruz Biotechnology, Inc.). Prior to immunostaining with the S1PR1-specific antibody, sections were pre-treated by autoclaving (120°C, 15 min.) in antigen-retrieval solution (DAKO, Ely, UK). Following the addition of primary antibody, streptavidin-conjugated or species-specific secondary antibodies coupled to Alexa Fluor 488 (green) or Alexa Fluor 594 (red) dyes (Invitrogen) were used. Sections were mounted in fluorescent mounting medium (DAKO) and examined using a Zeiss LSM5 confocal microscope (Zeiss, Welwyn Garden City, UK).
Brains were fixed in periodate-lysine-paraformaldehyde fixative and embedded in paraffin wax. Sections (thickness, 6 μm) were deparaffinised, and pre-treated to enhance the detection of PrP by hydrated autoclaving (15 min, 121 °C, hydration) and subsequent immersion formic acid (98%) for 5 min. Sections were then immunostained with 1B3 PrP-specific pAb. For the detection of astrocytes, brain sections were immunostained with anti-glial fibrillary acidic protein (GFAP; DAKO). For the detection of microglia, deparaffinised brain sections were first pre-treated with Target Retrieval Solution (DAKO) and subsequently immunostained with anti-ionized calcium-binding adaptor molecule 1 (Iba-1; Wako Chemicals GmbH, Neuss, Germany). Immunolabelling was revealed using HRP-conjugated to the avidin-biotin complex (Novared kit, Vector laboratories, Peterborough, UK).
PET immunoblot detection of PrPSc
PrPSc was detected in paraffin-embedded-tissue (PET) sections of lymph nodes as previously described (40). Briefly, tissues were fixed in periodate-lysine-paraformaldehyde and embedded in paraffin wax. Serial sections (thickness 6 μm) were mounted on polyvinylidine difluoride membrane (Bio-Rad, Hemel Hempstead, UK) and fixed by incubation at 55°C overnight. Membranes were then deparaffinised and digested with proteinase K (20 μg/ml) for 16 h at 55°C (to confirm the presence of PrPSc), washed in TBS/Tween (10 mM Tris-HCl pH 7.8, 100 mM NaCl, 0.5% Tween) and denatured in 3 M guanidine isothiocyanate (10 mM Tris-HCl pH 7.8) for 10 mins. Membranes were blocked in 2% casein, and PrP detected with PrP-specific pAb 1B3, followed by alkaline phosphatase-conjugated goat anti-rabbit antiserum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA). Bound alkaline phosphatase activity was detected with SigmaFast NBT/BCIP solution (Sigma, Poole, UK). Immunostained membranes were assessed using an Olympus dissecting microscope.
Immunoblot detection of PrPSc
Spleen fragments (~20 mg) were prepared as 10 % (wt/vol) tissue homogenates and PrPSc enriched by sodium phosphotungstic acid (NaPTA) precipitation (41), and treated in the presence of proteinase K (40 μg/ml, 60 min, 37°C; VWR, Lutterworth, UK). Following enrichment, pellets were re-suspended and diluted to an approximate protein concentration of 0.5 mg protein/ml and 10 μl electrophoresed through SDS/PAGE gels (12%) polyacrylamide gels (Invitrogen). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hemel Hemstead, UK) by semidry blotting. PrP was detected with the rabbit PrP-specific mAb (clone EP1802Y, Epitomics, Inc., Burlingame, CA) followed by horse radish peroxidase-conjugated goat anti-mouse antiserum. Bound horse radish peroxidase activity was detected with Supersignal West Dura Extended Duration Substrate (Pierce Biotechnology, Rockford, IL, USA).
Statistics
Data are presented as mean ± SD and significant differences between samples in different groups were sought by student’s t-test. Values of P < 0.05 were accepted as significant.
Results
Effect of FTY720-treatment on B and T cells
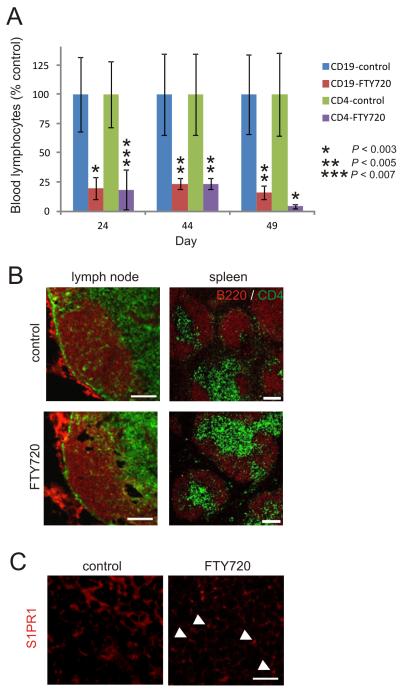
To study the requirement for recirculating lymphocytes in the dissemination of prions from the draining lymph node to non-draining lymph nodes and the spleen, S1PR1-blockade was used to induce lymphopenia in the blood and lymph by impeding the egress of B and T cells from SLO. Chronic S1PR1-blockade was achieved through continual exposure of C57BL/6 mice to the S1PR modulator FTY720 via drinking water. FTY720 is ideally suited for use in the experiments described below as it is extremely stable in aqueous solution and has been used in long-term studies (up to 12 mo.) without adverse affects (42-44). Parallel groups of mice were given normal drinking water as a control. As anticipated, the number of B and T cells (CD19+ and CD4+ cells, respectively) in the blood-stream of FTY720-treated mice was rapidly and significantly reduced when compared to controls (Fig. 1A; P < 0.007, n = 4) (27). The lymphopenia was maintained for the duration of the exposure to FTY720. Immunohistochemical (IHC) analysis suggested there was no observable effects of FTY720-treatment were observed in the density and overall distribution of B and T cells in lymph nodes and spleens (Fig. 1B). In control mice most lymphocytes appeared to exhibit a low-intensity homogenous membrane expression of S1P1 (Fig. 1C). In tissues from FTY720-treated mice this immunostaining appeared to be more punctate, implying internalization of S1PR1 (Fig. 1C) (29). Together, these data confirmed that chronic FTY720 treatment caused a prolonged lymphopenia by blocking the S1PR1-mediated egress of lymphocytes from SLO.
FIGURE 1.
FTY720 treatment causes lymphopenia. (A) Frequency of B cells and T cells (CD19+ and CD4+ cells, respectively) in the blood of mice administered FTY720 via drinking water. Data are presented as % control group. Each point represents mean ± SD (n = 4/group). *, P < 0.003; **, P < 0.005; ***, P < 0.007. (B) IHC analysis of the effect of FTY720 treatment on the distribution of B cells (B220+ cells, red) and T cells (CD4+ cells, green) in lymph nodes and spleen. Scale bar, 100 μm. (C) IHC analysis of the effect of FTY720 treatment on S1PR1 expression (red) by lymphocytes within lymph nodes. Arrows show apparent punctate immunostaining in cells from FTY720-treated mice indicative of the internalization of S1PR1. Scale bar 20 μm.
FTY720-treatment does not affect FDC status
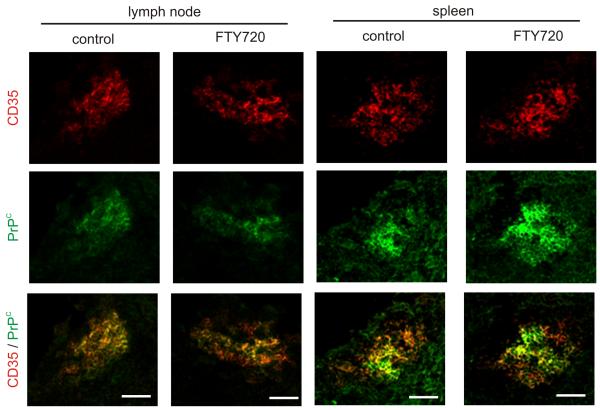
Following peripheral exposure prions first replicate upon the surfaces of FDC in the draining lymphoid tissue (3, 34, 36). Host cells must express cellular PrPC to sustain prion infection and FDC express high levels of PrPC on their surfaces (7, 45-46). FDC in mice also characteristically express high levels of CD35 (complement receptor 1) which has also been shown to be aid the retention of prions upon FDC (47-49). Therefore, we next determined the effect of FTY720 treatment on FDC status. IHC analysis suggested there was no observable difference in the status of CD35 and PrPC-expressing FDC within the lymph nodes and spleens of FTY720-treated and control mice (Fig. 2).
FIGURE 2.
FTY720 treatment does not affect the status of FDC within SLO. IHC analysis of the expression of CD35 (red) and PrPC (green) by FDC in lymph nodes and spleens from FTY720-treated and control mice. Data are representative of tissues from 4-6 mice/group. Scale bar, 100 μm.
S1PR1-blockade blocks prion dissemination between secondary lymphoid tissues
After exposure via skin scarification ME7 scrapie prions accumulate first upon FDC within the draining lymph node and weeks later spread to FDC within non-draining lymph nodes and the spleen (34, 36). Therefore, we next determined the effect of FTY720-mediated lymphopenia on the dissemination of prions between SLO. To do so, mice were first exposed to prions via skin scarification. Then 14 days later, when prion infection was established only within the draining lymph node (34), mice were continuously exposed to FTY720 via drinking water. A separate group of mice continued to receive normal drinking water as a control. Skin scarification was used as the route of prion exposure in this study to allow the delivery of the inoculum to be targeted directly and exclusively to the draining lymph node without initial contamination of the blood stream (34-36). This was important as it would be not be possible to study any potential effects on prion dissemination between SLO if the inoculation route itself directly contaminated the blood-stream causing haematogenous spread.
In this study, the normal cellular form of the prion protein is referred to as PrPC, and PrPSc is used to describe the disease-specific, abnormal accumulations of PrP that are characteristically found only in prion-affected tissues.(8) Prion disease-specific PrPScaccumulations are relatively resistant to proteinase K (PK) digestion, whereas cellular PrPC is destroyed. To confirm the presence of PrPSc in lymph nodes, histological sections were applied to nitrocellulose membrane, treated with PK and subsequently analysed by paraffin-embedded tissue (PET) immunoblot analysis (40).
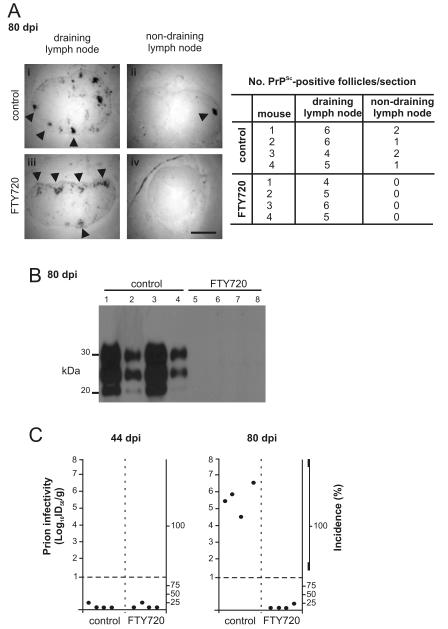
As anticipated, heavy PrPSc accumulations, consistent with localisation upon FDC (7, 34, 50), were detected at 80 days after prion exposure in the draining inguinal lymph nodes of control mice (Fig. 3Ai) and FTY720-treated mice (Fig. 3Aiii). Furthermore, FTY720-treatment had no significant effect on the number of PrPSc-positive follicles in the draining lymph node when compared to controls (P = 0.705, n = 4; student’s T test; Fig. 3A). These data show that S1PR1-blockade had no observable effect on the initial accumulation of prions within the draining lymph node. In control mice, heavy PrPSc accumulations were also observed in the non-draining inguinal lymph node (Fig. 3Aii) and the spleen (Fig. 3B) consistent with the dissemination of prions to non-draining SLO by this time after exposure. We also analysed prion infectivity levels in spleens from each group of mice. By 44 days after exposure only trace levels of prion infectivity were detected in spleens from either group of mice indicating that significant prion dissemination to non-draining SLO had not occurred at this time. However, high levels of prion infectivity were detected within control spleens by 80 days after exposure (Fig. 3C). In contrast, S1PR1-blockade prevented the dissemination of prions to non-draining SLO. In tissues from FTY720-treated mice taken at tissues at 80 days after prion exposure no PrPSc was detected in non-draining lymph nodes (Fig. 3Aiv), and no PrPSc or prion infectivity was detected in the spleen (Fig. 3B,C). Together, these data clearly show that the FTY720-mediated blockade of lymphocyte egress from SLO impeded the dissemination of prions from the draining lymph node to non-draining SLO.
FIGURE 3.
FTY720 treatment impedes prion dissemination between SLO. (A) PET-immunoblot analysis of PrPSc accumulation (blue/black) within the draining and non-draining lymph nodes of control mice (i-ii) and FTY720-treated mice (iii-iv) collected 80 days after prion exposure by skin scarification (n = 4/group). Arrows indicate sites of PrPSc accumulation in association with FDC. Scale bar = 0.5 mm. Right-hand panel shows the number of PrPSc-positive follicles/sections in the draining and non-draining lymph nodes from all mice from each group. (B) Western blot analysis of PrPSc accumulation within the spleens of control and FTY720-treated mice collected 80 days after prion exposure by skin scarification (n = 4/group). Samples were treated with PK prior to electrophoresis to destroy cellular PrPC. After PK treatment, a typical three-band pattern was observed between molecular mass values of 20 and 40 kDa, representing unglycosylated, monoglycosylated and diglycosylated isomers of PrP (in order of increasing molecular mass). Each lane represents an individual spleen (n = 4/group). (C) Prion infectivity levels were assayed spleens from control and FTY720 treated mice (n = 4/group) collected 44 and 80 days after exposure to ME7 scrapie prions via skin scarification. Prion infectivity titres were determined by transmission of tissue homogenates into groups of 4 indicator tga20 indicator mice. Each point represents data derived from an individual spleen. Data below the horizontal line indicate disease incidence in the recipient mice <100% and considered to contain trace levels of prion infectivity.
S1PR1-blockade does not influence prion neuroinvasion
We next determined the effect of chronic S1PR1-blockade on the spread of prions to the CNS (neuroinvasion). All control mice succumbed to clinical prion disease with a mean incubation period of 357 ± 10 days (n = 6). Chronic S1PR1-blockade had no significant influence on neuroinvasion as all FTY720-treated mice succumbed to clinical prion disease with similar incubation periods (359 ± 9 days, n = 6; P = 0.534, student’s T test when compared to controls).
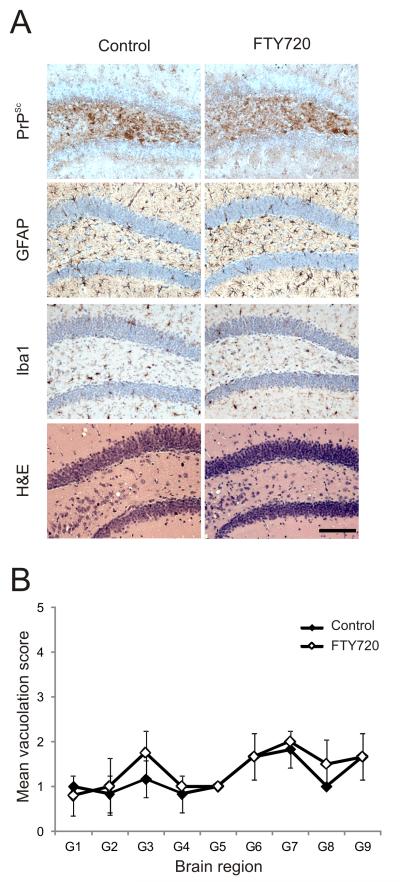
Characteristic disease-specific PrP accumulation, astrogliosis, microgliosis and spongiform pathology (vacuolation) typically associated with terminal infection with ME7 scrapie prions were detected in the brains of all clinically-affected control and FTY720-treated mice (Figure 4A). The severity and distribution of the spongiform pathology within the brains of the clinically-affected mice from each group was similar and typical of mice clinically affected with ME7 scrapie prions (Figure 4B).
FIGURE 4.
S1PR1-blockade does not influence prion neuroinvasion. Mice were first exposed to prions via skin scarification. Then 14 days later, mice were continuously exposed to FTY720 via drinking water. Normal drinking water was used as a control. Brains were collected from clinically scrapie-affected mice and the neuropathology within each brain compared. (A) Heavy accumulations of PrPSc (brown, top row), reactive astrocytes expressing GFAP (brown, second row), active microglia expressing Iba-1 (brown, third row) and spongiform pathology (H&E, bottom row) were detected in the brains of all clinically scrapie-affected control and FTY720-treated mice. Sections were counterstained with haematoxylin to detect cell nuclei. Scale bar, 100 μm (B) Pathological assessment of the spongiform change (vacuolation) in brains from terminally scrapie affected control mice (solid diamonds) and FTY720-treated (open diamonds) mice. Vacuolation was scored on a scale of 0-5 in the following grey matter areas: G1, dorsal medulla; G2, cerebellar cortex; G3, superior colliculus; G4, hypothalamus; G5, thalamus; G6, hippocampus; G7, septum; G8, retrosplenial and adjacent motor cortex; G9, cingulate and adjacent motor cortex. All data are representative of tissues from 6 mice/group.
Mice with B cell-restricted S1PR1-deficiency
To determine whether the effects of FTY720 treatment on prion pathogenesis were specifically due to the impairment of B cell egress from SLO, a Cre/LoxP approach was used to create mice in which S1PR1-deficiency was restricted to B cells (31). To do so, CD19cre mice, in which the Cd19 locus directs Cre recombinase expression in B cells, were crossed with mice containing a floxed S1pr1 allele which encodes S1PR1 (33). In the progeny CD19cre S1pr1flox/flox mice, S1pr1 expression is conditionally ablated in only in Cre recombinase-expressing cells (B cells). Cre recombinase-deficient littermates were used as controls.
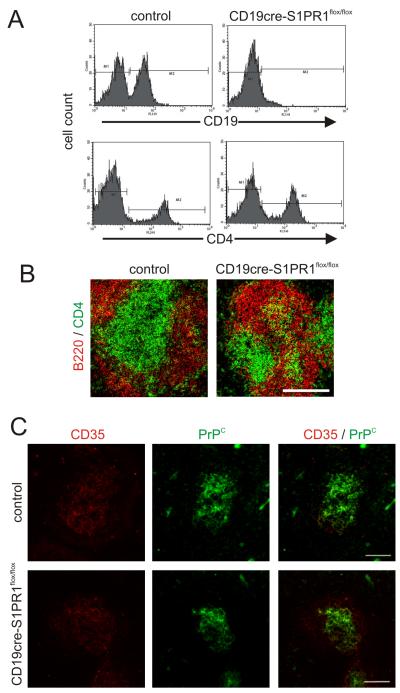
As anticipated, B cells were dramatically reduced in the blood of CD19cre S1pr1flox/flox mice when compared to Cre-deficient controls, whereas T cells were unchanged (Fig. 5A). No apparent differences were observed by IHC in the overall density and distribution of B and T cells in the spleens (Fig. 5B) and lymph nodes (data not shown). Consistent with data above (Fig. 2), IHC analysis suggested there was no observable difference in the status of CD35 and PrPC-expressing FDC in CD19cre S1pr1flox/flox and Cre-deficient control mice (Fig. 5C).
FIGURE 5.
Characterisation of CD19cre S1PR1flox/flox mice. (A) Frequency of B cells and T cells (CD19+ and CD4+ cells, respectively) in the blood of control (Cre-deficient) and CD19cre S1PR1flox/flox mice. (B) IHC comparison of the distribution of B cells (B220+ cells, red) and T cells (CD4+ cells, green) in the spleens of control and CD19cre S1PR1flox/flox mice. (C) IHC analysis of the expression of CD35 (red) and PrPC (green) by FDC in the spleens of control and CD19cre S1PR1flox/flox mice. Data are representative of tissues from 4-6 mice/group. Scale bars = 100 μm.
B cell-specific S1PR1-deficiency blocks prion dissemination between SLO
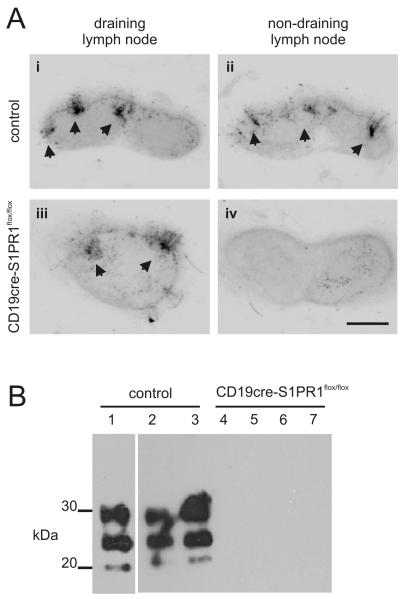
We next determined whether B cell-restricted S1PR1-deficiency impeded the dissemination prions to non-draining SLO. Groups of CD19cre S1pr1flox/flox mice and Cre-deficient control mice were exposed to prions via skin scarification and tissues collected 105 days after exposure. As anticipated, heavy PrPSc accumulations, consistent with localisation upon FDC, were detected within the draining inguinal lymph nodes of control and CD19cre S1pr1flox/flox mice (Fig. 6Ai,iii). Thus, as observed for FTY720-mediated S1PR1-blockade (Fig. 3A), B cell-restricted S1PR1-deficiency did not influence the initial delivery of prions to, and their accumulation within, the draining lymph node. In control mice the dissemination of prions to non-draining SLO had also occurred as heavy PrPSc accumulations were detected in the non-draining inguinal lymph node (Fig. 6Aii) and the spleen (Fig. 6B). In contrast, in CD19cre S1pr1flox/flox mice with B cell-restricted S1PR1-deficiency the dissemination of prions to the non-draining inguinal lymph node (Fig. 6Aiv) and the spleen (Fig. 6B) was blocked. These data clearly show that the inhibition of S1PR1-mediated B cell egress from SLO blocks the dissemination of prions from the draining lymph node to non-draining SLO. Taken together, these data suggest that the recirculation of B cells between SLO via the blood and lymph plays an important role in the initial dissemination of prions between SLO.
FIGURE 6.
Prion dissemination between SLO is blocked in mice with B cell-restricted S1PR1-deficiency. (A) PET-immunoblot analysis of PrPSc accumulation (blue/black) within the draining and non-draining lymph nodes of control (Cre-deficient) mice (i-ii) and CD19cre S1PR1flox/flox mice (iii-iv) collected 105 days after prion exposure by skin scarification (n = 4/group). Arrows indicate sites of PrPSc accumulation in association with FDC. Scale bar = 0.5 mm. (B) Western blot analysis of PrPSc accumulation within the spleens of control and CD19cre S1PR1flox/flox mice collected 105 days after prion exposure by skin scarification. Samples were treated with PK prior to electrophoresis to destroy cellular PrPC. After PK treatment, a typical three-band pattern was observed between molecular mass values of 20 and 40 kDa, representing unglycosylated, monoglycosylated and diglycosylated isomers of PrP (in order of increasing molecular mass). Each lane represents an individual spleen (n = 3-4/group).
S1PR1-signalling blockade displaces marginal zone B cells from the splenic marginal zone
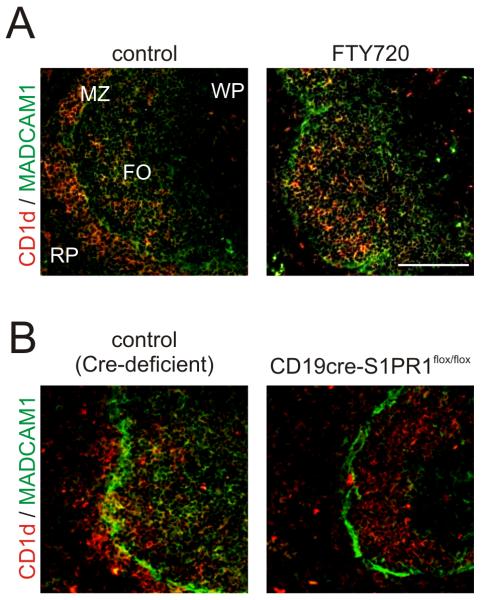
Within the spleen S1PR1-signalling has been shown to promote the positioning of B cells within the marginal zone (28). In mice, marginal zone B cells express high levels of the non-classical major histocompatibility complex molecule CD1d (28, 51). The splenic marginal zone is delineated by a distinct channel of mucosal vascular addressin cell-adhesion molecule 1 (MADCAM1)-expressing sinus lining cells. In C57BL/6 control mice (Fig. 7A) and Cre-deficient control mice (Fig. 7B) CD1d-expressing marginal zone B cells were present within the marginal zone and B cell follicles. In contrast, FTY720-mediated S1PR1 signalling blockade (Fig. 7A) or B cell-restricted S1PR1-deficiency (Fig. 7B) caused the displacement of marginal zone B cells into the B cell follicles.
FIGURE 7.
S1PR1-signalling blockade displaces marginal zone B cells from the splenic marginal zone. MADCAM1-expressing sinus-lining cells (green) form a distinct barrier between the marginal zone (MZ) and the white pulp (WP) (brown, arrows). In the spleens of control (C57BL/6) mice (A, left hand panel) and control (Cre-deficient) mice (B, left-hand panel), abundant CD1d-expressing marginal zone B cells (red) were present within the marginal zone and B cell follicles (FO). FTY720 treatment (A, right-hand panels) or B cell-restricted S1PR1-deficiency in CD19cre S1PR1flox/flox mice (B, right-hand panels) caused the displacement of marginal zone B cells into the B cell follicles. Data are representative of tissues from 4-6 mice/group. RP, red pulp. Scale bar = 100 μm.
Discussion
Following peripheral exposure prions accumulate first within the draining lymphoid tissue. However, after replication upon FDC within these tissues prions are subsequently propagated to most non-draining SLO including the spleen by a previously underdetermined mechanism. Lymphocyte S1PR1 stimulation controls the egress of T and B cells from SLO (27-29). When S1PR1 signalling is blocked, lymphocytes are sequestered within SLO resulting in lymphopenia in the blood and lymph. Here we show that in mice treated with the S1PR modulator FTY720 (52), or with S1PR1-deficiency restricted to B cells (31), the dissemination of prions from the draining lymph node to non-draining lymph nodes and the spleen is blocked. These data suggest that B cells interacting with and acquiring surface proteins from FDC (24), and recirculating between lymphoid tissues via the blood and lymph, play an important role in the initial propagation of prions to non-draining SLO.
Taken together, data from the current study and elsewhere suggest the following factors influence the initial propagation of prions within the periphery. Following peripheral exposure prions first accumulate and replicate upon the surfaces of FDC within the germinal centres of the draining lymphoid tissue (7, 53-54). FDC are considered to amplify the prions above the threshold level required for neuroinvasion. Following their expansion upon FDC, prions subsequently infect neighbouring nerve fibres of the peripheral nervous system from which they spread to the CNS where they ultimately cause neurodegeneration (11, 55-56). Within weeks of accumulating within the draining lymphoid tissue prions are subsequently propagated to most other SLO including the spleen, implying dissemination via the blood and lymph (3, 34). Within the germinal centres, B cells have been shown to often acquire FDC surface proteins during cognate antigen capture (24). Furthermore, B cells can recirculate between lymphoid tissues for several weeks (21), and can transfer antigen reactivity from the draining lymph node to non-draining lymph nodes within a few days of immunisation (22). In the current study when B cell egress from SLO was specifically blocked, the dissemination of prions to non-draining lymph nodes and the spleen was likewise impeded. These data clearly demonstrate that B cells recirculating between lymphoid tissues via the blood and lymph are crucial for the transfer of prions between the draining lymph node and non-draining SLO.
How B cells may acquire prions is uncertain. Prion disease does not invoke a specific humoural response (57), and disease pathogenesis is unaffected in mice deficient in antibody Fc-γ receptors or circulating immuoglobulins (48). This suggests that cognate (antigen [prion]-specific) capture by B cells via their B cell receptors is highly unlikely. Prions are considered to be initially acquired by FDC as complement-bound complexes (47-49, 58). B cells have also been shown to acquire complement-opsonized antigens in a noncognate (antigen-independent) manner via their complement receptors and deliver them intact to FDC (59-60), implying a potential mechanism by which B cells may also acquire prions as they travel through the germinal centre. Indeed, prion pathogenesis is impaired in the specific absence of complement receptors on B cells (47).
Data from the current study and elsewhere show that B cells influence the prion disease pathogenesis in the periphery in distinct ways. B cells themselves are not themselves sites of prion replication in SLO (7, 46, 61). Data also suggest that prion-contaminated B cells do not play a key role in the transfer of prions directly to the nervous system (62). Instead, B cells indirectly influence prion replication in SLO by their provision of important maturation stimuli, in the form of lymphotoxins and TNFα, which maintain FDC in their differentiated state (63). Without these stimuli, FDC rapidly de-differentiate and prion accumulation in SLO is blocked (1, 53-54). Data in the current study adds to this by showing how the migratory nature of B cells mediates the initial transfer of prions between SLO.
Data suggest that the intrasplenic positioning of immature hematopoietic classical dendritic cells (DC, a distinct cell lineage from FDC (6)) in the spleen is also regulated by S1P signalling (64). Furthermore, treatment with FTY720 impaired the migration of classical DC from the skin to the draining lymph node (65). Although other effects of S1PR1 blockade on prion pathogenesis cannot be entirely excluded, previous data suggest potential effects on the positioning or migration of classical DC are unlikely to be the major influence. Prion disease pathogenesis was not affected when Langerhans cell migration out of the skin was blocked (35), and in the current study FTY720 treatment was not initiated until 14 days after infection, by which time the prions had been delivered to the draining lymph node and had begun to replicate upon the FDC with them (34). Furthermore, in the current study the propagation of prions between SLO was also blocked in mice with S1PR1-deficiency restricted to B cells.
The positioning of marginal zone B cells in the marginal zone of the spleen is essential for their ability to capture blood-borne antigens. Marginal zone B cells rapidly shuttle back and forth between the marginal zone and follicles, providing an efficient mechanism for systemic antigen capture and delivery to FDC (66). The expression of SRP1 receptors on marginal zone B cells is important for their positioning within the splenic marginal zone (Fig. 7) (28, 66-67). Accordingly, in mice treated with FTY720 or with S1PR1-deficient B cells the capture of blood-borne antigen by marginal zone B cells and their subsequent deposition on FDC are diminished. These data suggest that a possible role for S1PR1-signalling in the capture of prions by marginal zone B cells in the spleen and their delivery to FDC cannot be excluded. However, data in the current study suggest this is unlikely to be the major influence on disease pathogenesis since the dissemination of prions to the non-draining lymph nodes was also blocked in mice treated with FTY720 or with S1PR1-deficient B cells.
Chronic S1PR1 signalling-blockade had no significant effect on prion neuroinvasion since control and FTY720-treated mice all developed clinical prion disease at similar times after exposure. These data are consistent with the conclusion that B cells do not directly mediate the transfer of prions to the nervous system (62). The SLO are highly innervated with sympathetic neurones (68) and their depletion dramatically impairs prion neuroinvasion (11). Data in the current study are also consistent with peripheral nervous system being the major route of prion transfer to the CNS (11), and the demonstration that following peripheral exposure (orally or via skin lesions) neuroinvasion appears to initially occur directly from the draining lymphoid tissue following replication upon the FDC within them (3, 34, 36, 69).
In conclusion, data in the current study suggest a novel B cell-dependent mechanism by which prions are initially propagated between SLO via the blood and lymph after peripheral exposure. Host inflammation has been shown to significantly influence prion disease pathogenesis; either through enhancing prion uptake or expanding their tissue distribution (3, 70-72). This implies that active germinal centre responses, for example after immunization or in response to congruent infection with a pathogen, may likewise influence prion pathogenesis by enhancing the propagation of prions within the host by stimulating their dissemination by circulating B cells.
Acknowledgements
We thank Bob Fleming, Fraser Laing, Simon Cumming, Irene McConnell and Mary Brady and the Pathology Services Group (University of Edinburgh, UK) for excellent technical support.
Abbreviations used in this paper
- BSE
bovine spongiform encephalopathy
- FDC
follicular dendritic cell
- IHC
immunohistochemistry
- PET
paraffin-embedded tissue blot
- PK
proteinase K
- PrP
prion protein
- S1PR1
sphingosine 1-phosphate receptor 1
- SLO
secondary lymphoid organ
- vCJD
variant Creutzfeldt-Jakob disease
Footnotes
This work was supported by grant funding from the Medical Research Council (Grant no. G0700640) and by Institute Strategic Programme Grant funding from the Biotechnology and Biological Sciences Research Council (N.A.M), and in part, by the Intramural Research Program of the NIH, NIDDK (S.L.P.).
References
- 1.Mabbott NA, Young J, McConnell I, Bruce ME. Follicular dendritic cell dedifferentiation by treatment with an inhibitor of the lymphotoxin pathway dramatically reduces scrapie susceptibility. J. Virol. 2003;77:6845–6854. doi: 10.1128/JVI.77.12.6845-6854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prinz M, Huber G, Macpherson AJS, Heppner FL, Glatzel M, Eugster H-P, Wagner N, Aguzzi A. Oral prion infection requires normal numbers of Peyer’s patches but not of enteric lymphocytes. Am. J. Pathol. 2003;162:1103–1111. doi: 10.1016/S0002-9440(10)63907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaysher BR, Mabbott NA. Role of the GALT in scrapie agent neuroinvasion from the intestine. J. Immunol. 2007;178:3757–3766. doi: 10.4049/jimmunol.178.6.3757. [DOI] [PubMed] [Google Scholar]
- 4.Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen J-M, Lantier F. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 2000;81:3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 5.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J. Gen. Virol. 1999;80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 6.Mabbott NA, Bailie JK, Kobayashi A, Donaldson DS, Ohmori H, Yoon S-O, Freedman AS, Freeman TC, Summers KM. Expression of mesenchyme-specific gene signatures by follicular dendritic cells: insights from the meta-analysis of microarray data from multiple mouse cell populations. Immunology. 2011;133:482–498. doi: 10.1111/j.1365-2567.2011.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCulloch L, Brown KL, Bradford BM, Hopkins J, Bailey M, Rajewsky K, Manson JC, Mabbott NA. Follicular dendritic cell-specific prion protein (PrPC) expression alone is sufficient to sustain prion infection in the spleen. PLoS Pathogens. 2011;7:e1002402. doi: 10.1371/journal.ppat.1002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 9.Legname G, Baskakov IV, Nguyen H-OB, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 10.Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, MacKenzie C, Houston F. Transmission of prion diseases by blood transfusion. J. Gen. Virol. 2002;83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- 11.Glatzel M, Heppner FL, Albers KM, Aguzzi A. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron. 2001;31:25–34. doi: 10.1016/s0896-6273(01)00331-2. [DOI] [PubMed] [Google Scholar]
- 12.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 13.Llewelyn CA, Hewitt PE, Knight RSG, Amar K, Cousens S, Mackenzie J, Will RG. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 14.Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;354:527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 15.Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, Lineham JM, Brandner S, Wadsworth JDF, Hewitt P, Collinge J. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368:2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 16.Health PA. vCJD abnormal protein found in a patient with haemophilia at post mortem. 2009.
- 17.Mabbott NA, MacPherson GG. Prions and their lethal journey to the brain. Nature Rev. Microbiol. 2006;4:201–211. doi: 10.1038/nrmicro1346. [DOI] [PubMed] [Google Scholar]
- 18.Hill AF, Desbruslais M, Joiner S, Sidle KCL, Gowland I, Collinge J. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 19.Hill AF, Butterworth RJ, Joiner S, Jackson G, Rossor MN, Thomas DJ, Frosh A, Tolley N, Bell JE, Spencer M, King A, Al-Sarraj S, Ironside JW, Lantos PL, Collinge J. Investigation of variant Creutzfeldt-Jakob disease and other prion diseases with tonsil biopsy samples. Lancet. 1999;353:183–189. doi: 10.1016/s0140-6736(98)12075-5. [DOI] [PubMed] [Google Scholar]
- 20.Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlington DM. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J. Exp. Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner M, Gulbranson-Judge A, Quinn ME, Walters AE, MacLennan IC, Tybulewicz VLJ. Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J. Exp. Med. 1997;186:2013–2021. doi: 10.1084/jem.186.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baine Y, Thornbecke GJ. Induction and persistence of local B cell memory in mice. J. Immunol. 1982;128:639–643. [PubMed] [Google Scholar]
- 23.Finke D, Baribaud F, Diggelmann H, Acha-Orbea H. Extrafollicular plasmablast B cells play a key role in carrying retroviral infection to peripheral organs. J. Immunol. 2001;166:6266–6275. doi: 10.4049/jimmunol.166.10.6266. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J. Exp. Med. 2009;206:1485–1493. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dilosa RM, Maeda K, Masuda A, Szakal AK, Tew JG. Germinal center B cells and antibody production in the bone marrow. J. Immunol. 1991;146:4071–4077. [PubMed] [Google Scholar]
- 26.Mathiason CK, Hayes-Klug J, Hays SA, Powers J, Osborn DA, Dahmes SJ, Miller KV, Warren RJ, Mason GL, Telling GC, Young AJ, Hoover EA. B cells and platelets harbour prion infectivity in the blood of deer infected with chronic wasting disease. J. Virol. 2010;84:5097–5107. doi: 10.1128/JVI.02169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 28.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster J. Spingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 29.Sinha RK, Park C, Hwang I-Y, Davis MD, Kehrl JH. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity. 2009;30:1–13. doi: 10.1016/j.immuni.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham THM, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2009;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu Y-P, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J. Exp. Med. 2010;207:1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer M, Rulicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO Journal. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 33.Allende ML, Yamashita T, Proia RL. G-Protein-coupled receptor S1P1 acts with endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 34.Mohan J, Bruce ME, Mabbott NA. Follicular dendritic cell dedifferentiation reduces scrapie susceptibility following inoculation via the skin. Immunology. 2005;114:225–234. doi: 10.1111/j.1365-2567.2004.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohan J, Bruce ME, Mabbott NA. Neuroinvasion by scrapie following inoculation via the skin is independent of migratory Langerhans cells. J. Virol. 2005;79:1888–1897. doi: 10.1128/JVI.79.3.1888-1897.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glaysher BR, Mabbott NA. Role of the draining lymph node in scrapie agent transmission from the skin. Immunology Letters. 2007;109:64–71. doi: 10.1016/j.imlet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Fraser H, Dickinson AG. Agent-strain differences in the distribution and intensity of grey matter vacuolation. J. Comp. Pathol. 1973;83:29–40. doi: 10.1016/0021-9975(73)90024-8. [DOI] [PubMed] [Google Scholar]
- 38.Fraser H, Dickinson AG. The sequential development of the brain lesions of scrapie in three strains of mice. J. Comp. Pathol. 1968;78:301–311. doi: 10.1016/0021-9975(68)90006-6. [DOI] [PubMed] [Google Scholar]
- 39.Farquhar CF, Somerville RA, Ritchie LA. Post-mortem immunodiagnosis of scrapie and bovine spongiform encephalopathy. J. Virol. Methods. 1989;24:215–222. doi: 10.1016/0166-0934(89)90023-2. [DOI] [PubMed] [Google Scholar]
- 40.Schulz-Schaeffer WJ, Tschoke S, Kranefuss N, Drose W, Hause-Reitner D, Giese A, Groschup MH, Kretzschmar HA. The paraffin-embedded tissue blot detects PrPsc early in the incubation time in prion diseases. Am. J. Pathol. 2000;156:51–56. doi: 10.1016/S0002-9440(10)64705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadsworth JDF, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 42.Dragun D, Fritsche L, Boehler T, Peters H, Budde K, Neumayer HH. FTY720: Early clinical experience. Transplant. Proc. 2004;36(Suppl 2S):544S–548S. doi: 10.1016/j.transproceed.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 43.Wijkstrom M, Kenyon NS, Kirchoff N, Kenyon NM, Mullon C, Lake P, Cottens S, Ricordi C, Hering BJ. Islet allograft survival in nonhuman primates immunosuppressed with basiliximab, RAD, and FTY720. Transplantation. 2004;77:827–835. doi: 10.1097/01.tp.0000116390.76425.20. [DOI] [PubMed] [Google Scholar]
- 44.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discovery. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 45.Brown KL, Stewart K, Ritchie D, Mabbott NA, Williams A, Fraser H, Morrison WI, Bruce ME. Scrapie replication in lymphoid tissues depends on PrP-expressing follicular dendritic cells. Nat. Med. 1999;5:1308–1312. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 46.Klein MA, Frigg R, Raeber AJ, Flechsig E, Hegyi I, Zinkernagel RM, Weissmann C, Aguzzi A. PrP expression in B lymphocytes is not required for prion neuroinvasion. Nat. Med. 1998;4:1429–1433. doi: 10.1038/4022. [DOI] [PubMed] [Google Scholar]
- 47.Zabel MD, Heikenwalder M, Prinz M, Arrighi I, Schwarz P, Kranich J, von Teichman A, Haas KM, Zeller N, Tedder TF, Weiss JH, Aguzzi A. Stromal complement receptor CD21/35 facilitates lymphoid prion colonization and pathogenesis. J. Immunol. 2007;179:6144–6152. doi: 10.4049/jimmunol.179.9.6144. [DOI] [PubMed] [Google Scholar]
- 48.Klein MA, Kaeser PS, Schwarz P, Weyd H, Xenarios I, Zinkernagel RM, Carroll MC, Verbeek JS, Botto M, Walport MJ, Molina H, Kalinke U, Acha-Orbea H, Aguzzi A. Complement facilitates early prion pathogenesis. Nat. Med. 2001;7:488–492. doi: 10.1038/86567. [DOI] [PubMed] [Google Scholar]
- 49.Mabbott NA, Bruce ME, Botto M, Walport MJ, Pepys MB. Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nat. Med.e. 2001;7:485–487. doi: 10.1038/86562. [DOI] [PubMed] [Google Scholar]
- 50.Brown KL, Gossner A, Mok S, Mabbott NA. The effects of host age on the transport of complement-bound complexes to the spleen and the pathogenesis of intravenous scrapie infection. J. Virol. 2012;86:1228–1237. doi: 10.1128/JVI.05581-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin F, Kearney JF. Marginal-zone B cells. Nature Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 52.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 53.Mabbott NA, Mackay F, Minns F, Bruce ME. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat. Med. 2000;6:719–720. doi: 10.1038/77401. [DOI] [PubMed] [Google Scholar]
- 54.Montrasio F, Frigg R, Glatzel M, Klein MA, Mackay F, Aguzzi A, Weissmann C. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science. 2000;288:1257–1259. doi: 10.1126/science.288.5469.1257. [DOI] [PubMed] [Google Scholar]
- 55.Prinz M, Heikenwalder M, Junt T, Schwarz P, Glatzel M, Heppner FL, Fu Y-X, Lipp M, Aguzzi A. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature. 2003;425:957–962. doi: 10.1038/nature02072. [DOI] [PubMed] [Google Scholar]
- 56.Beekes M, McBride PA. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007;274:588–605. doi: 10.1111/j.1742-4658.2007.05631.x. [DOI] [PubMed] [Google Scholar]
- 57.Iken S, Bachy V, Gourdain P, Lim A, Gregoire S, Chaigneau T, Aucouturier P, Carnaud C. Th2-polarized PrP-specific transgenic T-cells confer partial protection against murine scrapie. PLoS Pathogens. 2011;7:e1002216. doi: 10.1371/journal.ppat.1002216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mabbott NA, Bruce ME. Complement component C5 is not involved in scrapie pathogenesis. Immunobiology. 2004;209:545–549. doi: 10.1016/j.imbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 60.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montrasio F, Cozzio A, Flechsig E, Rossi D, Klein MA, Rulicke T, Raeber AJ, Vosshenrich CAJ, Proft J, Aguzzi A, Weissmann C. B-lymphocyte-restricted expression of the prion protein does not enable prion replication in PrP knockout mice. Proc. Natl Acad. Sci. USA. 2001;98:4034–4037. doi: 10.1073/pnas.051609398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raymond CR, Mabbott NA. Assessing the involvement of migratory dendritic cells in the transfer of the scrapie agent from the immune to peripheral nervous systems. J. Neuroimmunol. 2007;187:114–125. doi: 10.1016/j.jneuroim.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Mackay F, Browning JL. Turning off follicular dendritic cells. Nature. 1998;395:26–27. doi: 10.1038/25630. [DOI] [PubMed] [Google Scholar]
- 64.Czeloth N, Bernhardt G, Hoffmann F, Genth H, Förster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 65.Lan YY, Tokita D, Wang Z, Wang HC, Zhan J, Brinkmann V, Thomson AW. Sphingosine 1-phosphate receptor agonism impairs skin dendritic cell migration and homing to secondary lymphoid tissue: association with prolonged allograft survival. Transpl. Immunol. 2008;20:88–94. doi: 10.1016/j.trim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vora KA, Nichols E, Porter G, Cui Y, Keohane CA, Hajdu R, Hale J, Neway W, Zaller D, Mandala S. Sphingosine 1-phosphate receptor agonist FTY720-phosphate causes marginal zone B cell displacement. J. Leukoc. Biol. 2005;78:471–480. doi: 10.1189/jlb.0904487. [DOI] [PubMed] [Google Scholar]
- 68.Felten SY, Felten DL. Psychoneuroimmunology. 2nd ed Academic Press Inc.; 1991. Innervation of Lymphoid Tissue; pp. 27–69. [Google Scholar]
- 69.Kujala P, Raymond C, Romeijn M, Godsave SF, van Kasteren SI, H. W, Prusiner SB, Mabbott NA, Peters PJ. Prion uptake in the gut: identification of the first uptake and replication sites. PLoS Pathogens. 2011:e1002449. doi: 10.1371/journal.ppat.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, Seifert B, Miele G, Aguzzi A. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 71.Heikenwalder M, Kurrer MO, Margalith I, Kranich J, Zeller N, Haybaeck J, Polymenidou M, Matter M, Bremer J, Jackson WS, Lindquist S, Sigurdson CJ, Aguzzi A. Lymphotoxin-dependent prion replication in inflammatory stromal cells of granulomas. Immunity. 2008;29:998–1008. doi: 10.1016/j.immuni.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 72.Sigurdson CJ, Heikenwalder M, Manco G, Barthel M, Schwarz P, Stecher B, Krautler NJ, Hardt W-D, Seifert B, MacPherson AJS, Corthesy I, Aguzzi A. Bacterial colitis increases susceptibility to oral prion pathogenesis. J. Infect. Dis. 2009;199:243–252. doi: 10.1086/595791. [DOI] [PMC free article] [PubMed] [Google Scholar]