Abstract
EMBO J 31 10, 2261–2274 (2012); published online April 13 2012
Chávez-Gutiérrez et al (2012) examine the effects of Alzheimer’s disease (AD) causing presenilin (PSEN) and amyloid β precursor protein (APP) mutations on intramembrane cleavage catalyzed by γ-secretase. These data provide definitive insights that should settle the long-standing debate regarding the role of loss of function effects of PSEN mutations in AD etiology. They also provide additional insights into the complexities of γ-secretase cleavage that may help to guide future therapeutic discovery efforts not only in AD but in other indications.
AD, the leading cause of dementia, is pathologically characterized by the presence of two proteinopathies, aggregates of the amyloid β (Aβ) protein in senile plaques and aggregates of the microtubule associated protein tau in neurofibrillary tangles. AD can be caused by mutations in APP and PSEN1 or PSEN2 genes. Over the last 20 years, a great deal of data has shown that the common feature of these AD-linked mutations is to alter metabolism of Aβ, which is proteolytically derived from APP by the sequential actions of the β- and γ-secretase. This altered metabolism promotes aggregation of the normally soluble Aβ peptide, resulting in accumulation of toxic Aβ assemblies (Hardy and Selkoe, 2002).
Alternatively, some have proposed that altered metabolism of Aβ is not a causal factor, and that mutation in PSEN cause AD through a loss of function mechanism that reduces γ-secretase activity leading to altered signaling and accumulation of substrates (Shen and Kelleher, 2007). Chávez-Gutiérrez et al (2012) largely settle this debate by carefully performing detailed kinetic and processivity analysis of how AD-causing PSEN 1 and APP mutants alter γ-secretase cleavage. These elegant and comprehensive studies should shift the debate away from how PSEN/γ-secretase contributes to AD to how to safely target PSEN/γ-secretase to treat or prevent AD.
γ-Secretase is an intramembrane cleaving protease complex consisting of four protein subunits. It contains PSEN1 or PSEN2 as a catalytic core and three essential accessory proteins (APH1, Nicastrin (NCT), and PEN2) that play roles in complex maturation and stabilization (Takasugi et al, 2003). It is an unusual protease as it cleaves within the transmembrane domain (TMD) of many different type 1 membrane protein substrates. In most cases, γ-secretase cleavage occurs following ectodomain shedding of the substrate resulting in secretion of a small fragment (e.g., Aβ from APP) and untethering of the intracellular domain (ICD) from the membrane. Although it is still debated whether the fragments secreted following γ-cleavage have normal physiologic functions, there is no doubt that the liberated ICD often have essential roles. The classic example of the importance of the ICD release is evident in the essential role of γ-secretase cleavage in mediating Notch signaling (Koo and Kopan, 2004).
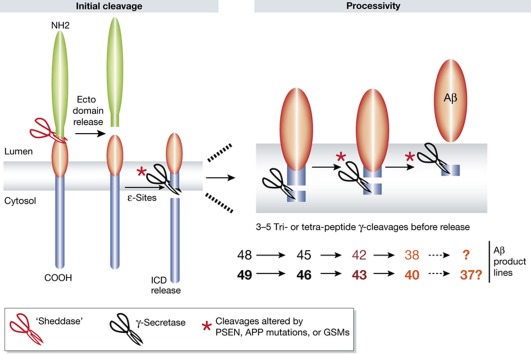
The current study builds on the model of γ-secretase cleavage proposed by Yasu Ihara and colleagues (Takami et al, 2009) (Figure 1). Cleavage begins at sites (termed the ε-site) near the cytoplasmic face of the substrate’s TMD followed by sequential cleavage of the TMD, via a series of tri- or tetra-peptide cleavages (γ-sites). Not only is the initial ε-cleavage site heterogeneous, but also the number of subsequent cycles, resulting in a range of final products. Current evidence indicates that the initial ε-cleavage site strongly influences subsequent cleavages, so that ‘product lines’ are established. Indeed, for APP, normal γ-secretase processing produces multiple Aβ peptides ranging from 42 (Aβ42) amino acids to less than 34 amino acids, with the major species typically being 40 amino acids (Aβ40). Depending on the initial ε-cleavage, the normal spectrum of Aβ peptides produced would necessitate either 3 sequential γ-cleavages to produce Aβ42, 4 cleavages to produce Aβ40 and 38, or even 5 cleavages to produce Aβ37, respectively.
Figure 1.
Schematic of the Ihara model of γ-secretase cleavage and effects of FAD-linked mutations or GSMs on Aβ production. Mutations in PSEN or APP alter both overall efficiency of the initial ε-cleavage as well as the ε-site (indicated by the asterisks). Two major ε-cleavages in APP produce two ‘product lines’ that influence subsequent cleavages, ‘processivity’. As the TMD is trimmed by the sequential cleavages, the substrate can either be further processed or released from the complex; this is indicated by the transition from black to red in the Aβ48/49 product lines. PSEN and APP mutants can alter the processivity resulting in release of increased Aβ42/43. GSMs are proposed to increase processivity resulting in less Aβ42 and more Aβ38.
Well over 100 mutations in PSEN, have been causally linked to autosomal dominant forms of familial AD (FAD). Initial data on FAD-linked PSEN mutants supports the notion that these mutations share a common pathological feature; they increased the relative production of Aβ42. As Aβ42 aggregates faster in vitro, is required for deposition in mouse models, and is typically the earliest and predominant species deposited in the AD brain, this data provided support for the Aβ aggregate (‘amyloid’) hypothesis of AD (Hardy and Selkoe, 2002; Younkin, 1998). As the net effect of both the PSEN and APP mutations was to increase the relative production of Aβ42, this finding coupled with the autosomal dominant mode of transmission resulted in many in the field stating that these mutations resulted in a toxic gain of function.
However, studies of the PSEN1 and 2 mutations suggested that some FAD-linked mutations did result in at least partial loss of function with respect to overall γ-secretase activity. Many also questioned how hundreds of different point mutations could result in the same gain of function. Furthermore elegant genetic studies in mice supported the notion that loss of PSEN function in the adult brain results in neurodegeneration. Collectivity, these data provided support for a presenilin loss of function-centric alternative to the amyloid hypothesis (reviewed in Ho and Shen, 2011).
Using in vitro assays to monitor the initial kinetics of γ-secretase cleavage at the ε-site and also the subsequent production of the various Aβ peptides, Chávez-Guitiérrez and colleagues demonstrate that six different PSEN1 and one PSEN2 FAD linked mutants variably effect the kinetics and site of the initial ε-cleavage but consistently altered the subsequent γ-cleavages resulting in an increase in the relative production of Aβ42/43. PSEN1 mutations also variably altered the kinetics of cleavage of different γ-secretase substrates; thus, overall efficiency of cleavage was often not decreased by PSEN mutation. Moreover, when similar studies were performed with wild-type PSEN1 and APP substrates with different FAD-linked mutations again variable effects on kinetics of initial cleavage were observed with consistent effects on altered production lines and increased release of longer Aβ peptides. Thus, these data strongly support the altered Aβ production hypothesis by demonstrating that different PSEN mutants alter γ-secretase cleavage by shifting the cleavage in complex and sometimes distinct fashions (Figure 1). Although the authors refer to these as qualitative differences, the differences are in fact quantitative; all of the cleavages are normal events they just appear to be shifted in various ways by PSEN and APP mutations. Notably, these studies are similar to a recent study by Quintero-Monzon et al, who also demonstrated that a panel of FAD-linked PSEN mutations showed large differences in overall activity and processivity (Quintero-Monzon et al, 2011).
These data, along with a large body of evidence from others, are difficult to reconcile with the alternative ‘loss of function’ hypothesis. At the biochemical level there is no consistent evidence that all FAD-linked mutations cause loss of catalytic function, but they all result in AD and they do increase relative levels of Aβ42. Genetic studies in model organisms and γ-secretase inhibitor studies showed that numerous phenotypes are associated with loss of γ-secretase function and primarily attributable to loss of Notch 1 signaling, including but not limited to gastrointestinal dysplasia, immune system dysfunction, and skin cancer. None of these features have been reported in humans with FAD-linked PSEN or APP mutations (Koo and Kopan, 2004). On the other hand true loss of function mutations in PSEN1, NCT and PEN2 that result in haploinsufficiency are linked to human acne inversa, a severe inflammatory disorder of the hair follicles (Wang et al, 2010).
In the final set of studies the authors extend their analyses to two classes of potential AD therapeutics that target γ-secretase: γ-secretase inhibitors (GSIs) and γ-secretase modulators (GSMs). The authors demonstrate that some GSIs preferentially inhibit Notch 1 and that previously reported APP selective inhibitors only show a marginal preference for APP versus Notch 1 inhibition. These data suggest that efforts to find APP selective inhibitors may be very challenging, and that differences in assays used to identify such ‘selective’ GSIs may result in misleading conclusions regarding selectivity. Data on GSMs suggest that they decrease the relative production of Aβ42 without altering overall γ-secretase activity by increasing the efficiency of the 4th cycle of γ-secretase cleavage, leading to more Aβ38. Although not a major feature of the manuscript the authors provide data suggesting that Aβ38 may be less effective at inhibiting Aβ42 aggregation than Aβ40. As different GSMs differentially shift γ-secretase cleavage to shorter Aβ peptides, such data suggest that different classes of GSMs may have subtle but important differences that could modulate their efficacy. Indeed, these data in the context of the Ihara model raise intriguing questions regarding processivity of γ-secretase cleavage that have important relevance to both pathogenesis of AD and development of GSMs. These include what controls the initial site of ε-cleavage and what controls processivity? Both of these factors influence the final product and both appear to be dissociable.
Though initially identified as a therapeutic target in Alzheimer’s (AD), γ-secretase has now been proposed to be a therapeutic target in various cancers, immunologic disorders, vasculitis, macular degeneration, diabetic nephropathy, ischemic reperfusion injury, traumatic brain injury, and fibrosis. There are many human trials testing the safety and efficacy of GSIs in cancer currently underway. Outside of the CNS, therapeutic inhibition of γ-secretase is most often associated with reduced Notch signaling; GSIs are often thought of in these settings as ‘Notch inhibitors’ (Rizzo et al, 2008). Because of this effort to repurpose GSIs, it is important not to disregard the potential consequences of loss of γ-secretase function. Prolonged, high-level, pharmacologic suppression of γ-secretase activity is almost certain to produce side-effects including negative effects on the CNS (but not induction of AD), as was seen in the halted Semagacestat trial. Thus, it will be critical to optimize therapeutic windows that maximize beneficial effects while limiting liabilities. Although this repurposing is moving rapidly, the current manuscript highlights how complex γ-secretase mediated cleavages can be even in a homogenous system. The biology underlying the repurposing efforts is likely to be highly complex; little is known about the exact composition of γ-secretase in the target cancers or organs and which substrates are mediating the beneficial biological effects of GSIs. Moreover, there has been insufficient comparison of the various GSIs with respect to inhibition of substrates besides Notch 1 and APP. We believe that it is important to consider the biological roles of other γ-substrates, be able to more easily monitor cleavage of some of these other substrates in vitro, ex vivo and in vivo, determine the inhibitory profile of various GSIs and account for the possible impact of heterogeneity of γ-secretase activity in different cells and tissues. The current manuscript and other recent studies highlight the importance of doing this correctly to insure that the optimal GSIs or other therapies targeting γ-secretase are evaluated for a given indication.
Footnotes
The authors declare that they have no conflict of interest.
References
- Chávez-Gutiérrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B (2012) The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J 31: 2261–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353–356 [DOI] [PubMed] [Google Scholar]
- Ho A, Shen J (2011) Presenilins in synaptic function and disease. Trends Mol Med 17: 617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Kopan R (2004) Potential role of presenilin-regulated signaling pathways in sporadic neurodegeneration. Nat Med 10: SupplS26–S33 [DOI] [PubMed] [Google Scholar]
- Quintero-Monzon O, Martin MM, Fernandez MA, Cappello CA, Krzysiak AJ, Osenkowski P, Wolfe MS (2011) Dissociation between the processivity and total activity of gamma-secretase: implications for the mechanism of Alzheimer's disease-causing presenilin mutations. Biochemistry 50: 9023–9035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L (2008) Rational targeting of Notch signaling in cancer. Oncogene 27: 5124–5131 [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ 3rd (2007) The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci USA 104: 403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y (2009) gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 29: 13042–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T (2003) The role of presenilin cofactors in the gamma-secretase complex. Nature 422: 438–441 [DOI] [PubMed] [Google Scholar]
- Wang B, Yang W, Wen W, Sun J, Su B, Liu B, Ma D, Lv D, Wen Y, Qu T, Chen M, Sun M, Shen Y, Zhang X (2010) Gamma-secretase gene mutations in familial acne inversa. Science 330: 1065. [DOI] [PubMed] [Google Scholar]
- Younkin SG (1998) The role of A beta 42 in Alzheimer's disease. J Physiol 92: 289–292 [DOI] [PubMed] [Google Scholar]