Abstract
Purpose
We examined the impact of hypofractionated radiation therapy and androgen suppression therapy (ast) on quality of life (qol) in high-risk prostate cancer patients.
Methods
Between March 2005 and March 2007, 60 patients with high-risk prostate cancer were enrolled in a prospective phase ii study. All patients received 68 Gy (2.72 Gy per fraction) to the prostate gland and 45 Gy (1.8 Gy per fraction) to the pelvic lymph nodes in 25 fractions over 5 weeks. Of the 60 patients, 58 received ast. The University of California–Los Angeles Prostate Cancer Index questionnaire was used to prospectively measure qol at baseline (month 0) and at 1, 6, 12, 18, 24, 30, and 36 months after radiation treatment. The generalized estimating equation approach was used to compare the qol scores at 1, 6, 12, 18, 24, 30, and 36 months with those at baseline.
Results
We observed a significant decrease in qol items related to bowel and sexual function. Several qol items related to bowel function were significantly adversely affected at both 1 and 6 months, with improvement toward 6 months. Although decreased qol scores persisted beyond the 6-month mark, they began to re-approach baseline at the 18- to 24-month mark. Most sexual function items were significantly adversely affected at both 1 and 6 months, but the effects were not considered to be a problem by most patients. A complete return to baseline was not observed for either bowel or sexual function. Urinary function items remained largely unaffected, with overall urinary function being the only item adversely affected at 6 months, but not at 1 month. Urinary function returned to baseline and remained unimpaired from 18 months onwards.
Conclusions
In our study population, who received hypofractionated radiation delivered using dynamic intensity-modulated radiotherapy with inclusion of the pelvic lymph nodes, and 2–3 years of ast prescription, qol with respect to bowel and sexual function was significantly affected; qol with respect to urinary function was largely unaffected. Our results are comparable to those in other published studies.
Keywords: Prostate cancer, quality of life, hypofractionation, imrt, toxicity, ast
1. INTRODUCTION
Prostate cancer is the leading cancer diagnosis in Canadian men, with 24,600 new cases diagnosed in 2010 1. It is also the third leading cancer-related cause of death, with an estimated 4300 cases in 2010 1. Although mortality has declined significantly in recent years, arguably because of earlier detection and improved treatment alike, prostate cancer remains the most prevalent cancer in men 1. By implication, then, an increasing number of survivors are living with the effects of cancer and its treatment, highlighting the importance of analyzing quality of life (qol) for new treatment regimens. Several studies have explored the relevance of treatment choice and qol to men being treated for prostate cancer 2–8.
Current treatment for high-risk prostate cancer usually consists of external-beam radiation therapy and long-term androgen suppression therapy (ast) 9,10. Studies using dose-escalated radiation 11–15 for prostate cancer have shown that higher radiation doses improve tumour control.
The linear quadratic model of exponential radiation cell killing has proved to be a robust theory that can be readily applied to clinical data. The linear quadratic cell survival equation includes the coefficients α and β (and their ratio α/β), which, according to the model, are mechanistically related to dna damage. Recent data suggest that the α/β ratio for prostate cancer is lower than had previously been assumed 16–19. That observation has encouraged the use of hypofractionated radiation schedules for the treatment of prostate cancer 20 to increase the biologic effect of delivered radiotherapy, while maintaining toxicity at an acceptable level. Published comparisons of conventional and hypofractionated radiation schedules for the treatment of prostate cancer indicate that recurrence-free survival is equal or improved with hypofractionation 21–23. Those studies also showed that the qol of prostate cancer patients is either similar or better with the hypofractionated schedules 24–30.
At our institution, we completed a phase ii prospective study for high-risk prostate cancer patients. All patients received a hypofractionated schedule to the prostate gland and a conventionally fractionated schedule to the pelvic lymph nodes using a dynamic intensity-modulated radiotherapy (imrt) technique with simultaneous integrated boost on a helical TomoTherapy Hi-Art system (Accuray, Sunnyvale, CA, U.S.A.). Most patients received variable-duration neoadjuvant, concomitant, and adjuvant ast. One of the study’s endpoints was to assess qol in those patients, and here, we report qol outcomes in patients treated using that protocol.
2. METHODS
2.1. Patient Selection
Between March 2005 and March 2007, the study enrolled 60 patients with a histologic diagnosis of prostate cancer involving localized disease with high-risk features [clinical stage T3a or higher, or initial prostate-specific antigen 20 ng/mL or higher, or Gleason score 8–10, or a combination of prostate-specific antigen greater than 15 ng/mL and Gleason score 7]. Staging was based on computed tomography (ct) imaging of the pelvis, a bone scan, and a standard clinical examination, including digital rectal examination.
2.2. Radiation Therapy Planning
The clinical target volumes (ctvs) and organs at risk were delineated on co-registered planning ct and 3-T magnetic resonance imaging images. The prostate gland and the proximal 1 cm of seminal vesicle were contoured to generate a volume designated as ctv68. The planning target volume, ptv68, was grown from the ctv68 by adding a 1-cm margin radially and a 5-mm margin posteriorly. The internal iliac, upper external iliac, and lower common iliac vessels were delineated on each slice up to the lower border of L5 and were encompassed within at least a 5-mm margin (avoiding bone, bladder, muscle, and mesorectum). The external iliac vessel contouring was stopped at the top of the femoral head. The obturator lymph node contouring was stopped at the beginning of the obturator foramen. The upper external iliac vessel delineation also included the lateral presacral nodal area. However, the medial portion of the presacral nodal area was not included in the lymph node delineation 31. These lymph node delineations are similar to those of the pelvic lymph nodes shown at the Radiation Therapy Oncology Group Web site 32, except for the exclusion of the medial portion of the presacral area. The pelvic lymph nodes and the distal seminal vesicles (beyond the proximal 1 cm) were included in the ctv45. The volume ptv45 was grown from the ctv45 by adding uniform 1-cm margins all around. Organs at risk, including the rectum from the anal canal to the rectosigmoid junction, the full volume of the bladder, the femora from head to the ischial tuberosities and peritoneal cavity, including all potential areas of small bowel and large bowel, were also drawn using ct images.
A single-phase inverse treatment plan was generated using helical tomotherapy. A dose of 68 Gy in 25 fractions (2.72 Gy per fraction) was prescribed to 95% of the ptv68, and a dose of 45 Gy in 25 fractions (1.8 Gy per fraction) to the ptv45 using a simultaneous integrated boost technique. Dose constraints of 55 Gy or less and 60 Gy or less to 50% and 30% respectively of the volume of rectum, and 60 Gy or less and 65 Gy or less to 50% and 30% respectively of the volume of bladder were used. Maximum dose to the peritoneal cavity was limited to 54 Gy or less. All patients underwent daily megavoltage ct image-guided verification before each treatment.
2.3. Study Design
Patients completed qol questionnaires before commencing radiation treatment (baseline) and at 1, 6, 12, 18, 24, 30, and 36 months after completion of radiation treatment. The questionnaires were self-administered by the patients on their own time with no assistance from clinical staff.
2.4. QOL Assessment
The qol assessments were obtained based on the University of California–Los Angeles Prostate Cancer Index questionnaire 27. The questionnaire consists of 18 items in 3 sections: urinary function (5 items), bowel function (5 items), and sexual function (8 items). Patient completion of the qol forms was optional at all time points.
2.5. Statistical Analysis
Each of the items on the qol questionnaire was assigned a nominal score of 1–6, depending on the number of possible responses available for that item. These nominal scores were converted to continuous scores to obtain a composite of all the scores. A poor nominal score was assigned a low continuous score and vice versa. The higher the continuous score, the better the qol, and vice versa. Table i explains the assignment of the continuous scores to the corresponding nominal scores of the Prostate Cancer Index (smaller numbers represent a poorer qol and higher numbers represent a better qol).
TABLE I.
Continuous scores assigned to the corresponding nominal scores 1–6 of the Prostrate Cancer Index, depending on the number of available responses per questiona
| Nominal variables | ||||||
|---|---|---|---|---|---|---|
| Categories | 1 | 2 | 3 | 4 | 5 | 6 |
| 3 | 33 | 66 | 100 | — | — | — |
| 4 | 25 | 50 | 75 | 100 | — | — |
| 5 | 20 | 40 | 60 | 80 | 100 | — |
| 6 | 17 | 34 | 51 | 68 | 83 | 100 |
Smaller numbers represent poorer quality of life, and higher numbers, better quality of life.
Means and standard errors were calculated for all 18 items of the Prostate Cancer Index at baseline and at 1 and 6 months after radiation therapy (Table ii). The 1- and 6-month follow-up was based on the scoring system described (Table i). The qol scores for each of the 18 items for each follow-up point (1, 6, 12, 18, 24, 30, and 36 months) were compared with the baseline scores using the generalized estimating equation approach 33. That approach accounts for the within-subject correlations arising because of repeated measurements of the same individual. It provides robust parameter estimates. Standard errors were then obtained (statistical analysis for repeated measurements). All statistical analyses were conducted using SAS (version 9.1.3: SAS Institute, Cary, NC, U.S.A.). Values of p < 0.05 were considered to be statistically significant.
TABLE II.
Mean score (± standard error) for each item on the Prostate Cancer Index quality-of-life (qol) questionnaire at baseline, 1 month, and 6 months
| qol item | Baseline | 1 Month | 6 Months |
|---|---|---|---|
| Urinary function | |||
| Leakage | 87±3.4 | 86±3.5 | 93±2.3 |
| Control | 91±2.3 | 90±1.9 | 94±1.6 |
| Number of pads or adult diapers | 99±0.6 | 97±1.6 | 100±0.0 |
| Dripping urine or wetting pants | 93±2.5 | 92±2.2 | 96±1.3 |
| Leakage interfering with sexual activity | 98±1.7 | 94±2.9 | 100±0.0 |
| As a problem overall | 39±3.2 | 42±2.9 | 32±2.7 |
| Bowel function | |||
| Rectal urgency | 95±2.5 | 73±4.0 | 82±4.2 |
| Stools loose or liquid | 82±2.3 | 73±2.6 | 77±2.8 |
| Distress secondary to bowl movements | 93±1.7 | 79±2.8 | 83±3.2 |
| Crampy pain | 93±2.1 | 85±3.1 | 90±3.1 |
| As a problem overall | 88±2.5 | 77±3.1 | 82±3.4 |
| Sexual function | |||
| Sexual desire | 34±3.0 | 27±2.3 | 27±2.3 |
| Ability to have an erection | 35±2.7 | 24±2.6 | 24±1.5 |
| Ability to reach orgasm | 35±2.9 | 28±2.8 | 25±1.7 |
| Quality of erections | 52±4.5 | 40±3.7 | 39±2.8 |
| Frequency of erections | 38±3.3 | 28±2.5 | 26±2.4 |
| Morning erections | 33±2.4 | 27±2.0 | 25±1.7 |
| Sexual intercourse | |||
| Unassisted by medical intervention | 43±3.2 | 38±2.4 | 37±2.0 |
| Assisted by medical intervention | 98±1.2 | 98±1.1 | 98±1.1 |
| Ability to function sexually | 31±2.5 | 25±1.8 | 24±1.6 |
| As a problem overall | 57±4.8 | 58±5.1 | 52±5.1 |
3. RESULTS
The mean age at enrollment was 68 years (range: 55–88 years), and all 60 patients completed the 36-month follow-up. In the analyses, 3 patients are excluded because, for administrative reasons, they were not approached to complete the qol questionnaire at baseline. Of the 60 patients, 58 received ast (leuprolide 22.5 mg subcutaneously every 3 months), prescribed for a total duration of 2–3 years. The baseline qol questionnaire was completed by 50 patients after they started ast (median time on therapy: 48 days) and by 7 patients before they started ast. Completion of the sexual function portion of the questionnaire was refused by 9 patients. On surveys completed by other patients, some questions went unanswered, mainly in the sexual function section, for unknown personal reasons. The missing responses were excluded from the statistical analysis for the applicable time points.
Table ii shows the means and standard errors for each questionnaire item score at baseline, 1 month, and 6 months. The qol scores for 1, 6, 12, 18, 24, 30, and 36 months were compared with the baseline score, and the results are summarized in Table iii. Significant and highly significant differences are noted. The differences between time points were deemed statistically significant if the p value was less than 0.05 and highly significant if the p value was less than 0.001.
TABLE III.
Parameter estimates and standard errors obtained from the quality-of-life (qol) scores using the generalized estimating equation approach (statistical analysis for repeated measurements)
| qol item |
Results by months since the end of radiation treatmenta |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 12 | 18 | 24 | 30 | 36 | ||||||||
| β | p Value | β | p Value | β | p Value | β | p Value | β | p Value | β | p Value | β | p Value | |
| Urinary function | ||||||||||||||
| Leakage | −0.35 | ns | 4.35 | ns | −3.42 | ns | −4.44 | ns | −3.62 | ns | −4.24 | ns | −1.24 | ns |
| Control | −0.40 | ns | 2.20 | ns | −3.26 | ns | −2.01 | ns | −0.02 | ns | −0.98 | ns | −1.61 | ns |
| Number of pads or adult diapers | −2.55 | ns | 0.64 | ns | −2.31 | ns | −2.98 | sd | −2.68 | ns | −2.17 | ns | −3.27 | ns |
| Dripping urine or wetting pants | 0.77 | ns | 2.99 | ns | −1.63 | ns | −2.83 | ns | −0.66 | ns | −1.35 | ns | −3.28 | ns |
| Leakage interfering with sexual activity | −2.25 | ns | 2.29 | ns | 0.71 | ns | −0.83 | ns | −1.78 | ns | −0.90 | ns | 1.50 | ns |
| As a problem overall | 4.56 | ns | −6.89 | sd | −0.80 | ns | −6.54 | ns | −6.76 | ns | −7.28 | ns | −3.54 | ns |
| Bowel function | ||||||||||||||
| Rectal urgency | −20.67 | hsd | −11.78 | sd | −17.92 | hsd | −8.55 | sd | −7.86 | sd | −12.01 | sd | −11.69 | sd |
| Stools loose or liquid | −9.67 | hsd | −6.54 | sd | −6.53 | sd | −0.10 | ns | 0.34 | ns | −2.98 | ns | −6.05 | ns |
| Distress secondary to bowl movements | −13.32 | hsd | −10.73 | hsd | −12.96 | hsd | −13.24 | hsd | −7.10 | sd | −11.35 | hsd | −11.42 | hsd |
| Crampy pain | −6.77 | hsd | −5.98 | sd | −6.34 | sd | −8.82 | sd | −2.82 | ns | −3.66 | ns | −5.97 | sd |
| As a problem overall | −10.86 | hsd | −7.99 | sd | −12.99 | sd | −8.51 | sd | −3.39 | ns | −8.50 | sd | −8.72 | sd |
| Sexual function | ||||||||||||||
| Sexual desire | −7.50 | hsd | −7.18 | sd | −6.56 | sd | −6.26 | sd | −1.75 | ns | −6.28 | ns | 1.33 | ns |
| Ability to have an erection | −6.19 | sd | −11.04 | hsd | −9.26 | hsd | −10.65 | hsd | −6.86 | sd | −9.22 | sd | −6.70 | ns |
| Ability to reach orgasm | −7.86 | sd | −10.83 | hsd | −9.85 | hsd | −10.28 | hsd | −7.18 | sd | −8.71 | ns | −5.52 | ns |
| Quality of erections | −11.71 | sd | −14.80 | hsd | −16.19 | hsd | −20.88 | hsd | −19.32 | hsd | −19.98 | hsd | −13.32 | sd |
| Frequency of erections | −10.20 | sd | −12.28 | hsd | −14.08 | hsd | −15.61 | hsd | −12.16 | sd | −11.30 | sd | −10.22 | sd |
| Morning erections | −6.84 | sd | −9.24 | hsd | −7.96 | sd | −6.97 | sd | −6.73 | sd | −7.56 | sd | −5.65 | sd |
| Sexual intercourse | ||||||||||||||
| Unassisted by medical intervention | −4.80 | ns | −5.97 | sd | −8.64 | sd | −7.96 | sd | −8.01 | sd | −7.86 | sd | −4.11 | ns |
| Assisted by medical intervention | 0.73 | ns | −0.16 | ns | 0.04 | ns | 0.65 | ns | −0.31 | ns | 0.54 | ns | 0.78 | ns |
| Ability to function sexually | −6.90 | sd | −7.24 | sd | −6.78 | sd | −7.82 | sd | −5.20 | ns | −6.71 | sd | −5.54 | ns |
| As a problem overall | 1.18 | ns | −2.48 | ns | −2.78 | ns | 6.66 | ns | 6.27 | ns | 4.95 | ns | 5.32 | ns |
A negative β indicates worsening quality of life.
ns = nonsignificant; sd = significant difference (p < 0.05); hsd = highly significant difference (p < 0.001).
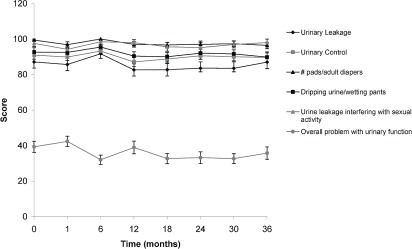
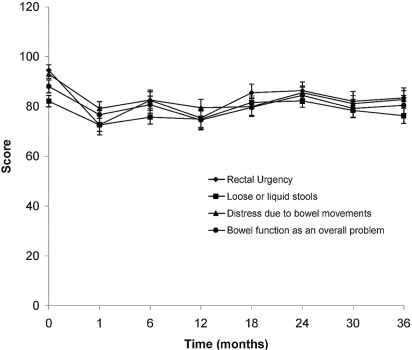
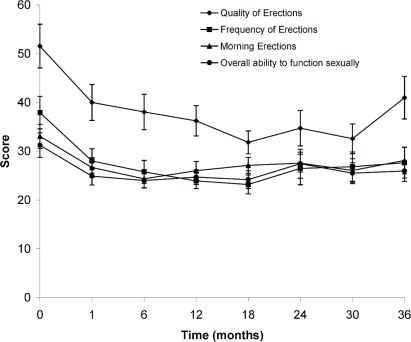
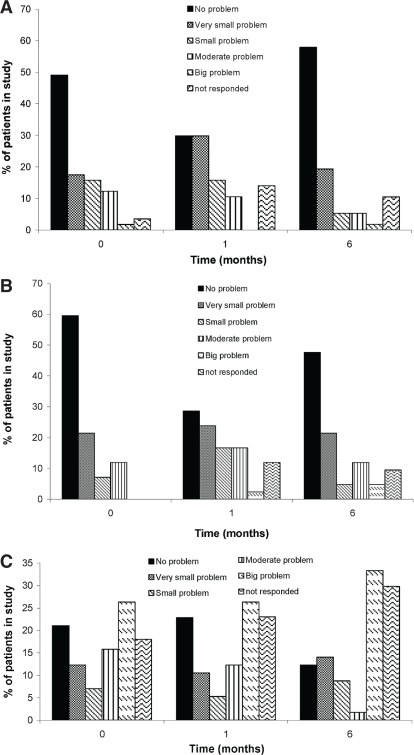
As shown in Figures 1 and 2, statistically significant trends toward a worse qol were observed for bowel and sexual function. Urinary function remained largely unaffected except for “urinary function as a problem overall” (Figure 3). That item in the questionnaire showed an initial improvement in qol at 1 month, with a subsequent decline at 6 months, and then a return to baseline leading up to 36 months. Figure 4 shows the percentage of patients choosing each available response on the qol questionnaire at 1 and 6 months.
FIGURE 1.
Statistically significant scores obtained in the gastrointestinal function portion of the quality-of-life questionnaire at baseline (0 months) and at 1, 6, 12, 18, 24, 30, and 36 months after completion of radiation treatment.
FIGURE 2.
Statistically significant scores obtained in the sexual function portion of the quality-of-life questionnaire at baseline (0 months) and at 1, 6, 12, 18, 24, 30, and 36 months after completion of radiation treatment.
FIGURE 3.
Statistically significant scores obtained in the urinary function portion of the quality-of-life questionnaire at baseline (0 months) and at 1, 6, 12, 18, 24, 30, and 36 months after completion of radiation treatment.
FIGURE 4.
The percentage of patients giving the indicated responses for (A) overall urinary function, (B) overall bowel function, and (C) overall sexual function at baseline (0 months) and at 1, 6, 12, 18, 24, 30, and 36 months after completion of radiation treatment.
Statistically significant differences in the scores were observed at 1, 6, 12, and 18 months for items concerning rectal urgency, loose bowel movements, and distress caused by bowel movements. Distress secondary to bowel movements remained significantly affected for the remainder of the follow-up period, as did bowel function as a problem overall.
In terms of sexual function, significant differences were observed for most items and for overall ability to function sexually, but not for the perception of sexual function as a problem overall. The adverse effect on qol for most items persisted for the remainder of the follow-up period.
4. DISCUSSION
To our knowledge, no published reports describe qol in high-risk prostate cancer patients when all patients received treatment to the pelvic lymph nodes using imrt delivery with a hypofractionated schedule and ast (hormonal treatment).
In our study, use of a hypofractionated schedule resulted in a significant decline in early post-treatment qol, largely in terms of bowel and sexual function. Urinary function was mostly unaffected, although qol increased in terms of urinary function at 1 month, and subsequently declined at 6 months. That decline recovered by 12 months and remained close to baseline thereafter (Figure 3). The general trend for most bowel and sexual function qol items (Figures 1 and 2) showed a lower score at 1 month, with bowel function improving somewhat toward baseline during subsequent follow-up, but with sexual function remaining lower in terms of quality and frequency of erections. These lower scores did not affect the patients’ perceptions of their ability to function sexually, which remain constant up to the 36-month follow-up.
No significant difference was observed in terms of urine leakage, urinary control, or the number of pads or adult diapers used. The calculated scores reveal that dripping urine, wetting pants, or urine leakage interfering with sexual activity did not affect qol (Figure 3). Overall statistical comparisons of scores at baseline with those at 1 month and 6 months show that more patients experienced decreased urinary function overall at 6 months (Table ii). The improvement in qol at 1 month was not statistically significant, but the decline at 6 months was. That finding likely represents not only the subjective nature of qol questionnaires, but also the changing attitudes of patients towards their qol with time. Patients might adjust to their new level of functioning after the 6-month mark and thus might be less likely to rate their overall function as poorly as before. The usual adverse effects of radiation treatment (increased frequency, nocturia, burning sensation, urgency, and slow urinary streams) are not separately listed in the questionnaire, which might be the reason for worsening urinary function overall without individual functions being affected. The urinary function questions were later included in a modified and newly validated expanded Prostate Cancer Index Composite questionnaire. The percentage of respondents who maintained that they had no difficulties with urinary function at baseline (49%) declined to 30% at 1 month, with patients distributing more into the “small problem” category. Still, fewer than 2% reported a “big problem” with urinary function at 6 months [Figure 4(A)]. Our data therefore show some urinary function impairment, but without necessarily affecting the patient’s qol.
A significant decline in qol was observed in terms of bowel function as it related to rectal urgency, loose bowel movements, distress with bowel movements, and bowel function as an overall problem. Worsening qol scores were observed at both 1 and 6 months compared with baseline (Table ii). The decrease in qol was less at 6 months than at 1 month. These bowel function qol items were still potentially below baseline at 6 months (Figure 1). Crampy pain showed a lower score (worse qol) at 1 month, with some improvement at 6 months (Figure 1). The results at 6 months were, however, not statistically significant (Table ii). Although lower qol scores persisted beyond the 6-month mark, especially for items such as distress caused by bowel movements, other items such as “stools loose or liquid,” crampy pain, and overall bowel function begin to approach baseline toward the 18- to 24-month mark. Whether that finding can be attributed to the patient’s ability to accommodate to a new level of function or to a true improvement in qol is difficult to ascertain.
Hanlon et al. 34 compared qol in patients receiving pelvic lymph node treatment with qol in those receiving prostate-only treatment. The results show a decrease in qol related to bowel function in patients with treated nodes. Our results, in patients who also received treatments to the pelvic lymph nodes, are similar. We delivered hypofractionated schedules using an imrt technique; Hanlon and colleagues delivered a conventional schedule using a 3-dimensional conformal radiotherapy technique.
Significantly decreased qol scores were observed for qol items dealing with ability, quality, frequency, and occurrence of morning erections, and also with the ability to function sexually. That finding is not surprising in a cohort of patients receiving continuous ast. Many patients (n = 50) had already received ast for a median duration of 48 days before they completed the baseline qol questionnaire, which may account for low scores at baseline. Of the 57 patients who were analyzed, 49 completed all items on the sexual function section of the questionnaire. That number declined to 36 by 36 months of follow-up and is reflected in the large standard deviations observed (Figure 2). The problem of obtaining responses to sexual function questions is reflected in a number of studies that analyzed sexual function 24,25,28. That problem is compounded by the fact that the qol questionnaire was optional.
Overall sexual function declined at 1 month and remained at the same level at 6 months. As an overall problem, sexual function was not significantly affected at either time point; however, the ability to function sexually was significantly decreased at 1 month and remained so at 6 months (Table ii) with no improvement toward baseline (Figure 2). There was no significant difference in the patient’s perception of lower sexual function being a problem. That observation is reflected in the fact that, although 73% of patients rated their ability to function sexually at baseline as “very poor” or “poor,” only 26% perceived that score as a big problem [Figure 4(C)]. At 36 months after treatment, the statistics remained about the same. Scores with respect to quality, frequency, and ability to achieve morning erections remained significantly lower at 36 months. The same trend is reflected in data from Namiki et al. 27 and Junius et al. 25. Thus, a large number of our patients did not perceive sexual function to be a problem despite significant impairment as discussed by Katz et al. 35.
The sexual function results may reflect the mean age of the patients included in the study (68 years). Men in that age group are both less likely to feel comfortable answering the questions and also more likely to experience sexual dysfunction secondary to age and comorbid medical conditions. According to Smith et al. 36 and Lindau et al. 37, only 37%–41% of men around the age of 70 are sexually active. Patients in our study may have felt confused at having to answer sexual function questions and may have perceived answering such questions as irrelevant to them. Most patients (79%) were not using any assistance (injection, vacuum pump, or phosphodiesterase type 5 inhibitor) to facilitate intercourse at baseline; that situation did not change over the course of treatment. Nearly all the patients in our study (58 of 60) were receiving ast; in a number of other studies, only some of the patients received ast 24,25,27. As previously documented, ast represents an independent risk factor for erectile dysfunction 2,38. In view of the use of ast and the hypofractionated radiation therapy schedule, the rates of erectile dysfunction over time will be interesting to observe and compare with those from existing studies 39,40.
Other studies have contemplated comparing imrt with either conformal radiation therapy or external-beam radiation therapy 24,26,27 (Table iv). The patient characteristics, the use of concurrent ast, and the dose to the prostate may vary, but overall, the studies show an equally decreased qol after both types of treatment, with improvement in some qol areas if the patient had undergone imrt. Lips et al. 24 showed better qol in a few domains (urinary symptoms and pain). Kupelian et al. 26 showed no difference in qol between imrt (78 Gy) and external-beam radiation therapy (69.6 Gy). Namiki et al. 27 showed no difference in urinary function, but worse bowel and sexual function with conventional external-beam radiation therapy. Junius et al. 25 did not compare methods, but using imrt (66 Gy to the prostate), they showed increased urinary symptoms at 1 month, with subsequent resolution at 6 months.
TABLE IV.
Comparison of our quality-of-life (qol) results with those in other published studies
| Reference (study period) | rt technique | Pts (n) | Filled qol (n) | Receivedast(n) | Dose per fraction (Gy) | Total dose (Gy) | Pelvicrt | Assessment type and time point (months) | qolquestionnaire type | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Kupelian et al., 2001 26 (1998–1999) |
imrt crt |
51 46 |
24 46 |
5 35 |
2.5 2.0 |
70 78 |
No | Cross-sectional Median: 24–27 (Range: 21–33) |
epic | No significant qol difference between the groups in terms of bowel, bladder, and sexual function |
| Namiki et al., 2006 27 (2000–2002) |
imrt xrt cort |
nr | 30 76 34 |
18 102 |
nr | 78 69.6 69.6 |
No | Longitudinal 0, 3, 6, 12, 18, 24 |
ucla pci SF-36 |
No difference in urinary function between groups at any time point. At 3 and 6 months, bowel function worse in xrt group than imrt group. At 3 months, sexual function lower after xrt, remaining substantially lower than baseline. No significant difference in sexual function from baseline to any other time point in the imrt group. At 18 months, sexual function better in imrt group than in xrt group. |
| Junius et al., 2007 25 (2002–2006) | imrt | 38 | 38 | 31 | 2.64 | 66 | No | Longitudinal 0, 1, 6, 12, 24, 36 |
qlq-C30 qlq-PR25 |
Urinary symptom scores reach peak at 1 month, normalized at 6 months, and stay stabilized afterward. Bowel symptom scores remain similar to baseline at 1 and 6 months, but worsen slowly at 1, 2, and 3 years. Sexual symptom score reaches nadir between 1 and 6 months, but improves between 2 and 3 years. |
| Lips et al., 2007 24 (1997–2004) |
imrt crt |
116 99 |
92 78 |
24 9 |
2.17 2 |
76 70 |
No | Longitudinal 0, 1, 6 |
qlq-C30 qlq-PR25 rand 36 |
No significant difference in qol between the imrt and 3dcrt groups. Sexual activity lower after treatment in both groups and remained lower after 6 months. Only 30 of 92 patients (33%) in imrt group and 28 of 78 patients (36%) in the crt group completed the sexual questionnaire. |
| Hanlon et al., 2001 34 (1992–1995) | 3dcrt | 95 100 |
66 73 |
None | 2.1 | 73 76 |
No Yes |
Cross sectional Median: 53–54 (Range: 35–71) |
aua spi bphii Author’s own |
Significant deterioration of bowel function in the group treated with pelvic rt compared with prostate rt only. Bladder and bowel function in general were comparable to a general population of similar age. |
| Pervez et al. (current study) (2005–2007) | imrt | 60 | 57 | 55 | 2.72 | 68 | Yes | Longitudinal 0, 1, 6 |
ucla pci | Urinary function items remained largely unaffected; urinary function overall was the only item adversely affected at 6 months, but not at 1 month. Bowel function was significantly adversely affected at both 1 and 6 months, with some improvement toward baseline at 6 months. Sexual function was significantly affected at baseline and 1 month, with no improvement observed at 6 months. Sexual function was not considered to be a problem by most patients. |
rt = radiation therapy; Pts = patients; ast = androgen suppression therapy; imrt = intensity-modulated radiotherapy; epic = Expanded Prostate Cancer Index Composite; xrt = external-beam radiotherapy; nr = not reported; ucla pci = University of California–Los Angeles Prostate Cancer Index; SF-36 = Medical Outcomes Study Short Form 36; cort = conventional radiotherapy; qlq-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire core module; qlq-PR25 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire prostate cancer module; crt = conformal radiotherapy; rand 36 = rand-36 Measure of Health-Related Quality of Life; 3dcrt = 3-dimensional conformal radiation therapy; aua = American Urological Association; spi = Symptom Problem Index; bphii = Benign Prostatic Hyperplasia Impact Index.
Although our questionnaire does not include the psychosocial categories present in other questionnaires, the responses from patients largely reflect the trends seen in other published data on the subject 24–29.
One limitation of the present study is the questionnaire, which was chosen at a time before recently validated questionnaires such as the Prostate Cancer Index Composite were available. It therefore fails to cover some of the psychosocial domains and irritable urinary symptoms included in other studies. But we have some concerns about increasingly long comprehensive questionnaires, in that patients might be less likely to complete them unless they are made mandatory. Another limitation is that many patients started ast before baseline qol was obtained, which may be why sexual function was scored low at baseline.
5. CONCLUSIONS
In our study population, in whom hypofractionated radiation was delivered using dynamic imrt (helical tomotherapy) with inclusion of the pelvic lymph nodes and with prescription of 2–3 years of ast, qol was significantly affected in terms of bowel and sexual function. Individual urinary functions were unaffected, but urinary function as a problem overall declined. Bowel function qol improved toward baseline with time, but sexual function did not improve. Those results are comparable to results from published studies in which hypofractionated schedules were used to treat the prostate only (no pelvic radiation) and in which conventional schedules were used to deliver treatment both to the pelvic lymph nodes and to the prostate. Further studies looking at the long-term effects on qol of treatment with hypofractionated schedules are needed and are in progress.
6. ACKNOWLEDGMENTS
The authors thank the Alberta Cancer Board for bridge and pilot funding, and Juliette Jordan and Michelle Encarnacao for data collection. All physician and physicist authors participated in study. Nadeem Pervez designed the study with assistance from Robert Pearcey. Sunita Ghosh analyzed the data. All authors helped to interpret the findings. Andrea Oprea and Nadeem Pervez wrote the manuscript, which was modified and approved by all authors.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to disclose.
8. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee . Canadian Cancer Statistics 2010. Toronto, ON: Canadian Cancer Society; 2010. [Google Scholar]
- 2.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 3.Penson DF. Quality of life after therapy for localized prostate cancer. Cancer J. 2007;13:318–26. doi: 10.1097/PPO.0b013e3181570121. [DOI] [PubMed] [Google Scholar]
- 4.Schulman C. Assessing the attitudes to prostate cancer treatment among European male patients. BJU Int. 2007;100(suppl 1):6–11. doi: 10.1111/j.1464-410X.2007.6976.x. [DOI] [PubMed] [Google Scholar]
- 5.Diefenbach MA, Mohamed NE. Regret of treatment decision and its association with disease-specific quality of life following prostate cancer treatment. Cancer Invest. 2007;25:449–57. doi: 10.1080/07357900701359460. [DOI] [PubMed] [Google Scholar]
- 6.Frank SJ, Pisters LL, Davis J, Lee AK, Bassett R, Kuban DA. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol. 2007;177:2151–6. doi: 10.1016/j.juro.2007.01.134. [DOI] [PubMed] [Google Scholar]
- 7.Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239–47. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 8.Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term follow-up. J Urol. 2010;183:2206–12. doi: 10.1016/j.juro.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an eortc study): a phase iii randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/S0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 10.Hanks GE, Pajak TF, Porter A, et al. Phase iii trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–8. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–101. doi: 10.1016/S0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 12.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the MD Anderson phase iii randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–105. doi: 10.1016/S0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 13.Storey MR, Pollack A, Zagars G, Smith L, Antolak J, Rosen I. Complications from radiotherapy dose escalation in prostate cancer: preliminary results of a randomized trial. Int J Radiat Oncol Biol Phys. 2000;48:635–42. doi: 10.1016/S0360-3016(00)00700-8. [DOI] [PubMed] [Google Scholar]
- 14.Eade TN, Hanlon AL, Horwitz EM, Buyyounouski MK, Hanks GE, Pollack A. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68:682–9. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Symon Z, Griffith KA, McLaughlin PW, Sullivan M, Sandler HM. Dose escalation for localized prostate cancer: substantial benefit observed with 3D conformal therapy. Int J Radiat Oncol Biol Phys. 2003;57:384–90. doi: 10.1016/S0360-3016(03)00569-8. [DOI] [PubMed] [Google Scholar]
- 16.Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50:1021–31. doi: 10.1016/S0360-3016(01)01607-8. [DOI] [PubMed] [Google Scholar]
- 17.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13. doi: 10.1016/S0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 18.Bentzen SM, Ritter MA. The alpha/beta ratio for prostate cancer: what is it, really? Radiother Oncol. 2005;76:1–3. doi: 10.1016/j.radonc.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Williams SG, Taylor JM, Liu N, et al. Use of individual fraction size data from 3756 patients to directly determine the alpha/beta ratio of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:24–33. doi: 10.1016/j.ijrobp.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 20.Amer AM, Mott J, Mackay RI, et al. Prediction of the benefits from dose-escalated hypofractionated intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2003;56:199–207. doi: 10.1016/S0360-3016(03)00086-5. [DOI] [PubMed] [Google Scholar]
- 21.Kupelian PA, Thakkar VV, Khuntia D, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: long-term outcomes. Int J Radiat Oncol Biol Phys. 2005;63:1463–8. doi: 10.1016/j.ijrobp.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 22.Kupelian PA, Willoughby TR, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;68:1424–30. doi: 10.1016/j.ijrobp.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 23.Yeoh EE, Holloway RH, Fraser RJ, et al. Hypofractionated versus conventionally fractionated radiation therapy for prostate carcinoma: updated results of a phase iii randomized trial. Int J Radiat Oncol Biol Phys. 2006;66:1072–83. doi: 10.1016/j.ijrobp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Lips I, Dehnad H, Kruger AB, et al. Health-related quality of life in patients with locally advanced prostate cancer after 76 Gy intensity-modulated radiotherapy vs. 70 Gy conformal radiotherapy in a prospective and longitudinal study. Int J Radiat Oncol Biol Phys. 2007;69:656–61. doi: 10.1016/j.ijrobp.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Junius S, Haustermans K, Bussels B, et al. Hypofractionated intensity modulated irradiation for localized prostate cancer, results from a phase i/ii feasibility study. Radiat Oncol. 2007;2:29. doi: 10.1186/1748-717X-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupelian PA, Reddy CA, Klein EA, Willoughby TR. Short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: preliminary results on late toxicity and quality of life. Int J Radiat Oncol Biol Phys. 2001;51:988–93. doi: 10.1016/S0360-3016(01)01730-8. [DOI] [PubMed] [Google Scholar]
- 27.Namiki S, Ishidoya S, Tochigi T, et al. Health-related quality of life after intensity modulated radiation therapy for localized prostate cancer: comparison with conventional and conformal radiotherapy. Jpn J Clin Oncol. 2006;36:224–30. doi: 10.1093/jjco/hyl002. [DOI] [PubMed] [Google Scholar]
- 28.Beckendorf V, Guerif S, Le Prisé E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of getug 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–63. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 29.Al-Mamgani A, van Putten WL, van der Wielen GJ, Levendag PC, Incrocci L. Dose escalation and quality of life in patients with localized prostate cancer treated with radiotherapy: long-term results of the Dutch randomized dose-escalation trial (ckto 96-10 trial) Int J Radiat Oncol Biol Phys. 2011;79:1004–12. doi: 10.1016/j.ijrobp.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 30.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The ucla Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:57–64. doi: 10.1016/j.ijrobp.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 32.Lawton CAF. Pelvic Nodal Consensus CTV Contours: High Risk/Locally Advanced Adenocarcinoma of the Prostate. Philadelphia, PA: Radiation Therapy Oncology Group; 2008. [Available for download at: http://www.rtog.org/CoreLab/ContouringAtlases/ProstatePelvicLymphNodes.aspx; cited: April 25, 2012] [Google Scholar]
- 33.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. doi: 10.2307/2531734. [DOI] [PubMed] [Google Scholar]
- 34.Hanlon AL, Watkins Bruner D, Peter R, Hanks GE. Quality of life study in prostate cancer patients treated with three-dimensional conformal radiation therapy: comparing late bowel and bladder quality of life symptoms to that of the normal population. Int J Radiat Oncol Biol Phys. 2001;49:51–9. doi: 10.1016/S0360-3016(00)01365-1. [DOI] [PubMed] [Google Scholar]
- 35.Katz G, Rodriguez R. Changes in continence and health-related quality of life after curative treatment and watchful waiting of prostate cancer. Urology. 2007;69:1157–60. doi: 10.1016/j.urology.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Smith LJ, Mulhall JP, Deveci S, Monaghan N, Reid MC. Sex after seventy: a pilot study of sexual function in older persons. J Sex Med. 2007;4:1247–53. doi: 10.1111/j.1743-6109.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 37.Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–74. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelefsky MJ, Cowen D, Fuks Z, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999;85:2460–8. doi: 10.1002/(SICI)1097-0142(19990601)85:11<2460::AID-CNCR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 39.Brown MW, Brooks JP, Albert PS, Poggi MM. An analysis of erectile function after intensity modulated radiation therapy for localized prostate carcinoma. Prostate Cancer Prostatic Dis. 2007;10:189–93. doi: 10.1038/sj.pcan.4500938. [DOI] [PubMed] [Google Scholar]
- 40.van der Wielen GJ, van Putten WL, Incrocci L. Sexual function after three-dimensional conformal radiotherapy for prostate cancer: results from a dose-escalation trial. Int J Radiat Oncol Biol Phys. 2007;68:479–84. doi: 10.1016/j.ijrobp.2006.12.015. [DOI] [PubMed] [Google Scholar]