1. INTRODUCTION
We read with great interest the recent article by Madernas et al. describing the clinical experience of four regional cancer centres in Ontario with rates of febrile neutropenia (fn) stemming from adjuvant fec-d (5-fluorouracil–epirubicin–cyclophosphamide, then docetaxel) for early-stage breast cancer 1. Those authors retrospectively reviewed the electronic and paper records of 671 patients treated at the Ottawa Hospital Cancer Centre, the Cancer Centre of Eastern Ontario, the London Regional Cancer Program, and the Northeastern Ontario Regional Cancer Centre, who had completed adjuvant fec-d chemotherapy between June 1, 2006, and December 31, 2008. They observed an overall fn event rate of 22.7% (152 in 671), which is considerably higher than that reported in the pivotal pacs 01 trial (11.2%) that led to the widespread adoption of adjuvant fec-d for node-positive early-stage disease 2. Similar observations have been reported for tc (docetaxel–cyclophosphamide) chemotherapy, another recently introduced adjuvant regimen for early-stage breast cancer 3–5.
Clinical practice guidelines from the American Society of Clinical Oncology and the European Society for Medical Oncology both recommend that primary prophylaxis with granulocyte colony–stimulating factors (g-csf) be considered for treatment regimens with the probability of a fn event rate of 20% or higher 6,7. With clinical experience suggesting that fn rates during fec-d treatment are more common than reported in pacs 01, 35% of patients in the study by Madernas et al. did receive primary prophylaxis with g-csf, leading to a statistically significant reduction in the observed fn rates for those who received primary prophylaxis compared with those who did not (6.4% vs. 31.4%; relative risk: 0.20; p < 0.001).
2. METHODS
Because of similar concerns, and in the context of a paucity of prospectively collected data about fn event rates among patients treated with either fec-d or tc, we conducted a prospective, real-time assessment of a consecutive cohort of patients receiving standard adjuvant fec-d, tc, or fec 100 for early-stage breast cancer and beginning therapy between April 12 and October 20, 2010. Real-time prospective review of electronic records was conducted continuously, encompassing the entire duration of each patient’s treatment, with data abstracted at each treatment cycle for the entire cohort.
The Nova Scotia Cancer Centre, based in Halifax, is the largest tertiary cancer centre in Atlantic Canada, with a catchment area of roughly 700,000. Because of an excellent network of peripheral hospitals, a proportion of the patients seen in Halifax for their initial consultation receive their systemic therapy elsewhere, under the supervision of the consulting medical oncologist. The real-time electronic record review captured event rates regardless of where the patients actually received their systemic therapy.
3. RESULTS
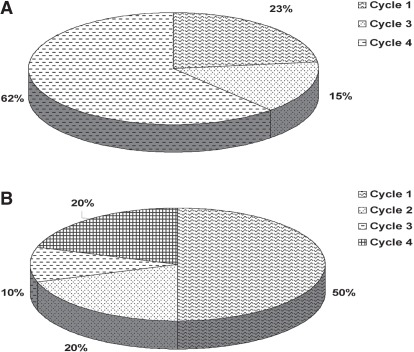
All 79 patients who started adjuvant chemotherapy between the specified dates were included in the data capture process. Table i presents patient characteristics. Table ii presents regimen-specific fn event rates, the rates of primary and secondary g-csf prophylaxis, and the event rates after each type of prophylaxis. Figure 1 presents the distribution of the timing of fn events for fec-d and tc. Of patients experiencing a fn event, 5 did not receive secondary prophylaxis: 3 because the fn episode occurred after the last planned cycle of chemotherapy, and 2, because of early treatment discontinuation. No treatment-related deaths occurred.
TABLE I.
Patient characteristics
| Variable |
Treatment group |
|||
|---|---|---|---|---|
| tc | fec-d | fec100 | All | |
| Patients (n) | 36 | 37 | 6 | 79 |
| Median age (years) | 54 | 54 | 49.5 | 54 |
| Median body surface area (m2) | 1.77 | 1.73 | 1.79 | 1.76 |
| One or more comorbidities (%) | 63.9 | 41.6 | 50 | 52.6 |
| Median tumour size (cm) | 2.3 | 2.8 | 1.9 | 2.4 |
| Node positivity (%) | 43 | 100 | 0 | 64.4 |
| Grade 3 (%) | 69.4 | 54 | 60 | 61.8 |
| er positive or negative, pr positive (%) | 69.4 | 80.5 | 80 | 75.3 |
| her2 positive (%) | 13.9 | 23.5 | 40 | 20 |
tc = docetaxel–cyclophosphamide; fec = cyclophosphamide–epirubicin–5-fluorouracil; -d = followed by docetaxel.
TABLE II.
Incidence of febrile neutropenia and use of prophylaxis
| Variable |
Treatment group |
|||
|---|---|---|---|---|
| tc | fec-d | fec100 | All | |
| Patients (n) | 36 | 37 | 6 | 79 |
| Primary prophylaxis (n)a | 6 | 9 | 1 | 16 |
| Secondary prophylaxis (n)b | 5 | 13 | 0 | 18 |
| Febrile neutropenia events | ||||
| Overall (%) | 27.8 | 35.1 | 0 | 29.1 |
| With no primary prophylaxis (%) | 33.3 | 46.4 | 0 | 36.5 |
| After any prophylaxis (n) | 0 | 0 | 0 | 0 |
All primary prophylaxis was with pegfilgrastim.
Secondary prophylaxis was distributed equally between filgrastim and pegfilgrastim.
tc = docetaxel–cyclophosphamide; fec = cyclophosphamide–epirubicin–5-fluorouracil; d = followed by docetaxel.
FIGURE 1.
Incidence distribution of febrile neutropenia events, by cycle. (A) With administration of fec-d (cyclophosphamide–epirubicin–5-fluorouracil, then docetaxel). (B) With administration of tc (docetaxel–cyclophosphamide).
Previous work has suggested that certain patient subsets may be at higher risk of fn events and that primary prophylaxis might be considered at a lower regimen-specific fn probability (for example, 10%–20%) if certain demographic or clinical risk factors are present 8.9. We assessed fn event rates as a function of two of the factors most commonly reported to increase fn risk; age and the presence of comorbidities. We observed no obvious differences in fn rates according to age [<50 years: 9 in 27 (33.3%); 50–60 years: 7 in 31 (22.6%); >60 years: 7 in 21 (33.3%)] or number of comorbidities [0: 12 in 37 (32.4%); 1–2: 10 in 39 (25.6%); >2: 1 in 3 (33.3%)].
4. DISCUSSION
In our prospectively-assessed cohort receiving standard adjuvant chemotherapy for breast cancer, the fn incidence exceeded American Society of Clinical Oncology and European Society for Medical Oncology thresholds for consideration of primary g-csf prophylaxis and was greater than the incidence reported in the relevant clinical trials (tc: 4% for <65 years of age and 8% for >65 years of age; fec-d: 11.2%) 2,3,5,6. The fn event rates were similar across age groups and were not observed to vary by presence of 1 or more comorbidities, suggesting that elucidation of risk factors may not select patients preferentially at risk for fn because of adjuvant tc or fec-d. In the context of data reported by Madernas et al. 1 and others 4,5, our data suggest that fn event rates during adjuvant fec-d and tc in clinical practice are substantially higher than those reported in the pivotal clinical trials and commonly exceed clinical guideline thresholds for consideration of primary prophylaxis with g-csf for patients choosing to receive those adjuvant systemic regimens for moderate- to high-risk early-stage breast cancer.
5. ACKNOWLEDGMENT
The authors gratefully acknowledge the breast medical oncology nursing staff—Helen Searle, Darlene Arsenault, Lynn Baxter–MacPherson, Lynn Shore, Laverne McDaniels, Jodi McIssac, and Tara Shaw—for their assistance in patient identification and ongoing care, and also Ms. Sandra Bellefontaine for expert administrative assistance.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no relevant conflicts of interest to declare.
7. REFERENCES
- 1.Madarnas Y, Dent SF, Husain SF, et al. Real-world experience with adjuvant fec-d chemotherapy in four Ontario regional cancer centres. Curr Oncol. 2011;18:119–25. doi: 10.3747/co.v18i3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for nodepositive breast cancer patients: the fnclcc pacs 01 trial. J Clin Oncol. 2006;24:5664–71. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 3.Jones SE, Savin MA, Holmes FA, et al. Phase iii trial comparing doxorubicin plus cyclophosphamide to docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–7. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 4.Soong D, Haj R, Leung MG, et al. High rate of febrile neutropenia in patients with operable breast cancer receiving docetaxel and cyclophosphamide. J Clin Oncol. 2009;27:e101–2. doi: 10.1200/JCO.2009.23.0508. [DOI] [PubMed] [Google Scholar]
- 5.Myers R, Higgins B, Jeffrey M, et al. Chemotherapy induced febrile neutropenia of docetaxel with cyclophosphamide (tc) for adjuvant therapy of breast cancer in the community—reality check [abstract 2092]. Presented at the San Antonio Breast Cancer Symposium; San Antonio, TX. December 9–11, 2009; [Available online at: http://www.abstracts2view.com/sabcs09/view.php?nu=SABCS09L_848; cited April 21, 2012] [Google Scholar]
- 6.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 7.Greil R, Psenak O, on behalf of the esmo Guidelines Working Group Hematopoietic growth factors: esmo recommendations for the application. Ann Oncol. 2007;18(supp 2):ii89–91. doi: 10.1093/annonc/mdm052. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH. A novel approach to maintain planned dose chemotherapy on time: a decision making tool to improve patient care. Eur J Cancer. 2000;36(suppl 1):S15–21. doi: 10.1016/S0959-8049(99)00257-9. [DOI] [PubMed] [Google Scholar]
- 9.Hosmer W, Malin J, Wong M. Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal and prostate cancer. Support Care Cancer. 2011;19:333–41. doi: 10.1007/s00520-010-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]