Abstract
During the seminiferous epithelial cycle of spermatogenesis, the ectoplasmic specialization (ES, a testis-specific adherens junction, AJ, type) maintains the polarity of elongating/elongated spermatids and confers adhesion to Sertoli cells in the seminiferous epithelium, and known as the apical ES. On the other hand, the ES is also found at the Sertoli-Sertoli cell interface at the blood-testis barrier (BTB) known as basal ES, which together with the tight junction (TJ), maintains Sertoli cell polarity and adhesion, creating a functional barrier that limits paracellular transport of substances across the BTB. However, the apical and basal ES are segregated and restricted to the adluminal compartment and the BTB, respectively. During the transit of preleptotene spermatocytes across the BTB and the release of sperm at spermiation at stage VIII of the seminiferous epithelial cycle, both the apical and basal ES undergo extensive restructuring to facilitate cell movement at these sites. The regulation of these events, in particular their coordination, remains unclear. Studies in other epithelia have shown that the tubulin cytoskeleton is intimately related to cell movement, and MARK [microtubule-associated protein (MAP)/microtubule affinity-regulating kinase] family kinases are crucial regulators of tubulin cytoskeleton stability. Herein MARK4, the predominant member of the MARK protein family in the testis, was shown to be expressed by both Sertoli and germ cells. MARK4 was also detected at the apical and basal ES, displaying highly restrictive spatiotemporal expression at these sites, as well as co-localizing with markers of the apical and basal ES. The expression of MARK4 was found to be stage-specific during the epithelial cycle, structurally associating with α-tubulin and the desmosomal adaptor plakophilin-2, but not with actin-based BTB proteins occludin, β-catenin and Eps8 (epidermal growth factor receptor pathway substrate 8, an actin bundling and barbed end capping protein). More importantly, it was shown that the expression of MARK4 tightly associated with the integrity of the apical ES because a diminished expression of MARK4 associated with apical ES disruption that led to the detachment of elongating/elongated spermatids from the epithelium. These findings thus illustrate that the integrity of apical ES, an actin-based and testis-specific AJ, is dependent not only on the actin filament network, but also on the tubulin-based cytoskeleton.
Keywords: Actin filament, cell adhesion, ectoplasmic specialization, germ cells, MARK4, microtubule, seminiferous epithelial cycle, Sertoli cells, spermatogenesis, spermiogenesis, testis
Introduction
Polarity-inducing kinases of the MARK [microtubule-associated protein (MAP)/microtubule affinity-regulating kinase] family are Ser/Thr protein kinases initially identified in Caenorhabditis elegans, Drosophila melanogaster and Saccharomyces cerevisiae, and they arerelated to the PAR (partitioning-defective) gene products.1-3 MARK is also known as Par-1 in nematodes and fruit flies.4 Four MARK isoforms, namely MARK1, 2, 3 and 4, have been identified in humans to date, which form a subfamily of the calcium/calmodulin-dependent protein kinase (CAMP) group of kinases.5 MARK4 is the human ortholog of Par-1, and unlike Par3 and Par6 which are restricted to the apical region of epithelial cells, MARK4 is located mostly at the basal region of cells, conferring apico-basal polarity during cellular events such as embryonic development,4,6 illustrating its significance in cell polarity. MAPKs were first shown to be capable of phosphorylating tau and related microtubule-associated proteins (MAPs) at Ser residues within KXGS motifs present in microtubule binding repeats, triggering the disruption of the microtubule network.7 For instance, it is known that tau [Note: Tau proteins are products of alternative splicing from a single gene known as MAPT8,9 (microtubule-associated protein tau), which are crucial to stabilize microtubules in cells, such as neurons] is highly soluble under normal physiological conditions. Hyperphosphorylation of tau by MAPK (mitogen activated protein kinase) is one of the initial events for the abnormal/pathological aggregation of insoluble tau to neurofibrillary tangles.10 Coupled with amyloid plaques, these are the two hallmarks of Alzheimer disease, since they lead to the degradation of neurons in the brain and result in Alzheimer disease. Other studies have shown the substrates of MARKs to include many cellular regulatory proteins, such as cell cycle regulating phosphatase Cdc25, class IIa histone deacetylases, MAPK, scaffolding protein KSR1 (kinase suppressor of Ras 1), and desmosomal armadillo protein plakophilin 2 (PKP2).11-13 Thus, since the early findings on the effects of MARKs on tau protein phosphorylation in 19977 which illustrated their role in microtubule dynamics, MARKs are now known to be involved in regulating a diverse array of cellular processes such as cell cycle, cell polarization, neuronal function, and cell signaling.1-3 In this context, it is of interest to note that recent studies have shown that a simultaneous knockdown of connexin 43 (Cx43) and PKP2 by RNAi, which are components of the gap junction (GJ) and the desmosome at the BTB, affects distribution of TJ-proteins at the BTB (e.g., occludin, ZO-1), causing their mis-localization, thereby perturbing the BTB integrity.14 Since PKP2 is a substrate of MAPK4,13 MAPK4 may play a critical role in BTB dynamics via its effects on the gap junction (GJ) and desmosome, such as an interaction with PKP2, at the BTB during spermatogenesis.
During the seminiferous epithelial cycle of spermatogenesis, highly polarized Sertoli cells, and spermatids arising from meiosis II undergo spermiogenesis.15,16 For instance, the tight junction (TJ), basal ES, GJ and desmosome that constitute the BTB are restricted near the basement membrane, and these junctions segregate the seminiferous epithelium into the basal and the adluminal compartments.17,18 Additionally, during spermiogenesis, spermatids that derive from meiosis II undergo extensive morphological transformations via 19 steps in rats (16 in mice) to form elongated spermatids (spermatozoa).19,20 Beginning from step 8 spermatids that appear at stage VIII of the cycle, the apical ES forms, and its function is to anchor step 8–19 spermatids onto the Sertoli cell so that germ cells can obtain structural and nourishment. Once the apical ES appears, it remains as the only anchoring device that persists throughout spermiogenesis until its degeneration prior to spermiation,16,21 illustrating that the ES is the critical ultrastructure that confers polarity to developing spermatids (apical ES) and Sertoli cells (basal ES). Both the apical and basal ES are typified by the presence of conspicuous actin filament bundles that lie perpendicular to the apposing plasma membranes of the Sertoli-spermatid and Sertoli-Sertoli cell interface, respectively, and they are sandwiched in-between the cisternae of endoplasmic reticulum and the cell membrane,16,17,21 illustrating the significance of the actin filament network to ES function. Moreover, the apical ES is considered to be one of the strongest adhesive junctions, significantly stronger than the desmosome22 , which is probably due to the unusual actin filament network at the site,23 yet spermatids move progressively up-and-down the epithelium at spermiogenesis during the epithelial cycle, this thus requires the presence of microtubules at the apical ES to serve as a “track” for spermatid migration.21,24 However, there is no study in the literature reporting the biology, maintenance and regulation of the microtubule network at the ES. We thus thought it pertinent to examine the localization of MARK4 at the apical and basal ES and its likely interacting partners at these sites as our initial attempt to understand the role of microtubules in ES function. Based on the restrictive temporal and spatial expression of MARK4 in the seminiferous epithelium during the epithelial cycle, MARK4 appears to be a crucial protein that stabilizes microtubules in Sertoli cells at the ES. This is the subject of this report.
Results
Stage-specific localization of MARK4 at the apical ES and BTB in the seminiferous epithelium of adult rat testes
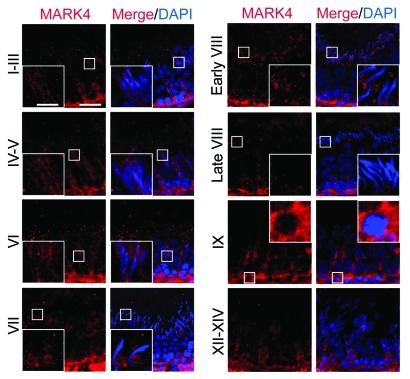
Using a specific antibody against MARK4 (Fig. 1A, Table 1), MARK4 was detected in the testis by immunoblotting (Fig. 1A–C), and it was found to be more abundant in germ cells than in Sertoli cells. These findings were consistent with results from immunofluorescence microscopy experiments, in which MARK4 localized prominently to spermatogonia and early spermatocytes throughout the seminiferous epithelial cycle (Fig. 2). However, the expression of MARK4 by more advanced germ cells, such as round spermatids and elongating/elongated spermatids, was found to be highly stage-specific during the epithelial cycle (Fig. 2). The localization of MARK4 at the BTB appeared to be stage-specific since its presence at this site was prominent in most stages of the epithelial cycle except for stages XII-XIV when its expression was considerably reduced. Moreover, the intense localization of MARK4 at the site of the BTB, as well as with migrating leptotene spermatocytes, was clearly visible at stage IX of the cycle (Fig. 2). At the apical ES, MARK4 was also expressed stage-specifically, being highest from stage I to early VIII, its level of expression diminished considerably to an almost non-detectable level by late stage VIII, coinciding with the release of sperm at spermiation (Fig. 2). Also, MARK4 displayed unusual changes in its spatiotemporal localization at the apical ES, surrounding the head of elongating spermatids during the epithelial cycle. For instance, MARK4 was found to surround the entire elongating spermatid head from stages I-V. However, its localization began to shift as the cycle progresses, limited almost exclusively to the concave side of the spermatid head. This restrictive localization was clearly visible at stage VII, and at early stage VIII; the pattern of restricted localization at the concave side of the spermatid head gradually vanished, moving away from the site, and became almost completely undetected by late stage VIII (Fig. 2). This restrictive spatiotemporal expression of MARK4 seemingly suggests that this protein may be related to the stability of the ES during spermatogenesis, such as apical ES during spermiogenesis or basal ES at the BTB via its effects in maintaining the tubulin network at both sites. It appears that its virtual “disappearance” at the apical ES correlates with the apical ES degeneration to facilitate the release of sperm at spermiation. The stage-specific and cellular localization of MARK4 at the apical ES and the BTB was further confirmed by immunohistochemistry (IHC) using an anti-MARK1–4 antibody (Fig. 3A–F). It was also shown that MARK4, at 79 kDa, is the predominant form of MARK proteins in the testis, such as in germ cells (Fig. 3A). Also, the cellular localization of MARK1–4 at the BTB and the apical ES shown in Figure 3C–F vs. Figure 3A (negative control) is consistent with findings using the specific MARK4 antibody shown in Figure 2.
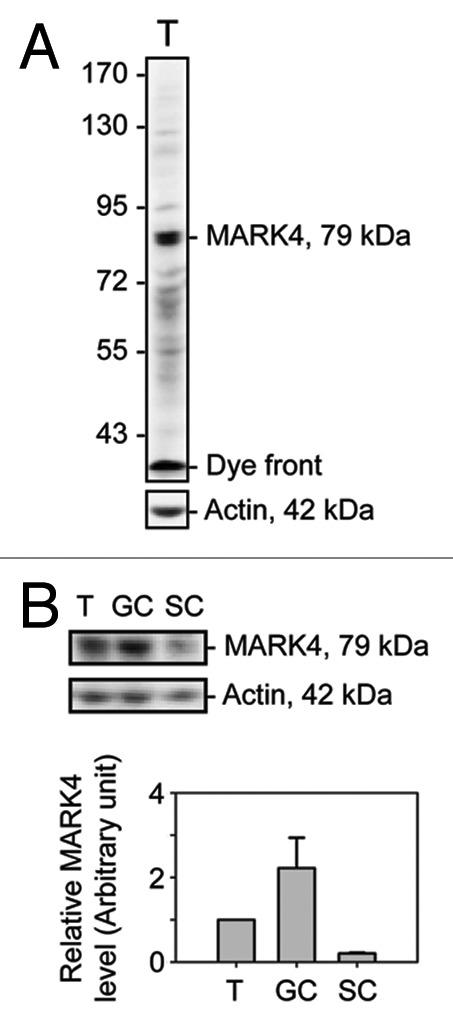
Figure 1. A study to characterize the anti-MARK4 antibody and the cellular distribution of MARK4 in the rat testis. The specificity of the anti-MARK4 antibody was demonstrated by immunoblotting using ~60 μg protein from lysates of adult rat testes using a 6.5% 7 SDS-polyacrylamide gel, with actin as a loading control (A). A prominent band with an electrophoretic mobility of ~79 kDa corresponding to the predicted apparent Mr of MARK4 was detected by immunoblotting. The relative level of MARK4 in lysates from adult rat testes (T), germ cells (GC) isolated from 90-d-old rat testes and cultured in vitro for ~16 h, and Sertoli cells (SC) isolated from 20 d-old rat testes and cultured in vitro for ~4 d were shown in (B), and the composite data were shown in a histogram in (C). Each bar = mean ± SD of n = 3 independent experiments.
Table 1. Antibodies used for different experiments in this report.
| Antibody* | Vendor | Catalog number | Application | Working dilution for IB, (IF), IHC, Co-IP |
|---|---|---|---|---|
| Rabbit anti-MARK4 |
Abcam (Cambridge, MA) |
ab32529 |
IB, IF, Co-IP |
1:1000, (1:100), 1:150 |
| Rabbit anti-MARK1–4 |
Abcam |
ab74131 |
IHC |
1:100 |
| Rabbit anti-MARK4 |
Cell Signaling Technology (Danvers, MA) |
4834 |
IB |
1:500 |
| Mouse anti-N-cadherin |
Invitrogen (Carlsbad, CA) |
33–3900 |
IF |
(1:100) |
| Mouse anti-ZO-1 |
Invitrogen |
33–9188 |
IF |
(1:100) |
| Mouse anti-Arp3 |
Sigma-Aldrich (St. Louis, MO) |
A5979 |
IF |
(1:100) |
| Mouse anti-laminin α2 |
EMD Millipore (Billerica, MA) |
MAB1922Z |
IF |
(1:100) |
| Rabbit anti-β-catenin |
Invitrogen |
71–2700 |
Co-IP |
1:50 |
| Rabbit anti-occludin |
Invitrogen |
71–1500 |
IB |
1:250 |
| Mouse anti-Eps8 |
BD Biosciences (San Jose, CA) |
610143 |
IB |
1:5000 |
| Goat anti-plakophilin-2 |
Santa Cruz Biotechnology (Santa Cruz, CA) |
sc-18976 |
Co-IP |
1:400 |
| Goat anti-actin |
Santa Cruz Biotechnology |
sc-1616 |
IB |
1:300 |
| Rabbit anti-β-tubulin | Abcam | ab6046 | Co-IP | 1:150 |
Antibodies used in this study cross-reacted with the corresponding proteins in rats as indicated by the manufacturers. IB, immunoblotting; IF, immunofluorescence microscopy; IHC, immunohistochemistry; Co-IP, co-immunoprecipitation.
Figure 2. Stage-specific and restrictive temporal and spatial expression of MARK4 in the seminiferous epithelium during the seminiferous epithelial cycle of spermatogenesis. Frozen cross-sections of testes (7 μm in thickness) were obtained with a cryostat at -20°C, fixed in Bouin’s fixative and processed for fluorescence microscopy using an anti-MARK4 antibody (see Table 1).59,71 Cell nuclei were visualized by DAPI (4’,6-diamidino-2-phenylindole) staining. Different stages of the seminiferous epithelial cycle are labeled. MARK4 was detected at the apical ES, BTB and the basement membrane. The expression of MARK4 was found to be stage-specific, with its expression at the BTB being the highest at stages VI-IX. Its expression at the apical ES appeared in stage IX and persisted through early stage VIII, but declined considerably to an almost non-detectable level by late stage VIII. Its localization at the apical ES also changed during the epithelial cycle. For instance, immunoreactive MARK4 was found to surroundthe entire head of elongating spermatids at stages IV-VI, but its localization was shifted and it was found to be restricted to the concave side of the spermatid head at stage VII, and diminished in early stage VIII. In some micrographs, a selected area is boxed and magnified in the inset. Bar in the first micrograph = 50 μm, which applies to all low magnification micrographs; bar = 20 μm in inset, which also applies to all insets.
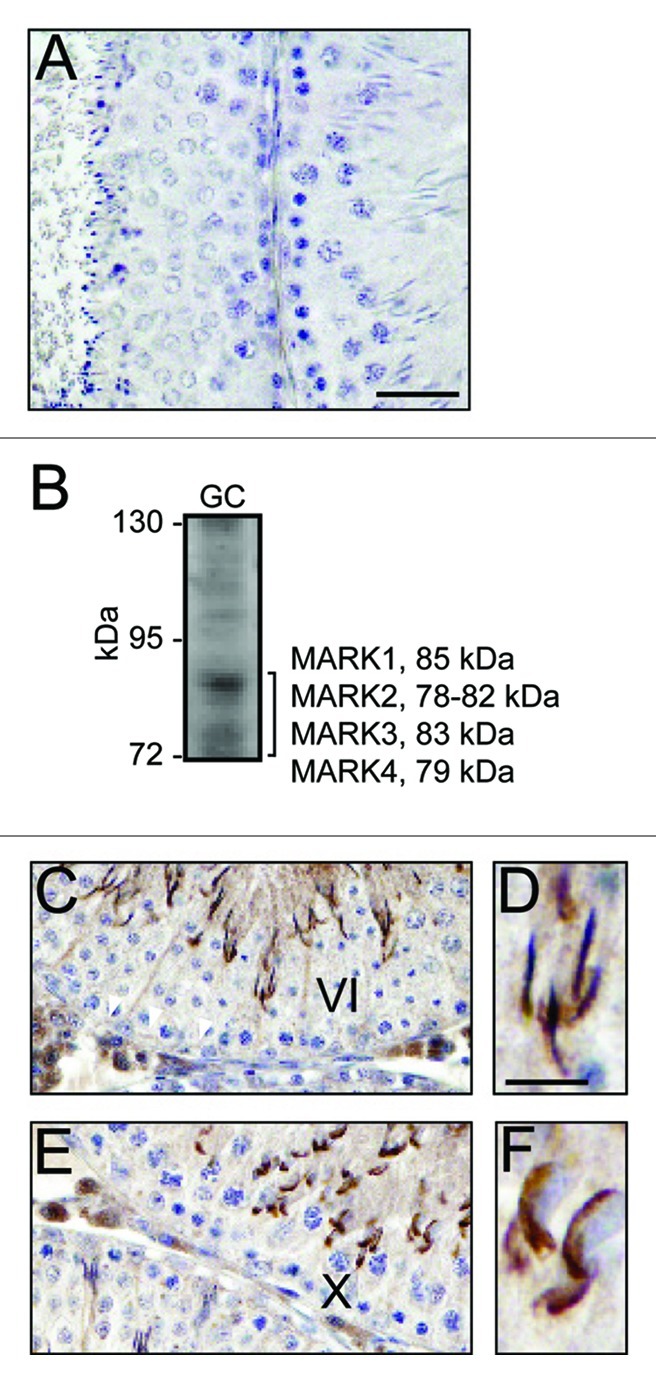
Figure 3. Localization of MARK1–4 in the seminiferous epithelium and its association with the apical and basal ES. The localization of MARK4 at the apical ES and the BTB in the seminiferous epithelium was further confirmed by using an anti-MARK1–4 antibody (see Table 1) with (A) illustrating the staining of the cross-section of adult rat testes with normal rabbit IgG to substitute the anti-MARK1–4 antibody to serve as a negative control. The specificity of this anti-MARK1–4 antibody (against the peptide NH2-LDTFC-COOH that was a consensus stretch of sequence shared by all MARKs proteins in humans) was shown by immunoblotting using lysates of germ cells (GC) (60 μg protein) and a 7% SDS-polyacrylamide gel (B); and it is apparent that this anti-MARK1–4 antibody recognizes MARK4, 79 kDa, as the major MARK protein in the testis (see also Figure 1A) (B). Similar to the localization of MARK4 in the testis (see Figure 2), MARK1–4 was detected at the apical ES (C, D), surrounding the entire elongating spermatid head in a stage VI (C, D) and a stage X (E, F) tubule, as well as at the BTB (see “white” arrowheads in C). Bar in A = 40 μm, which also applies to C and E; bar in D = 15 μm, which also applies to F.
Co-localization of MARK4 with markers of the hemidesmosome, BTB, and apical ES in the seminiferous epithelium
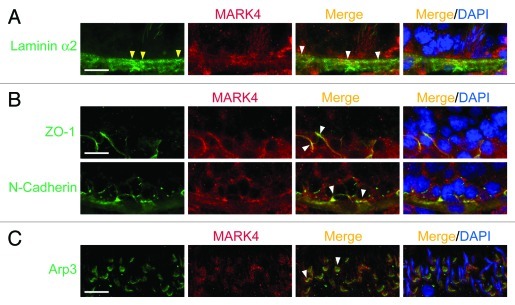
The prominent localization of MARK4 at the BTB and apical ES in the seminiferous epithelium is further supported by using an antibody against MARK1, 2, 3 and 4 (Fig. 3A–F). However, MARK4 was found to be the most predominant MARK protein among MARK1–4 in the testis since an anti-MARK antibody specifically recognized a consensus stretch of sequence of NH2-LDTFC-COOH among MARK1, 2, 3 and 425 (see Table 1) was found to detect a prominent 79 kDa band of MARK4 (Fig. 3B vs. Fig. 1A). Using this MARK1-4 antibody, members of MARK were found to be restricted to the apical ES and BTB (Fig. 3C–F), however, spermatogonia and spermatocytes were also stained positive by this antibody as shown in Figure 3C and E. Recent studies have shown that there is a functional axis known as the “apical ES-BTB-hemidesmosome/basement membrane” axis,16,26 so we next examined if MARK4 can be a likely regulator in this axis by first examining its localization with corresponding marker proteins along this axis (Fig. 4). It was found that MARK4 co-localized with laminin α2 (a maker of hemidesmosome),26 ZO-1 (a TJ adaptor) and N-cadherin (a basal ES integral membrane protein) (both are markers of the BTB),16,27 and Arp3 (actin-related protein 3, which together with Arp2 forms the Arp2/3 protein complex that regulates branched actin polymerization),28,29 implicating its likely involvement in regulating this functional axis via its effects to maintain tubulin cytoskeleton stability,1,30,31 such as at the ES.
Figure 4. A study by dual-labeled immunofluorescence analysis to illustrate the localization of MARK4 at the hemidesmosome/basement membrane, the BTB, and the apical ES. Precise localization of MARK4 in the seminiferous epithelium was further investigated by its co-localization with corresponding markers of the hemidesmosome/basement membrane (e.g., laminin α2) (A), the BTB (e.g., ZO-1, a TJ adaptor; N-cadherin, a basal ES protein) (B), and the apical ES (e.g., Arp3)(C). The general site where hemidesmosomes are found is annotated by “yellow” arrowheads, and selected merged signals are annotated by “white” arrowheads. Bar in the first micrograph in A, B or C = 40 μm, which applies to remaining micrographs.
Structural interactions between MARK4 and proteins at the apical ES and the BTB
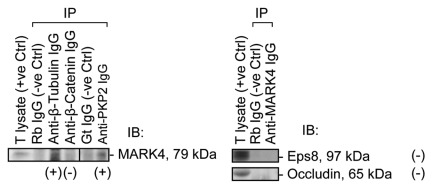
To identify the interacting partners of MARK4, co-immunoprecipitation (Co-IP) using lysates of rat testes was performed (Fig. 5). It was found that MARK4 structurally associated with β-tubulin (Fig. 5), which together with α-tubulin are known to constitute microtubules in mammalian cells.32 MARK4 also interacted with desmosomal armadillo protein plakophilin-2, an adaptor of desmosome at the BTB, which was shown to form a regulatory protein complex with connexin-43 that modulated protein distribution at the BTB since the simultaneous knockdown of connexin-43 and plakophilin-2 by RNAi impeded the distribution of TJ-proteins (e.g., occludin, ZO-1) at the Sertoli cell BTB, rendering these proteins to re-distribute from the Sertoli cell-cell interface into the cell cytosol, destabilizing the TJ-permeability barrier function.14 However, MARK4 did not structurally interact with TJ- (e.g., occludin), and basal ES- (e.g., β-catenin, Eps8) proteins at the BTB, or apical ES-protein (e.g., Eps8) (Fig. 5).
Figure 5. A study by co-immunoprecipitation (Co-IP) to identify the binding partners of MARK4 in adult rat testes. Using about 800 μg protein of lysates from adult rat testes for each Co-IP reaction as described in Materials and Methods with the corresponding antibodies, MARK4 was found to structurally interact with β-tubulin and desmosomal armadillo protein plakophilin-2, but not with any actin-based adhesion proteins at the basal ES (e.g., β-catenin), apical ES (e.g., Eps 8) and TJ (e.g., occludin, Eps8). T lysate, testis lysate; IP, immunoprecipitation; IB, immunoblotting; Rb, rabbit; Gt, goat; Ctrl, control; IgG, immunoglobulin G; (+), positive protein-protein interaction; (-), negative protein-protein interaction.
MARK4 supports the integrity of the apical ES via its effects on the tubulin stability
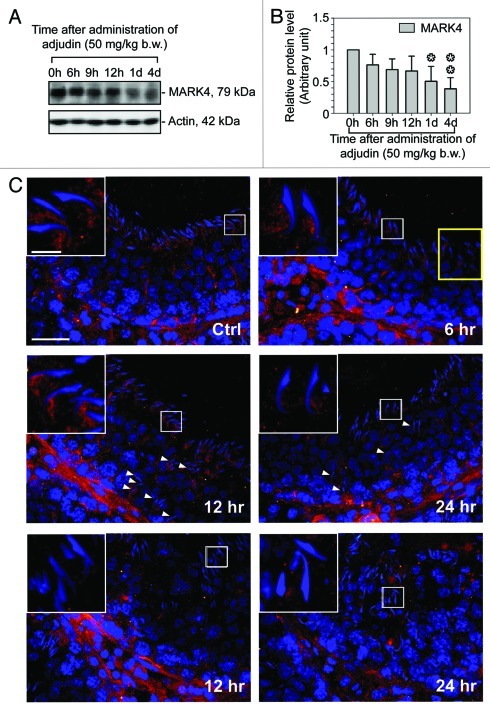
In order to further define the role of MARK4 in maintaining the integrity of the apical ES via its effects in stabilizing the tubulin cytoskeleton, we sought to use the adjudin model to examine changes in the restrictive spatiotemporal expression of MARK4 at the apical ES during adjudin-induced loss of cell polarity and premature release of spermatid from the seminiferous epithelium, mimicking spermiation.20,33,34 It was noted that the adjudin-induced disruption of spermatid polarity and spermatid loss from the seminiferous epithelium was found to associate with a significant decline in the steady-state level of MARK4 (Fig. 6A and B). There was a considerable “shift,” to be followed by a considerable loss of expression of MARK4 at the apical ES, mimicking the subtle decline and disappearance of MARK4 at the apical ES during the release of sperm at spermiation (Fig. 6C vs. Fig. 2). For instance, in both mis-oriented spermatids in which cell polarity was disrupted with their heads no longer pointing toward the basement membrane and prematurely departing elongating/elongated spermatids, MARK4 was found to diminish considerably, virtually disappearing from the apical ES (Fig. 6C), effectively mimicking elongated spermatids that undergo spermiation at late stage VIII of the epithelial cycle (Fig. 6C vs. Fig. 2). These findings thus support the notion that MARK4 is relevant to maintain apical ES stability via its effects in stabilizing tubulin cytoskeleton.
Figure 6. A study using the adjudin model to illustrate MARK4 maintains the tubulin cytoskeleton stability at the apical ES. Adult rats (~300 g b.w.) were treated with a single dose of adjudin (50 mg/kg b.w.) at time 0h (hour), which is known to induce germ cell loss,34 initially elongating/elongated spermatids, to be following by round spermatids and spermatocytes. Changes in the steady-state level of MARK4 was quantified by immunoblotting shown in (A) using β-actin as a protein loading control. (B) This histogram summarizes the composite data shown in (A) with each bar = mean ± SD of n = 3 rats. Changes in the localization of MARK4 in the seminiferous epithelium was also assessed by immunofluorescence microscopy as shown in (C). In the control (Ctrl) testis, such as an early stage VIII tubule, MARK4 localized at the concave side of the elongating spermatids began to move away from this site, similar to data shown in Figure 2 to prepare the release of sperm at spermiation during the epithelial cycle. However, in rats treated with adjudin by 6 h, while MARK4 was still found at the concave side of the elongating spermatid head (see inset), MARK4 diminished considerably in the apical ES in most elongating spermatids (see spermatids encircled by the “yellow” box). By 12- and 24-h after adjudin treatment (middle panel), many elongating spermatids remained “trapped” in the seminiferous epithelium, many of which lost their polarity and became mis-oriented with their heads no longer pointing toward the basement membrane and the level of MARK4 was also considerably diminished or even absence (see “white” arrowheads). However, in many tubules at 12- and 24-h after adjudin treatment (bottom panel), mis-oriented elongating spermatids, round spermatids and even spermatocytes were depleting from the epithelium and the staining of MARK4 vanished surrounding the heads of these spermatids, noticeably different from the control rat testis (see Ctrl micrograph). Selected area in each of these micrographs was encircled by a “white” box which was magnified and shown in an inset. In Ctrl, bar = 50 μm, which applies to all other micrographs; bar in inset in Ctrl = 10 μm, which also applies to all other insets.
Discussion
In the rat testis, there is a functional axis known as the “apical ES-BTB-hemidesmosome axis” which coordinates cellular events that take place at the opposite ends of the seminiferous epithelium during spermatogenesis,16,26 such as the degeneration of the apical ES to facilitate the release of sperm at spermiation and restructuring of the BTB to facilitate the transit of preleptotene spermatocytes at the immunological barrier that occur simultaneously at stage VIII of the epithelial cycle. Biologically active fragments of laminin chains, such as domain IV of the laminin γ3 and β3 chains, that were released at the apical ES, likely via the action of MMP-2 (matrix metalloprotease-2) that was highly expressed at the apical ES prior to spermiation35 on the α6β1-integrin/laminin-α3β3γ3 adhesion protein complex,36-39 were found to perturb the Sertoli cell TJ-permeability barrier function at the BTB,26 illustrating that the presence of a functional axis between the apical ES and the BTB. Furthermore, these biologically active laminin fragments were also found to perturb the hemidesmosome function by reducing the expression of β1-integrin at the site.26 More important, a knockdown of β1-integrin at the hemidesmosome by RNAi was found to perturb the BTB function, illustrating a functional loop exists between the BTB and the hemidesmosome.26 Since other studies have shown that a disruption of the basement membrane function40,41 could perturb the integrity of the seminiferous epithelium, and the use of anti-collagen antibody [note: collagen α3 (IV) is a major component of the basement membrane] was found to perturb the Sertoli cell BTB function,42 illustrating a regulatory axis indeed exists between components of the basement membrane and the BTB. These findings coupled with the fact that hemidesmosomes (a cell-extracellular matrix anchoring junction type) are an integrated component of the basement membrane,16,43,44 and that basement membrane is a modified form of extracellular matrix in the testis,45 we thought it pertinent to rename the axis as the “apical ES-BTB-hemidesmosome/basement membrane” functional axis. Recent findings in the field, such as the use of the phthalate-induced Sertoli cell injury model, have supported the presence of this axis that coordinates and regulates cellular events that take place across the seminiferous epithelium during spermatogenesis.46-48 While the identification of this axis is crucial to understand the intriguing regulation of cellular events across the axis, the major players along these axis that coordinate these cellular events, such as spermiation and BTB restructuring, remain unknown.
The ES, both the apical ES at the Sertoli-spermatid (step 8–19 in the rat testis), and the basal ES at the Sertoli-Sertoli cell interface at the BTB, is morphologically similar, in which extensive network of bundles of actin filaments that lie perpendicular to the plasma membrane, and sandwiched in-between the cisternae of the endoplasmic reticulum and the apposing plasma membranes of: (1) Sertoli-spermatid interface at the apical ES or (2) Sertoli-Sertoli interface at the basal ES.16,21 This network of filament bundles, however, is limited only to the Sertoli cell. These actin filament bundles also confer to the ES its unusual adhesive strength.22,23 However, the ES must undergo restructuring to facilitate the timely movement of developing spermatids (i.e., step 8–19 in the rat testis) during spermiogenesis, as well as BTB restructuring during the epithelial cycle. This spermatid movement is possible only in the presence of the tubulin-based microtubule network that is found adjacent to the apical and basal ES,21,49 which serves as the “tracks” for spermatid movement during spermiogenesis.21,24 Earlier studies have demonstrated that Eps8 (epidermal growth factor receptor pathway substrate 8),50 Arp3 (actin-related protein 3),51 and drebrin E52 are the three actin regulatory and actin binding proteins that elicit changes in the actin filament configuration at the apical ES to regulate spermatid movement. However, the regulation of the tubulin-based microtubule network in the testis remains unknown. Herein, we demonstrate for the first time the presence of a tubulin-stabilizing regulatory protein MARK4, which can be found in the testis, but displaying highly restrictive spatiotemporal expression at the ES, in particular the apical ES, during spermiogenesis. However, at stage VII or early stage VIII when the apical ES begins to undergo degeneration to prepare for spermiation, MARK4 was intensively localized at the concave side of the apical ES where extensive endocytic vesicle-mediated protein trafficking takes place, creating an ultrastructure formerly known as the apical tubulobulbar complex (apical TBC),53-55 suggesting that MARK4 is being used to facilitate protein trafficking events at the site, consistent with the reported functional role of MARKs in other epithelia.1,3 However, the expression of MARK4 at the apical ES was found to reduce to a level almost non-detectable at late stage VIII when spermiation occurs, illustrating a complete loss of MARK4-mediated microtubule network stability at the site correlates with spermatid loss. This conclusion was supported by the study using the adjudin model in which spermatids were depleted from the epithelium prematurely. Using this in vivo model, it was found that a loss of spermatid polarity, in which the heads of the elongating/elongated spermatids were pointing randomly instead of unidirectionally toward the basement membrane as of control rat testes, was associated with a subtle loss of MARK4 at the apical ES and the premature release of elongating/elongated spermatids from the epithelium. These findings thus demonstrate unequivocally that the tubulin-based cytoskeleton regulated by MARK4 is working in concert with other polarity proteins, such as PAR3 (partitioning-defective protein 3), PAR6, Cdc42, and 14–3-3 (also known as PAR5), associated with the actin-based cytoskeleton to regulate spermatid polarity and adhesion along the apical ES-BTB-hemidesmosome/basement membrane axis. Furthermore, the demonstration that MARK4 structurally interacts with desmosomal adaptor plakophilin-2 illustrates that this protein may also play a role in regulating desmosome at the BTB. In summary, these findings reported herein thus provide the basis for designing functional experiments to understand and resolve unanswered questions concerning the role of tubulin cytoskeleton in this axis. For instance, does the restrictive spatiotemporal expression of MARK4 in the seminiferous epithelium serve as the molecular “switch” that turns “on” and “off” regarding the stability of the tubulin cytoskeleton to induce changes in adhesion at the apical ES and basal ES during the epithelial cycle of spermatogenesis?
Materials and Methods
Animals and antibodies
The use of Sprague-Dawley rats for studies reported herein was approved by the Rockefeller University Institutional Animal Care and Use Committee (IACUC) with Protocol Numbers 09-016 and 12-506. Rats were housed at the Rockefeller University Comparative Bioscience Center (CBC) with free access to standard rat chow and water ad libitum at 22 ± 1°C with a light:dark cycle of 12 h:12 h, according to the applicable portions of the Animal Welfare Act and the guidelines in the Department of Health and Human Services publication “Guide for the Care and Use of Laboratory Animals.”
The adjudin animal model
Adult rats (~300 g b.w.) were treated with a single dose of adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, a potential male contraceptive known to induce germ cell loss, most notably elongating spermatids by disrupting the apical ES,20,34,56,57 at 50 mg/kg b.w. by gavage (working dilution of adjudin at 20 mg/ml, suspended in 0.05% methylcellulose/99.5% MilliQ water) as described.58 Thereafter, groups of rats with n = 3–6 animals per time point, including both treatment and control groups, were euthanized by CO2 asphyxiation. Testes were removed, and one testis from each rat was fixed in Bouin’s fixative to be embedded in paraffin wax, and the other testis from the same rat was snap-frozen in liquid nitrogen for at least 5 min, before it was stored at -80°C until used for either preparation of lysates for immunoblotting59,60 or to obtain frozen sections (7-µm in thickness at -22°C in a cryostat) for immunohistochemistry. This animal model was selected so that changes in the expression and/or localization of MARK4 (note: MARK4 is a regulator of microtubule dynamics) during adjudin-induced spermatid loss from the seminiferous epithelium could be correlated with apical ES disruption (note: ES is an actin-based testis-specific adherens junction type). This thus assesses the functional relationship between actin filament bundles and the microtubule filaments at the apical ES since the movement of spermatids during spermiogenesis and the release of spermatid at spermiation16,61 involves the apical ES, and this is intimately related to microtubules besides actin filaments,21,24 yet there is no report in the literature to address the functional relationship between these two cytoskeletons in the testis.
Primary testicular cell cultures
Sertoli cells were isolated from 20-d-old rat testes.62 These cells were differentiated, ceased to divide, and mimicked Sertoli cells isolated from adult rat testes morphologically, physiologically and functionally.63,64 Sertoli cells were cultured in serum-free F12/DMEM (Ham’s F12 nutrient mixture/Dulbecco’s modified Eagle’s medium) supplemented with growth factors62 at 35°C in a humidified CO2 incubator with 95% air/95% CO2 (v/v)62 for 4 d to allow the assembly of a functional TJ-permeability barrier that mimicked the Sertoli cell BTB in vivo.65,66 Thereafter, these cultures were terminated and lysates were obtained.59 Germ cells were isolated from adult rat testes using a mechanical procedure,67 and cultured in F12/DMEM supplemented with 2 mM sodium pyruvate and 6 mm sodium DL-lactate for ~16 h. The viability of these germ cells was at least 96% as judged by erythrosine red dye exclusion test68 when these cultures were terminated for lysate preparation.
Immunofluorescence microscopy
Immunofluorescence microscopy and dual-labeled immunofluorescence analysis was performed essentially as earlier described51,59 using frozen sections (7 μm in thickness) of testes obtained in a cryostat at -22°C. To avoid inter-experimental variations, all sections of testes from rats within an experimental group including both treated vs. control rats were processed in a single experimental session by mounting all sections on a minimal number of poly-l-lysine-coated microscope slides (Polysciences). Sections were fixed in paraformaldehyde (w/v), permeabilized in 0.1% Triton X-100 (v/v), blocked in 1% BSA (w/v), followed by an overnight incubation with anti-MARK4 antibody (Table 1), and then an Alexa Fluor-conjugated secondary antibody (Invitrogen; Alexa Fluor 555). Fluorescence images were acquired using a Nikon Eclipse 90i Fluorescence Microscope System and Nikon NIS Elements Advanced Research software package and converted to JEPG format. All images were subsequently assembled using Adobe InDesign in Adobe Creative Suite 3.0.
Immunohistochemistry (IHC)
IHC was performed essentially as earlier described65 with minor modifications. In brief, testes were fixed in Bouin’s fixative (Polysciences) for 24 h, dehydrated using increasing concentrations of ethanol, cleared with xylene, embedded in paraffin and sectioned. After deparaffinization in xylene and rehydration, antigen retrieval was performed in 10 mM citrate buffer, pH 6.0 at 22°C, for 10 min in a microwave. Other procedures, including incubation with the anti-MARK1–4 antibody (see Table 1), were performed as earlier described.65 Thereafter, sections were incubated with biotinylated goat anti-rabbit IgG at 1:400 dilution, followed by VECTASTAIN Elite ABC (Avidin: Biotinylated enzyme Complex) reagent (Vector Labs). Color development was performed using DAB (3,3′-diaminobenzidine). Micrographs were obtained using the Nikon Eclipse 90i microscope and Nikon NIS Elements Advanced Research Imaging Software package in JEPG format. All micrographs were subsequently analyzed in PhotoShop using Adobe Creative Suite 5.0.
Immunoblotting
Lysates from Sertoli and germ cell cultures, and testes were obtained using lysis buffer [10 mM Tris, 0.15M NaCl, 1% NP40 (Tergitol-type nonyl phenoxypolyethoxylethanol, v/v), and 10% glycerol (v/v), pH 7.4 at 22°C]. Protease and phosphatase inhibitor cocktails (Sigma-Aldrich) were added to the lysis buffer immediately prior to its use at 1:100 dilution according to instructions from the manusfacturer. Protein concentration was estimated by DC Protein Assay (Bio-Rad Labs). SDS-PAGE and immunoblotting were performed59 to visualize MARK1–4 or MARK4 in blots. Enhanced chemiluminescence was performed using a luminol/p-coumaric acid-based home-made kit as described.69 Chemiluminescence images were obtained using a Fujifilm LAS-4000 mini imaging system, and images were acquired in TIFF format using Multi Gauge (Version 3.1) in Science Lab 2005 software package (Fujifilm Corp).
Co-immunoprecipitation (Co-IP)
To identify binding partners of MARK4 in the adult testis, co-immunoprecipitation was performed essentially as earlier described59,70 to assess the binding partners of MARK4 in adult rat testes. In brief, rat testis lysates (~800 μg protein in each reaction tube) were first pre-cleared with the corresponding animal serum (or IgG) as described59 to eliminate non-specific antibody-antigen interactions. Thereafter, the immunoprecitiation antibody was added, samples were incubated overnight at room temperature and the immunocomplexes were precipitation using Protein A/G Plus (Santa Cruz). Binding partners were then identified by immunoblotting using corresponding antibodies (see Table 1).
Statistical analysis
Each experiment was repeated at least three times with n = 3–6 rats or using different batches of testicular cells. Statistical analysis was performed by one-way ANOVA and post hoc Dunnett’s test using GB-STAT (Version 7.0, Dynamic Microsystems).
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056034 to C.Y.C.; U54 HD029990 Project 5 to C.Y.C.); and Government of India, Department of Biotechnology, BT/BI/03/015/2002 to P.P.M., Department of Information Technology, DIT/R&D/BIO/15(9)/2007 to P.P.M., and University Grants Commission to P.J. for University Research Fellowship.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/20724
References
- 1.Marx A, Nugoor C, Panneerselvam S, Mandelkow E. Structure and function of polarity-inducing kinase family MARK/Par-1 within the branch of AMPK/Snf1-related kinases. FASEB J. 2010;24:1637–48. doi: 10.1096/fj.09-148064. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–22. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matenia D, Mandelkow EM. The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem Sci. 2009;34:332–42. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Kemphues KJ. PARsing embryonic polarity. Cell. 2000;101:345–8. doi: 10.1016/S0092-8674(00)80844-2. [DOI] [PubMed] [Google Scholar]
- 5.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 6.Brajenovic M, Joberty G, Küster B, Bouwmeester T, Drewes G. Comprehensive proteomic analysis of human Par protein complexes reveals an interconnected protein network. J Biol Chem. 2004;279:12804–11. doi: 10.1074/jbc.M312171200. [DOI] [PubMed] [Google Scholar]
- 7.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/S0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 8.Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988;85:4051–5. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–26. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 10.Mocanu MM, Nissen A, Eckermann K, Khlistunova I, Biernat J, Drexler D, et al. The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. J Neurosci. 2008;28:737–48. doi: 10.1523/JNEUROSCI.2824-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dequiedt F, Martin M, Von Blume J, Vertommen D, Lecomte E, Mari N, et al. New role for hPar-1 kinases EMK and C-TAK1 in regulating localization and activity of class IIa histone deacetylases. Mol Cell Biol. 2006;26:7086–102. doi: 10.1128/MCB.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang SH, Kobayashi R, Graves PR, Piwnica-Worms H, Tonks NK. Serine phosphorylation-dependent association of the band 4.1-related protein-tyrosine phosphatase PTPH1 with 14-3-3beta protein. J Biol Chem. 1997;272:27281–7. doi: 10.1074/jbc.272.43.27281. [DOI] [PubMed] [Google Scholar]
- 13.Müller J, Ritt DA, Copeland TD, Morrison DK. Functional analysis of C-TAK1 substrate binding and identification of PKP2 as a new C-TAK1 substrate. EMBO J. 2003;22:4431–42. doi: 10.1093/emboj/cdg426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci U S A. 2009;106:10213–8. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–53. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–95. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CY, Wong EW, Lie PP, Li MW, Mruk DD, Yan HH, et al. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins: An unexpected turn of events. Spermatogenesis. 2011;1:105–15. doi: 10.4161/spmg.1.2.15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 20.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–80. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 22.Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–9. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- 23.Russell LD, Goh JC, Rashed RMA, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod. 1988;39:105–18. doi: 10.1095/biolreprod39.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development? Hum Reprod Update. 2004;10:349–69. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- 25.Trinczek B, Brajenovic M, Ebneth A, Drewes G. MARK4 is a novel microtubule-associated proteins/microtubule affinity-regulating kinase that binds to the cellular microtubule network and to centrosomes. J Biol Chem. 2004;279:5915–23. doi: 10.1074/jbc.M304528200. [DOI] [PubMed] [Google Scholar]
- 26.Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A. 2008;105:8950–5. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–63. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–62. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–92. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9:860–73. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 31.Moritz M, Agard DA. Gamma-tubulin complexes and microtubule nucleation. Curr Opin Struct Biol. 2001;11:174–81. doi: 10.1016/S0959-440X(00)00187-1. [DOI] [PubMed] [Google Scholar]
- 32.Dutcher SK. The tubulin fraternity: alpha to eta. Curr Opin Cell Biol. 2001;13:49–54. doi: 10.1016/S0955-0674(00)00173-3. [DOI] [PubMed] [Google Scholar]
- 33.Cheng CY, Mruk DD. New frontiers in nonhormonal male contraception. Contraception. 2010;82:476–82. doi: 10.1016/j.contraception.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MK, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–61. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–64. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 36.Yan HHN, Cheng CY. Laminin α 3 forms a complex with β3 and γ3 chains that serves as the ligand for α 6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 37.Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β 1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–82. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- 38.Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F. Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain α6β1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod. 1998;58:371–8. doi: 10.1095/biolreprod58.2.371. [DOI] [PubMed] [Google Scholar]
- 39.Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, et al. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–18. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lustig L, Denduchis B, González NN, Puig RP. Experimental orchitis induced in rats by passive transfer of an antiserum to seminiferous tubule basement membrane. Arch Androl. 1978;1:333–43. doi: 10.3109/01485017808988354. [DOI] [PubMed] [Google Scholar]
- 41.Denduchis B, Satz ML, Sztein MB, Puig RP, Doncel G, Lustig L. Multifocal damage of the testis induced in rats by passive transfer of antibodies prepared against non-collagenous fraction of basement membrane. J Reprod Immunol. 1985;7:59–75. doi: 10.1016/0165-0378(85)90021-X. [DOI] [PubMed] [Google Scholar]
- 42.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–87. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 43.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays. 2004;26:978–92. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 44.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 45.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–15. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 46.Yao PL, Lin YC, Richburg JH. Transcriptional suppression of Sertoli cell Timp2 in rodents following mono-(2-ethylhexyl) phthalate exposure is regulated by CEBPA and MYC. Biol Reprod. 2011;85:1203–15. doi: 10.1095/biolreprod.111.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod. 2010;82:516–27. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao PL, Lin YC, Richburg JH. TNF α-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 2009;80:581–9. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell LD, Malone JP, MacCurdy DS. Effect of the microtubule disrupting agents, colchicine and vinblastine, on seminiferous tubule structure in the rat. Tissue Cell. 1981;13:349–67. doi: 10.1016/0040-8166(81)90010-0. [DOI] [PubMed] [Google Scholar]
- 50.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–67. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci U S A. 2010;107:11411–6. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li MWM, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, et al. Actin-binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–36. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009;80:162–74. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- 54.Young JS, Guttman JA, Vaid KS, Shahinian H, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009;80:153–61. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 55.Young JS, Vogl AW. Focal adhesion proteins Zyxin and Vinculin are co-distributed at tubulobulbar complexes. Spermatogenesis. 2012;2:63–8. doi: 10.4161/spmg.19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mok KW, Mruk DD, Lie PPY, Lui WY, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction. 2011;141:571–80. doi: 10.1530/REP-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng CY, Lie PPY, Wong EWP, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis. 2011;1:291–7. doi: 10.4161/spmg.1.4.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64:1500–8. doi: 10.1095/biolreprod64.5.1500. [DOI] [PubMed] [Google Scholar]
- 59.Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–65. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su L, Mruk DD, Lee WM, Cheng CY. Drug transporters and blood--testis barrier function. J Endocrinol. 2011;209:337–51. doi: 10.1530/JOE-10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011;763:237–52. doi: 10.1007/978-1-61779-191-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li JCH, Lee TW, Mruk TD, Cheng CY. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl. 2001;22:266–77. [PubMed] [Google Scholar]
- 64.Lui WY, Lee WM, Cheng CY. Transforming growth factor β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 65.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–47. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 66.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–14. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aravindan GR, Pineau CP, Bardin CW, Cheng CY. Ability of trypsin in mimicking germ cell factors that affect Sertoli cell secretory function. J Cell Physiol. 1996;168:123–33. doi: 10.1002/(SICI)1097-4652(199607)168:1<123::AID-JCP15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 68.Pineau C, Syed V, Bardin CW, Jégou B, Cheng CY. Germ cell-conditioned medium contains multiple factors that modulate the secretion of testins, clusterin, and transferrin by Sertoli cells. J Androl. 1993;14:87–98. [PubMed] [Google Scholar]
- 69.Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis. 2011;1:121–2. doi: 10.4161/spmg.1.2.16606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–86. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012;35:86–101. doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]