Abstract
This study reports on the use of a microsystem for evaluation of photodynamic therapy (PDT) procedures on the “mixed” (carcinoma-normal) cultures. Balb/3T3 (normal mouse embryo) and A549 (human lung carcinoma) cells were tested in separated and “mixed” cultures. Interactions and migration of cells cultured together were observed. The PDT procedures were examined in the hybrid (PDMS/glass) microsystem which contains cell culture microchambers integrated with network of microchannels. We investigated that the number of dead cells after PDT procedures is dependent on the kind of cell culture. Moreover, the influence of the carcinoma cells on the viability of normal cells in the “mixed” culture was observed.
Development of new anticancer drugs is associated with investigation of the influence of many compounds on the various cells. In vitro culture of various cell lines in the same environment/medium allows to investigate inhibitory effect of many compounds and monitor cell-cell interactions. The interactions between tumor and normal cells must also be tested, because they indicate cellular functions such as survival/apoptosis, migration, proliferation, and differentiation. Cell co-cultures can resemble dependences existing in a live organism, so they give important information about cells’ behavior.1, 2 Many different cells interactions were investigated in conventional methods, where behavior of the cells was tested on a tissue culture substrate.3, 4 Nowadays, scientists are searching for new methods (for example, microsystems) which enable to control the degree of cellular interactions. The application of the microdevices allows to control the environment of cell culture systems. Besides many advantages resulting from the miniaturization, microsystems provide reductions in cost of equipment and higher-throughput information. The usage of low cells’ number and small volumes of reagents enable flexibility in the control of cell culture conditions. Moreover, the application of microsystems allows to investigate cells interactions in various stages of a tumor. They provide a possibility of developing personalized therapy, in which drug dosages and their combinations of therapies can be patient-defined to treat an individual disease.5
Poli(dimetylosiloxane)- PDMS is a material the most often used for fabrication of microchips. PDMS properties such as: biocompatibility, transparency, gas permeability enable its application in cell engineering. Up to now, any negative effect of PDMS exerted on the various cells (e.g., adherent or spheroids) was observed, if this material was applied for the microsystem fabrication. The viability of the cells cultured on PDMS surface was comparable to polystyrene microplates used in the conventional tests. In the literature, many studies focused on the comparison of proliferation and viability cells in microscale and macroscale were described.6, 7 Moreover, the response of cells in both scales is similar and comparable, although different culture conditions are applied. Flow of medium and others reagents in microchips is one of the differences between microscale and macroscale. Flow conditions, applied in the microdevices produce shear stress on the cells. The effect of shear stress depends on both the cell type and the local hydrodynamic environment. The usage of quantitative intracellular Ca2+ analysis of the cells can be used as a model system demonstrating influence of flow conditions on the cells. It was observed that even a brief exposure to low levels of shear stress induced an intracellular Ca2+ flux in the cells, although no morphological changes were noted.8 The microsystems were often used for measure interactions between normal and carcinoma cells on the fundamental knowledge of these phenomena and also enable drug discovery and high-throughput screening with time and cost benefits. Cooper et al. investigated the normal and chronic myeloid leukaemia (CML) stem cell responses to tyrosine kinase inhibitor and dasatinib in the microfluidic single cell arrays.9 They confirmed that the microsystem can be utilized for the study of patient-derived non-adherent cells, which are generally tested on the macroscopic level. Many microsystems are dedicated for cell cytotoxicity tests as automated drug screening routines. In the literature, there are described investigations on various cell lines, drugs, and geometry of microsystems.5, 10 Various patterned co-cultures as a useful tool for studying cell–cell interactions were described in the literature.11-14 The interactions and migration in both adherent cells and spheroids were investigated.15, 16
The microsystem can be a new faster and cheaper tool applied for examination of photodynamic therapy (PDT) procedures.17 PDT is an anticancer therapy, where the light of specific wavelength is used to induce a photosensitizer, which is accumulated in the cells. The induced photosensitizer in the presence of intracellular oxygen produces reactive oxygen species (ROS), which are toxic for the cells.18 Because of the significant difference in the activities of key enzymes in the heme pathway between tumor and normal cells, porphyrins accumulation in tumor cells is generally higher than in the normal cells. The toxic effect of the same parameters of PDT procedures must be studied on normal and carcinoma cells.19 There is also important to test interaction between normal and carcinoma cells cultured in “mixed” culture and investigation of toxic effect after PDT procedure. Chiu et al. determined PDT results in cell death in co-culture of keloid fibroblasts and keratinocytes.20 The other group investigated the effects of PDT and additional administration of heme on the treatment of melanoma cells in comparison to nonmalignant keratinocytes.21 In this co-culture, 65% melanoma cells and 35% normal cells were present before PDT, whereas only 41% melanoma cells and 59% normal cells after PDT. This kind of research indicates that PDT might also be interesting for melanoma treatment.
We propose an investigation, which combines examination of PDT procedures, studies of cells interactions and microtechnology. This connection gives a possibility to test toxic effect on the “mixed” culture in the microdevice. Moreover, interaction between the normal and carcinoma cells in the simple model can also be used to evaluate effectiveness of PDT procedure.
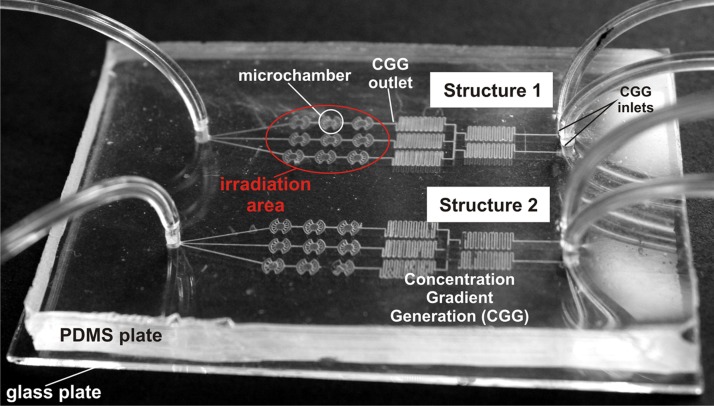
The microsystem with two microstructures for investigation of two various cell cultures was used in our experiments. The geometry of the microstructure includes the microchambers matrix (3 × 3) integrated with microchannels, which form concentration gradient generator (CGG) (Figure 1). PDMS (poly(dimetylosiloxane)) and glass were used as constructional materials to fabricate the microsystem. The microchambers in the glass were obtained by photolithography and wet etching technique. Whereas the microstructures in PDMS were fabricated using photolithography and replica molding methods. The geometry of the fabricated microsystem and hydrophilic properties of glass enabled the growth of adherent cells (both normal and carcinoma). All technical details and experimental protocols (such as geometry or principles of work of concentration gradient generator) were described in our previous papers.10, 17 Moreover, the CGG allowed to prepare different concentrations of the introduced reagent (precursor of photosensitizer) and it can also create a “mixed” culture with different density of the various cells.
Figure 1.
The microfluidic system (75 mm × 55 mm) used for evaluation of PDT procedures. Two separate microstructures are fabricated in the same microsystem for simultaneous experiments on various cell culture. The microdevice consists of culture microchambers (a diameter of 1 mm and a depth of 30 μm), and microchannels (a width of 100 μm and a depth of 50 μm) creating also CGG. The microchambers were fabricated in the sodium glass plate, whereas microchannels in the PDMS.
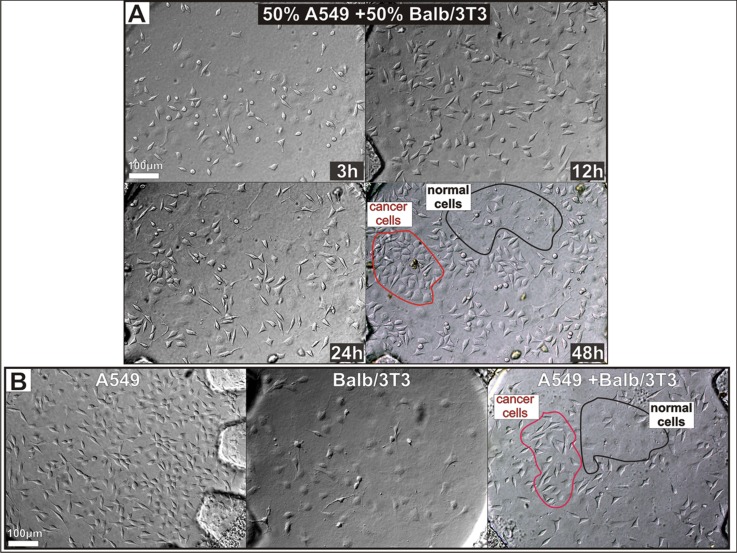
The PDT procedures were examined on the Balb/3T3 (normal mouse embryo) and A549 (human lung carcinoma) cells cultured in the separated and the “mixed” culture. The designed microsystem was sterilized and flushed with the culture medium. After that the various cell cultures were obtained in the microsystem. Besides separated (Balb/3T3 and A549) cultures, three “mixed” cultures in the ratio: 25:75; 50:50, and 75:25 of A549:Balb/3T3 cells were obtained in the microchambers. The ratio of the cells was established by preparing suitable suspension of two cell lines. The density of each cell line was independently calculated using cell counter (Cell Counter - Invitrogen). After that the cells were mixed and introduced into the microchambers. The usage of various densities of two cell types allowed to test how density of the carcinoma cells affects on the normal cells after photodynamic therapy. In Figure 2a, growth of the “mixed” culture in the ratio: 50:50 of A549:Balb/3T3 cells is shown. In the next hours, divisions of two cell lines were observed, and the same kind of cells approaching each other. The cells were migrated and characteristic clusters between proliferated cells were formed. Forty-eight hours “mixed” culture (Figure 2a) indicates that both cell lines divide and grow suitable in common microchamber. For comparison, the microchambers with carcinoma A549 cells, normal Balb/3T3 cells and “mixed” A549 and Bab/3T3 are shown in Figure 2b. Each kind of cells has different morphology and size. Balb/3T3 cells are larger than A549 and have thinner cell membranes. These cultures were used for PDT procedures examination according to procedure elaborated previously.17
Figure 2.
The “mixed” culture growth. Balb/3T3 : A549 ratio is 50:50. The cells were monitored over time, and their migration was observed. Finally they created characteristic clusters. (b) The separated culture of A549 and Balb/3T3 cells, and the “mixed” culture of A549: Balb/3T3 cells. The separated Balb/3T3 and A549 are attached to the glass one by one, whereas “mixed” culture creates cluster between the cells in the same kind.
In order to perform PDT procedures, exogenous 5-aminolevulinic acid (ALA) (0 and 0.75 mM) was introduced with a flow rate of 1.2μl/min (the value of flow rate was established in our previous work.10) into the microchambers with various cell cultures. The cells with ALA were incubated for 4 h. After that the cells were irradiated trough the PDMS cover using a high power LED (λ = 625 nm, t = 60 s, energy dose = 30 J/cm2). Viability of the cells was determined using an inverted fluorescence microscope in the presence of calceine AM (CAM) and propidium iodide (PI). Cell viability was determined by counting the number of green objects (corresponding to live cells) and red (dead cells) with an image processing software (cell^f, olympus). The cells were counted in the whole microchamber (a diameter of 1 mm and a depth of 30 μm). First, the toxic effect of PDT procedures on the separated Balb/3T3 and A549 cell was investigated. The number of dead cells was increasing with higher concentration of ALA for both separated culture. However, the differences between the numbers of dead cells for kind of cell lines were observed. Both 0.375 and 0.75 mM concentration of ALA indicates stronger inhibition of A549 cells proliferation after PDT procedure. The usage just only 0.375 mM ALA in PDT procedures indicates death higher than 70% carcinoma cell, whereas lower than 20% of normal cells.
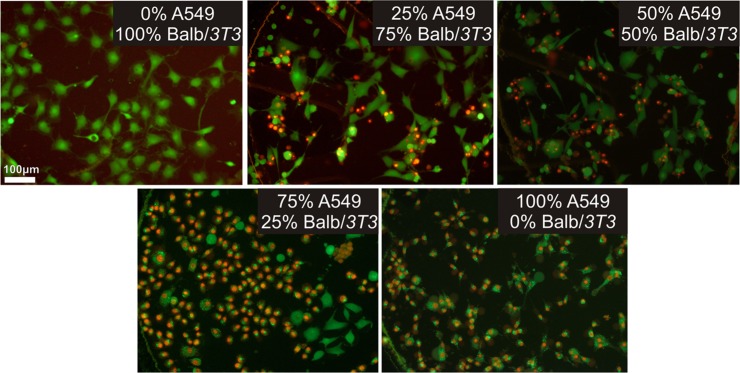
The PDT procedure performed on the “mixed” culture caused that the number of the dead cells was the highest in the culture with higher density of the carcinoma cells. In Figure 3, staining with CAM and PI of cell cultures after PDT procedures PI is shown. The number of dead cells (red cells) in the “mixed” culture was the highest for the 75%A549:25% Balb/3T3 cell culture. Moreover, for this culture, we observed that the number of living cells is higher for the normal cells. The same tendency was also observed in others “mixed” cultures. These conclusions (distinguish between the normal and carcinoma cells) were determined based on morphology of carcinoma and normal cells. Although, PDT procedure has no toxic effect on the normal cells in the separate cultures, toxic effect was observed in “mixed” cultures. A549 cells produce ROS, which can have toxic effect also on the normal cells, cultured in the same environment. We examined that the number of the dead cells after PDT is dependent on the kind of cell culture.
Figure 3.
Viability of the cells in “mixed” cultures in microchambers after PDT procedure. Viability is examined after LIVE/DEAD cell staining with PI and CAM. For each ALA concentration three images of the interested area were taken at 10 x magnification using a fluorescence microscope. Cell viability was determined by counting the number of green objects (corresponding to live cells) and red (dead cells) with an image processing software (cell^f, olympus).
In Figure 4, the number of the dead cells in the separated and “mixed” culture after PDT procedure is shown. The toxic effect on the cells in the “mixed” culture with 0 mM ALA was not observed. The data gives information that “mixed” type of cell culture with no ALA addition does not influence on the proliferation and the viability of the cells. Both 0.75 and 0.375 mM ALA cause death of the normal and carcinoma cells cultured together. The usage of the lower ALA concentration causes slightly lower cells lethality in the “mixed” cultures. The number of dead cells increased with the density of A549 cells. We suggest that the presence of signalling events from dead cells will impact on the other cells. The PDT procedures in the “mixed” culture were also performed in macroscale. Although, some differences between viability in microscale and macroscale were observed, the tendency was similar. The results are shown in supplementary materials.22
Figure 4.
The number of the dead cells in the “mixed” culture 24 h after PDT procedure performed in microsystem. Each data corresponds to mean ± SD (n = 4).
In this work, we described the microdevice, which allows to study the interactions between different cell lines and the cellular response to external stimuli. The presented results show a possibility of examination of interaction between two kind of the cells (carcinoma and normal) and evaluation of the photodynamic therapy process in vitro in microscale. The density of the normal and carcinoma cells influences on the toxic effect after PDT procedure. However, the number of living normal cells is higher than carcinoma. It can indicate that this procedure is more selective on the carcinoma cells. Our further studies will be focused on testing PDT procedures for different values of PDT parameters. Moreover, we will test simultaneous influence of two different photosensitizers and various concentration obtained in the CGG. In our future investigations, we will label the normal and cancer cells with different markers. Application of this type of microfluidic device is expected to have a significant influence on biological and engineering studies. The therapy effects will allow the medical doctors to optimize parameters of the patient treatment, i.e., the dose of irradiation, time of exposition, and concentration of photosensitizers.
ACKNOWLEDGMENTS
This work has been supported by the European Union in the framework of European Social Fund through the Warsaw University of Technology Development Programme, realized by Center for Advanced Studies.
References
- Kaji H., Camci-Unal G., Langer R., and Khademhosseini A., Biochim. Biophys. Acta. 3, 239 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani S. R. and Bhatia S. N., Curr. Opin. Biotechnol. 17, 524 (2006). 10.1016/j.copbio.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Smith C. W., Eskin S. G., and McIntire L. V., Blood 75, 227 (1990). [PubMed] [Google Scholar]
- Schrode W., Mecke D., and Gebhardt R., Eur. J. Cell Biol. 53, 35 (1990). [PubMed] [Google Scholar]
- Wlodkowic D., Skommer J., McGuinness D., Faley S., Kolch W., Darzynkiewicz Z., and Cooper J. M., Anal. Chem. 81, 6952 (2009). 10.1021/ac9010217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodkowic D., Faley S., McGuinness D., and Cooper J. M., Anal. Chem. 81, 9828 (2009). 10.1021/ac902010s [DOI] [PubMed] [Google Scholar]
- Yin H., Pattrick N., Zhang X., Klauke N., Cordingley H. C., Haswell S. J., and Cooper J.M., Anal. Chem. 80, 179 (2008). 10.1021/ac701958z [DOI] [PubMed] [Google Scholar]
- Yin H., Zhang X., Pattrick N., Klauke N., Cordingley H. C., Haswell S. J., and Cooper J. M., Anal. Chem. 79, 7139 (2007). 10.1021/ac071146k [DOI] [PubMed] [Google Scholar]
- Faley S. L., Copland M., Wlodkowic D., Kolch W., Seale K. T., Wikswo J. P., and Cooper J. M., Lab Chip 9, 2659 (2009). 10.1039/b902083g [DOI] [PubMed] [Google Scholar]
- Ziolkowska K., Jedrych E., Kwapiszewski R., Lopacinska J., and Skolimowski M., Chudy M. Sens. Actuat. B 145, 533 (2010). 10.1016/j.snb.2009.11.010 [DOI] [Google Scholar]
- Ciofani G., Migliore A., Raffa V., Menciassi A., and Dario P., J. Biosci. Bioeng. 105, 536 (2008). 10.1263/jbb.105.536 [DOI] [PubMed] [Google Scholar]
- Yamazoe H., Okuyama T., Suzuki H., and Fukuda J., Acta Biomater. 6, 526 (2010). 10.1016/j.actbio.2009.07.036 [DOI] [PubMed] [Google Scholar]
- Chiu D. T., Jeon N. L., Huang S., Kane R. S., Wargo C. J., Choi I. S., Ingber D. E., and Whitesides G. M., Proc. Natl. Acad. Sci. U.S.A., 97, 2408 (2000). 10.1073/pnas.040562297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Kong B., and Li D. , Biotechnol. Lett. 30, 1537 (2008). 10.1007/s10529-008-9725-2 [DOI] [PubMed] [Google Scholar]
- Yeh Ch. H., Tsai S. H., Wu L. W., and Ch. Lin Y., Lab Chip 11, 2583 (2011). 10.1039/c1lc20113a [DOI] [PubMed] [Google Scholar]
- Torisawa Y. S., Mosadegh B., Luker G. D., Morell M., O’Shea K. S., and Takayama S., Integr. Biol. 1, 649 (2009) 10.1039/b915965g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrych E., Pawlicka Z., Chudy M., Dybko A., and Brzozka Z., Anal. Chim. Acta 2, 149 (2011). 10.1016/j.aca.2010.10.005 [DOI] [PubMed] [Google Scholar]
- Atif M., Firdous S., Khurshid A., Noreen L., Zaidi S. S. Z., and Ikram M., Laser Phys. Lett. 6, 886 (2009). 10.1002/lapl.200910087 [DOI] [Google Scholar]
- Lee J. B., Choi J. Y., Chun J. S., Yun S. J., Lee S. C., Oh J., and Park H. R. B., J Dermatol. 159, 61, (2008). 10.1111/j.1365-2133.2008.08611.x [DOI] [PubMed] [Google Scholar]
- Chiu L. L., Sun C. H., Yeh A. T., Torkian B., Karamzadeh A., Tromberg B., and Wong B. J., Lasers Surg. Med. 37, 231 (2005). 10.1002/lsm.v37:3 [DOI] [PubMed] [Google Scholar]
- Kastle M., Grimm S., Nagel R., Breusing N., and Grune T., Free Radic Biol. Med. 50, 305 (2011). 10.1016/j.freeradbiomed.2010.11.012 [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.3658842 for comparison of viability after PDT procedures in the “mixed” culture in micro and macroscale.