Abstract
We experimentally investigate the effects of high electric field on living cells inside a charged droplet under electrophoretic actuation. When an aqueous droplet suspended in a dielectric liquid contacts with electrified electrode, the droplet acquires charge. This charged droplet undergoes electrophoretic motion under strong electric field (1–3 kV/cm), which can be used as a droplet manipulation method in biomicrofluidic applications. However, because strong electric field and use of dielectric oil can be a harmful environment for living cells, the biological feasibilities have been tested. Trypan blue test and cell growth test have been performed to check the viability and proliferation of cells in a droplet under various electric field strengths and actuation times. We have not observed any noticeable influence of electric field and silicone oil on the viability and proliferation of cells, which indicates that electrophoresis could be safely used as a manipulation method for a droplet containing living biological system.
INTRODUCTION
Microfluidic cell biology in the multidisciplinary field of micro total analysis systems (μTAS) has been extensively studied recently.1, 2 The application area of this biomicrofluidic technology has been diversified from biomedical areas such as clinical diagnostics, cancer research, assays, drug discovery, and tissue engineering to basic cell biology fields such as stem cell research, single cell analysis, microbiology, and cell mechanics.1 Especially, the in vitro compartmentalization of cells in a water-in-oil droplet offers a great number of opportunities in cell biology.3, 4, 5 Microdroplets provide an environment in which cells or biological reactions can be isolated minimizing cross contamination. Each droplet can be considered as an equivalent of the test tube or Petri dish having tiny volume with reduced reagent consumption and rapid mixing. These advantages unique to droplet microfluidics have been exploited in single cell analysis,6, 7 cell encapsulation and screening,8 and cell sorting.9
In recent times, active researches on electrostatic charging and the subsequent manipulation of a charged droplet have been reported.10, 11, 12, 13, 14, 15, 16 The electrostatic charging and sorting of droplets can be used as an on-demand sorting tool in microchannel where carrier fluid transports microdroplets.13, 14, 15, 16 It is also possible to control individual droplet without microchannel and carrier fluid, like electrowetting on dielectrics (EWOD) or dielectrophoresis (DEP). Using the electrophoresis of a charged droplet (ECD) actuation, a single droplet transportation,12 the coalescence of droplets, and the corresponding biochemical reactions11 have been demonstrated. The consistent actuations of various aqueous droplets, including a cell suspension droplet, have been shown.10 Minimal contact with electrode surface for droplet actuation and fast droplet velocity are suitable features of this ECD actuation method for biological applications.10
In spite of its advantages in biological applications, there are some concerns on the biocompatibility of the ECD as a droplet manipulation method for living cells. Direct current (dc) electric potential application can induce changes of cellular morphology and growth of living cells.17, 18 Serious electric effects on cell viability, membrane permeability, and cytoskeletal morphology of HeLa cells were reported.17 Negative effects of pulsed high electric field (2–5 kV/cm) on yeast cells in aqueous suspension were also investigated.19 On the other hand, some cells like Bacillus stearothermophilus is known to show strong resistance to high electric field up to 40 kV/cm. There is also a report that the application of electric field to living cells can promote metabolic responses.20 The ECD actuation needs high electric field (1–3 kV/cm) and there flows electric current when a droplet contacts with the electrode for charging (O(10−9) A). For that reason, we are concerned with the electric field effects mentioned above for the cells in a droplet under the ECD actuation.
Therefore in this work, the effects of the ECD actuation on the viability and proliferation of cells in a water-in-oil droplet have been investigated. For a water-in-oil droplet, high electric field can be mostly reduced inside a droplet due to Faraday cage effect. However, quantitative description on the effects of electric field on cells in a water-in-oil droplet has not been reported yet. In this regards, the effects of electric field strength and exposure time on the viability and proliferation of cells in a water-in-oil droplet have been considered. Because the use of silicone oil and the encapsulation in the form of microdroplet can have an effect on the cell viability or proliferation, for comparison, the effect of silicone oil has also been investigated separately.
MATERIALS AND METHODS
Experimental setup and materials
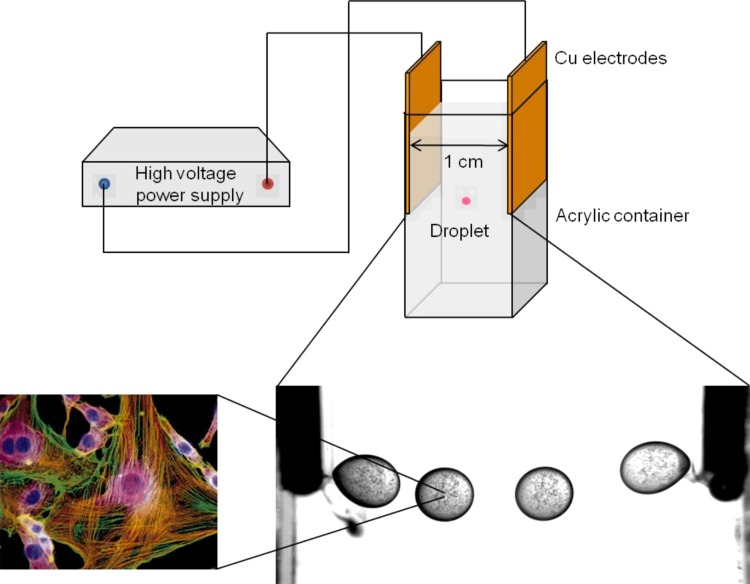
The schematic view of an ECD experimental system is shown in Fig. 1. A transparent rectangular 10 × 10 × 50 mm3 (Internal dimension) acrylic chamber is prepared for oil container. Two thin planar 10 × 30 mm2 copper electrodes are fixed on the inner wall of acrylic container to make uniform electric field. The distance between the electrodes is 1 cm and these electrodes are connected to a dc high voltage power supply (Treck Model 610E). The vertical length of the two parallel electrodes covers half of the full vertical length of acrylic container. Because a cell suspension droplet falls down in a dielectric oil due to density difference, this electrode design prevents falling of a droplet and makes consistent ECD actuation be possible. As a result of the balance between gravitational and electrical forces, a cell suspension droplet shows pendulum motion as illustrated at the bottom of Fig. 1.
Figure 1.
Schematic view of the experimental setup. The time step between sequential images of a 2.0 μl cell suspension droplet is 0.12 s.
As a dielectric suspending medium, silicone oil (Shin-Etsu KF 96, 50 cSt kinematic viscosity, 2.75 dielectric constant, 960 kg/m3 density) is used without any surfactant. Mouse 3T3-J2 fibroblast is used as a cell model. We did not use Escherichia coli or yeast as a cell model to avoid the contamination effect from outside environment. Because fibroblast is easy to handle and less sensitive to environment change, we can expect more consistent experimental results. Fibroblast is cultured in 90% air/10% CO2 at 37 °. Culture medium is the mixture of Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal bovine serum (FBS), and 100 g/ml Penicillin and streptomycin. The cultures are replenished with fresh medium every 3 days. After fibroblast covers more than 70% of the culture dish surface, cells are detached using trypsin–ethylenediaminetetraacetic acid (EDTA) and are collected to prepare cell suspension of 2.5 × 106 cells/ml concentration.
Experimental procedure
The acrylic oil container with the copper electrodes is prepared filled with silicone oil. The two electrodes are connected to a dc power supply. A 2.0 μl cell suspension droplet is dispensed by micropipette (Eppendorf) and the power supply is turned on. Due to initial positive charge of the droplet, the droplet goes to the negative electrode. Upon contact with the negative electrode, the droplet acquires negative charge and moves to the positive electrode. After several bouncing motion between the electrodes, due to gravity, the droplet reaches to the bottom edge of electrode and shows pendulum motion as in Fig. 1. After the ECD actuation for a given exposure time, the droplet on the bottom of acrylic container is collected by another micropipette and is put on each tube (Eppendorf) for preparation of the viability and the proliferation tests. For accurate analysis, the amount of sample should be more than 20 μl, however a droplet larger than 2 μl easily breaks up under usual ECD actuation. Therefore, the above experiment is repeated until sufficient amount of sample is obtained in each experimental condition.
To evaluate the effect of high electric field on cell viability and proliferation, three different electric fields (1, 2, 3 kV/cm) are considered. Under electric field less than 1 kV/cm, consistent ECD actuation is not possible under current experimental setup. The electrical force is not strong enough to overcome the gravity. Under electric field higher than 4 kV/cm, a droplet breaks up easily into smaller droplets. For the effect of ECD actuation time, three different conditions (10, 30, 90 s) under 3 kV/cm are considered. The velocity of droplet under ECD actuation is quite fast (2–3 cm/s). If we use less viscous oil than 50 cSt, we can make a droplet move even faster. Therefore, in an ECD based biomicrofluidic application, it is less probable to encounter the situation when a droplet should be continuously actuated for more than a minute.
Viability and proliferation tests
Right after all the ECD experiments, samples are centrifuged with 900 g for 3 min. After centrifugation, the supernatant is suctioned out and the cell pellets are suspended in 20 μl culture media. Among the 20 μl, 10 μl of sample and 0.4% trypan blue (Gibco) are mixed by 1:1 ratio and 10 μl of mixed sample is loaded in a hemocytometer and the number of live and dead cells are counted using a microscope (Olympus, Japan). Only cells in hemocytometer grids are counted and among them, dead cells are indicated with blue color.
The remaining 10 μl samples are suspended in 3 ml culture media again and each sample is seeded in three marked Petri dish which is used to take an image of samples in the same position. Culture medium is changed every two days and sample images are taken three times for 6 days at the same six positions using phase-contrast microscope (X71, Olympus Japan). Cell numbers are counted based on the obtained images. Cells are displayed with black spots in microscope images, and by counting the black spots, cell numbers are counted. Based on cell numbers at each day, cell growth curves are obtained.
For comparison, three different control groups are prepared. One is positive control group which is not exposed to any electric field or silicone oil. When the cell suspension is prepared, 20 μl of sample is divided and situated in a normal cell culture condition during the experiments. Another group is negative control group which is heated in 95 °C for 1 min. The other group is silicone oil group which is exposed to silicone oil for 1 h. The detailed explanations on the silicone oil group are described in the following section.
RESULTS AND DISCUSSION
Effects on cell viability
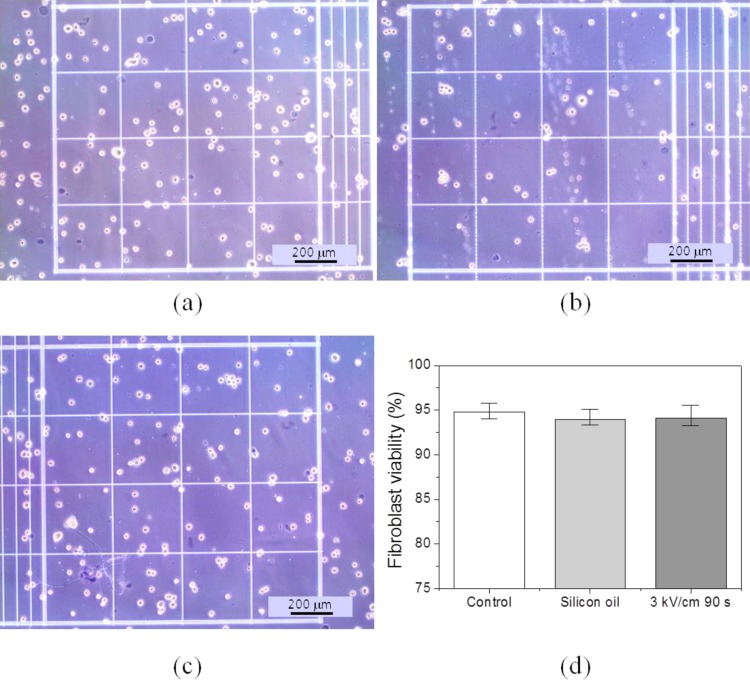
Cells encapsulated in water-in-oil droplets have shown to remain viable in several droplet microfluidic devices.3 Various cells like bacteria, yeast, mammalian cell, and even multicellular organisms can survive and proliferate inside a droplet for several days.3, 8 In the present work, to distinguish and quantify the effect of silicone oil and the encapsulation in the form of microdroplet, the silicone oil group is prepared. 20 μl sample is made by dispensing 2 μl cell suspension droplets in silicone oil. After 1 h, the cell suspension sample is taken out from silicone oil. The silicone oil group can be considered as 0 kV/cm for 1 h. As shown in Fig. 2, there is no noticeable change in fibroblast viability by silicone oil (the P value of the t test with control group is 0.16). However, the total number of cells in silicone oil group slightly decreases and as a result, the cell concentration decreases a little as in Fig. 2b.
Figure 2.
Trypan blue test results. (a) Control group (without any treatment). (b) Silicone oil group (Silicone oil in 1 h). (c) Experimental group under 3 kV/cm for 90 s. (d) Effects of silicone oil and ECD actuation on fibroblast viability (control group: 94.7% ± 0.59%, silicone group: 93.8% ± 1.2%, and experimental group: 94.0% ± 2.1%).
In the previous research, we had found that the cells in a droplet under ECD actuation with 3 kV/cm are viable.10 For that reason, we have checked the viability of fibroblast under 3 kV/cm for 90 s only, in the present study. Except for slight decrease in cell concentration as in the silicone oil group, no visible change in fibroblast viability is observed (the P value of the t test with control group is 0.49). The slight decrease in the percentage of living cells and the increase in the standard deviation of silicone (1.2%) and experimental (2.1%) groups are supposed to be attributed to the loss of cells during experiments. Therefore, the use of silicone oil and high electric field has little effect on the viability of fibroblast in a water-in-oil droplet under ECD actuation. However, the viability test result is not enough to confirm the biocompatibility of ECD actuation. In this regard, further proliferation of fibroblast has been conducted.
Effects on cell proliferation
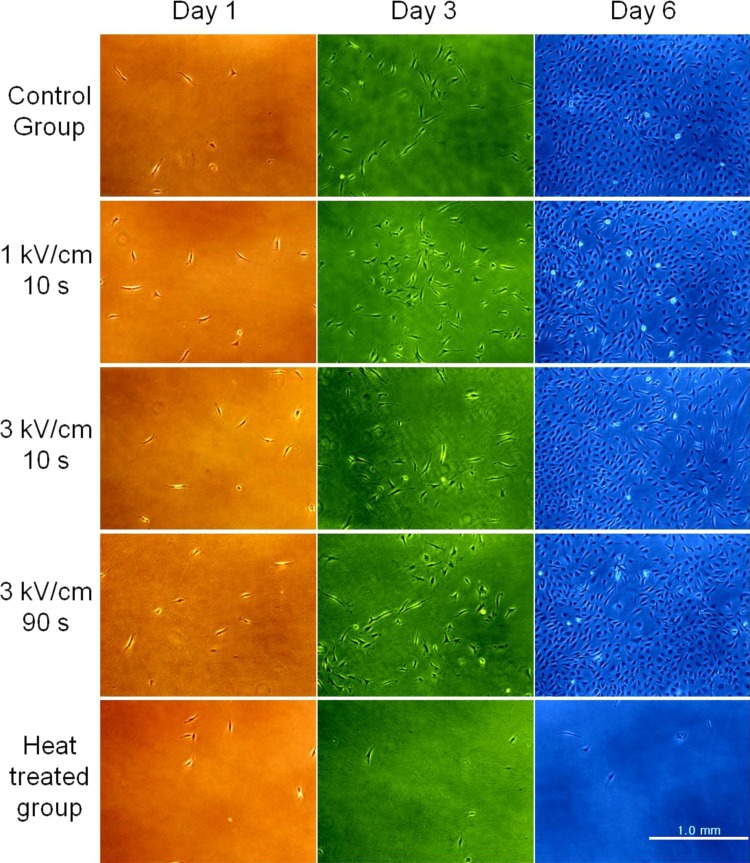
In the live and dead viability test, it is difficult to discriminate between healthy and damaged cells. Therefore, it is necessary to grow cell to check the function and the physical condition of cell even though there is little visible effect on cell viability. Including the control and the silicone oil groups, all the cells of the experimental groups are proliferated. In Fig. 3, the microphotographs of proliferated fibroblasts under different conditions are shown at days 1, 3, and 6. We can clearly see the proliferation of fibroblasts for 6 days of culture except for the heat treated group. There is little apparent effect of electric field strength and ECD actuation time on the proliferation of fibroblasts in Fig. 3. The results of the silicone oil group and the other experimental groups (2 kV/cm for 10 s, 3 kV/cm for 30 s) are not different from that of the control group.
Figure 3.
Phase contrast microscopic images for proliferated fibroblasts under different experimental conditions at different days. Colors are added artificially to distinguish columns easily.
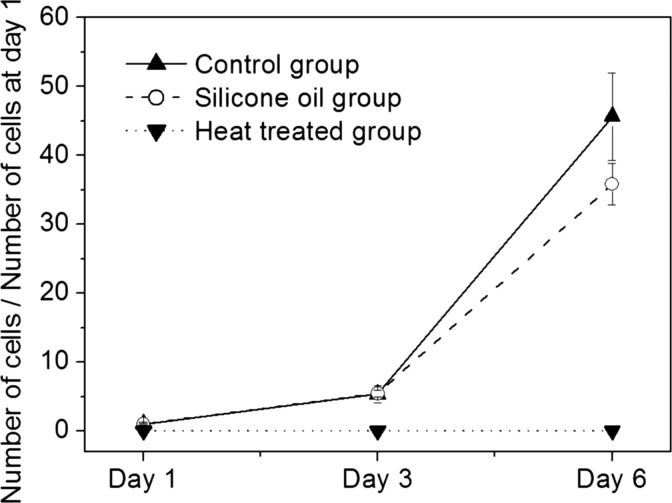
In Fig. 4, the cell growth rates of the control, the silicone oil, and the heat treated groups are compared to quantitatively show the effect of silicone oil on the proliferation of fibroblast. Even though we use the same cell suspension for making each droplet, the resulting cell concentration can be slightly different due to coagulation of cells during the experiments. As a result, the initial number of cells in each group is different. Therefore, the cell growth rate is estimated by dividing the number of cells at days 3 and 6 by the number of cells at day 1. Until the third day, the cell growth rates of the control and the silicone oil groups are nearly identical. However, at day 6, the cell growth rate of the silicone oil group is slightly lower than that of the control group (the P value of the t test is 0.04). This can be thought as a negative effect of silicone oil on the proliferation. However, this can be also explained by contact inhibition. Because the number of cells of the silicone oil group at day 1 (mean 72) is much higher than that of the control group (mean 41.5), more detailed explanations on the contact inhibition are given at the end of this section.
Figure 4.
Influence of silicone oil on the proliferation of fibroblast. The average number of cells in sample images at day 1 is 41.5 ± 15.8 for the control group and 72 ± 9.3 for the silicone oil group and at day 6, it is 1895.7 ± 393 for the control group and 2578.7 ± 322.4 for the silicone oil group, respectively.
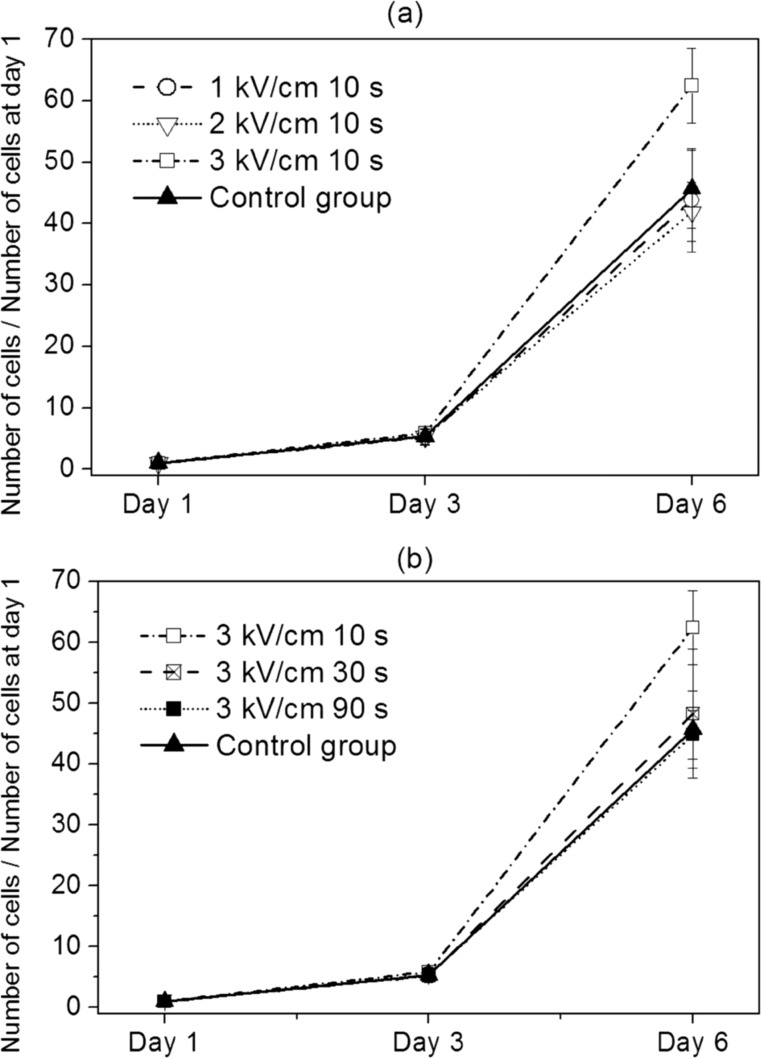
The effect of electric field strength of ECD actuation on the proliferation of fibroblast is illustrated in Fig. 5a. The ECD actuation time is fixed to be 10 s. As in the previous result for the silicone oil group, there are little differences in the growth rate of each group until day 3. The proliferation rates of 1 kV/cm (the P value of the t test with control group is 0.78) and 2 kV/cm (the P value of the t test with control group is 0.46) are slightly lower but not significantly different from that of the control group at day 6. Whereas for 3 kV/cm, the proliferation rate at day 6 is higher than that of the control group (the P value of the t test is 0.01). This can be thought as a positive effect of ECD actuation on the proliferation of fibroblast. However, as in the silicone oil group result, this can be also explained by contact inhibition effect. Because the number of cells of 3 kV/cm 10 s case at day 1 (mean 32) is lower than that of the control group (mean 41.5), in this regard, there is little dependency of electric field strength on the proliferation of fibroblast.
Figure 5.
Influence of the ECD actuation on the proliferation of fibroblast. (a) The effect of electric field strength. (b) The effect of ECD actuation time.
The effect of ECD actuation time on the proliferation is shown in Fig. 5b. The electric field strength is set to be 3 kV/cm. The overall trend is very similar to that of the previous results. As the ECD actuation time increases, the proliferation rate at day 6 decreases. However, it is difficult to explain this as the negative effect of increasing ECD actuation time. Because the two 30 and 90 s groups are not statistically different from the control group (the P value of the t test with the control group is 0.73 for 30 s and 0.87 for 90 s, respectively). Even though the proliferation rate of 10 s group is higher than the control group, this can be attributed to the contact inhibition as explained previous. Therefore, there is little effect of increasing the ECD actuation time on the proliferation of fibroblast. In all the experimental groups, we are not able to find any negative effect of ECD actuation on the proliferation of fibroblast in a water-in-oil droplet.
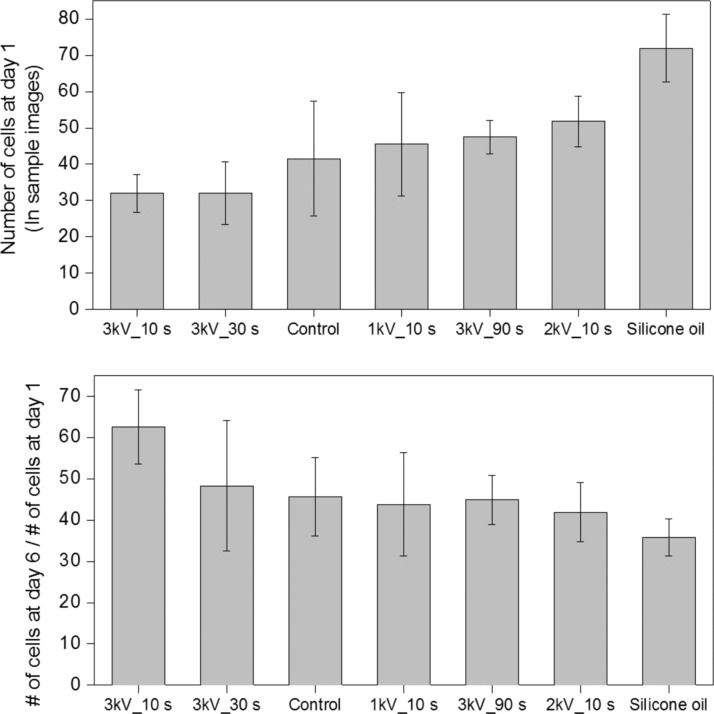
To show the contact inhibition effect more clearly, the number of cells at day 1 and the proliferation rate of cells at day 6 are illustrated in Fig. 6. Because all the groups are arranged in ascending order of the number of cells in sample images at day 1, the 3 kV/cm 10 s group (mean 32) appears leftmost and the silicone oil group (mean 72) located rightmost. Regardless of experimental conditions, the proliferation rate at day 6 shows clear negative correlation with the number of cells at day 1. For 3 kV/cm 10 s and 3 kV/cm 30 s groups, the average number of cells at day 1 is the same; however, the smaller relative standard deviation of 3 kV/cm 10 s group (16.3% for 10 s and 27.2% for 30 s, respectively) seems to play positive role on the proliferation. Because an even distribution of cells is more helpful for proliferation in the view point of contact inhibition, the relatively low proliferation rate of 1 kV/cm 10 s group at day 6 can be understood in the same context. All these findings imply that the proliferation of fibroblast in the current experimental groups mainly depends on the initial number of cells rather than experimental conditions. Therefore, we can conclude that there is no effect of the use of silicone oil and the ECD actuation of current conditions on the viability and proliferation of fibroblast in a water-in-oil droplet.
Figure 6.
The dependency of the proliferation rate of fibroblast at day 6 on the number of cells at day 1.
The current experimental observations are perfectly in line with the physical expectations: External high electric fields are screened from the inside of the droplet within the Debye length (Faraday cage effect). Because the conductivity of the cell culture solution is high enough (1.4 S/m) and the approximate value of the Debye length is sufficiently small (0.75 nm), the experimental results agree with the theory. The total charge deposited on a 2 μl droplet by the direct charging is about 5.5 × 10−11 C and the contact time with electrode is several millisecond.21 Therefore, the current upon charging is several tens of nanoampere. Furthermore, most charges are concentrated at the droplet surface. As a result, the cells inside a droplet are little affected by the direct charging.
Effects of cell concentration
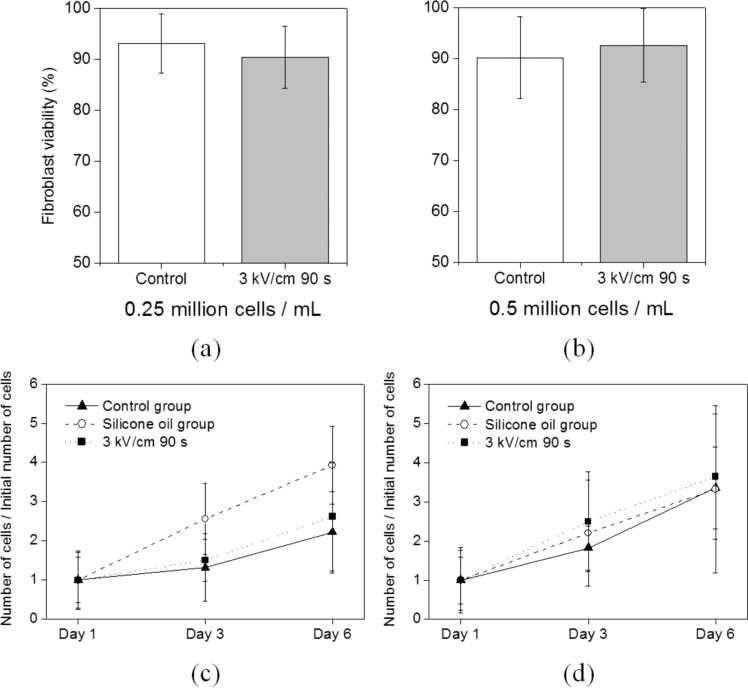
In droplet microfluidics for cellular assays, cell concentration is an important parameter. Therefore, we have also investigated the effects of cell concentration on the viability and proliferation of fibroblast under ECD actuation. Because the initial cell concentration of the previous results is fixed (2.5 × 106 cells/ml), the two different cell suspensions with lower cell concentrations (2.5 × 105 and 5.0 × 105 cells/ml) are used. As shown in Fig. 7, there is no noticeable change in the viability and proliferation of fibroblast for both the concentrations. Because of low cell concentration, the standard deviations of the cell growth rate data are higher than that of the previous results (the relative standard deviation ranges from 20% to 78%). Therefore, the previous conclusion for the viability and proliferation of fibroblast steel holds for lower cell concentration.
Figure 7.
The effects of cell concentration on the viability and proliferation of fibroblast. (a) The viability test results for 2.5 × 105 cells/ml. The P value of the t test is 0.49 (control group: 93.2% ± 5.8%, experimental group: 90.5% ± 6.1%). (b) The viability test results for 5.0 × 105 cells/ml. The P value of the t test is 0.62 (control group: 90.2% ± 8.0%, experimental group: 92.7% ± 7.2%). (c) The proliferation test results for 2.5 × 105 cells/ml. The P value of the t test with the control group is 0.88 for the experimental group and 0.16 for the silicone oil group. (d) The proliferation test results for 5.0 × 105 cells/ml. The P value of the t test with the control group is 0.42 for the experimental group and 0.83 for the silicone oil group.
Even though we have shown some biocompatibility of the ECD actuation, there are some limitations in the current work. For more consistent experimental results, we have used a fibroblast as a cell model because it is less sensitive to environmental change. However, this insensitivity to environmental change, in some way, can hide the effects of the ECD actuation and there is a risk for misunderstanding. We have several choices for droplet packaging: a droplet can be submerged, floated, or sandwiched between oils.10 Depending on the packaging, we also have many choices for dielectric oil. Therefore, additional studies on the biocompatibility using various cells with different droplet packaging can be beneficial for further confirmation. We have not used surfactant in the current system. The absence of surfactant in the system positively favors cell viability. However, this may limit the practical applicability because surfactant is useful for preventing adhesion to solid wall and uncontrolled merging of droplets. Therefore, researches relating to surfactant are also necessary for more practical applications.
CONCLUSION
We have investigated the effects of ECD actuation on the viability and proliferation of cells in a droplet. Trypan blue viability and the cell growth rate tests show that there is little effect of the ECD actuation and the use of silicone oil on the viability and proliferation of fibroblast in a water-in-oil droplet under various ECD actuation conditions. This biocompatibility of ECD actuation is partly attributed to Faraday cage effect: an aqueous droplet surrounding cells plays a role of shielding external high electric field. Unlike our initial concerns about the current flow induced by charging of a droplet, the amount of current seems to be small enough not to affect the viability or function of living biological system. We hope the current biocompatibility results can be a basis for the use of a water-in-oil droplet and the ECD actuation method in various biomicrofluidic applications for living cells.
ACKNOWLEDGMENTS
This work has been supported by Grant R01-2010-0027653 from National Research Foundation (NRF) of Korea, and by BK21 program of the Ministry of Education, Science and Technology (MEST) of Korea.
References
- Salieb-Beugelaar G. B., Simone G., Arora A., Philippi A., and Manz A., Anal. Chem. 82, 4848 (2010). 10.1021/ac1009707 [DOI] [PubMed] [Google Scholar]
- Lu H., Rivet C., Lee H., Hirsch A., and Hamilton S., Chem. Eng. Sci. 66, 1490 (2011). 10.1016/j.ces.2010.08.015 [DOI] [Google Scholar]
- Hollfelder F. and Schaerli Y., Mol. Biosyst. 5, 1392 (2009). 10.1039/b907578j [DOI] [PubMed] [Google Scholar]
- Huck W. T. S., Theberge A. B., Courtois F., Schaerli Y., Fischlechner M., Abell C., and Hollfelder F., Angew. Chem. Int. Ed. 49, 5846 (2010). 10.1002/anie.200906653 [DOI] [PubMed] [Google Scholar]
- Demello A. J., Huebner A., Sharma S., Srisa-Art M., Hollfelder F., and Edel J. B., Lab Chip 8, 1244 (2008). 10.1039/b806405a [DOI] [PubMed] [Google Scholar]
- Mellors J. S., Jorabchi K., Smith L. M., and Ramsey J. M., Anal. Chem. 82, 967 (2010). 10.1021/ac902218y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler A. R., Throndset W. R., Whelan R. J., Leach A. M., Zare R. N., Liao Y. H., Farrell K., Manger I. D., and Daridon A., Anal. Chem. 75, 3581 (2003). 10.1021/ac0340758 [DOI] [PubMed] [Google Scholar]
- Clausell-Tormos J., Lieber D., Baret J. C., El-Harrak A., Miller O. J., Frenz L., Blouwolff J., Humphry K. J., Koster S., Duan H., Holtze C., Weitz D. A., Griffiths A. D., and Merten C. A., Chem. Biol. 15, 875 (2008). 10.1016/j.chembiol.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Chabert M. and Viovy J. L., Proc. Natl. Acad. Sci. U.S.A. 105, 3191 (2008). 10.1073/pnas.0708321105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D. J., Noh J., moon D., and Kang I. S., Anal. Chem. 83, 5168 (2011). 10.1021/ac200248x [DOI] [PubMed] [Google Scholar]
- Jung Y. M. and Kang I. S., Biomicrofluidics 4, 024104 (2010). 10.1063/1.3427356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y. M. and Kang I. S., Biomicrofluidics 3, 022402 (2009). 10.1063/1.3122299 [DOI] [Google Scholar]
- Link D. R., Grasland-Mongrain E., Duri A., Sarrazin F., Cheng Z. D., Cristobal G., Marquez M., and Weitz D. A., Angew. Chem. Int. Ed. 45, 2556 (2006). 10.1002/anie.v45:16 [DOI] [PubMed] [Google Scholar]
- Li C. M., Wang W., Yang C., and Liu Y. S., Lab Chip 10, 559 (2010). 10.1039/b924929j [DOI] [PubMed] [Google Scholar]
- Zhao X. Z., Guo F., Ji X. H., Liu K., He R. X., Zhao L. B., Guo Z. X., Liu W., and Guo S. S., Appl. Phys. Lett. 96, 193701 (2010). 10.1063/1.3360812 [DOI] [Google Scholar]
- Ahn B., Lee K., Panchapakesan R., and Oh K. W., Biomicrofluidics 5, 024113 (2011). 10.1063/1.3604393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita M., Ikariyama Y., and Aizawa M., J. Biotechnol. 14, 321 (1990). 10.1016/0168-1656(90)90116-S [DOI] [PubMed] [Google Scholar]
- Toh S. L., Sahoo S., Lee W. C., and Goh J. C. H., Biotechnol. Bioeng. 106, 690 (2010). 10.1002/bit.22734 [DOI] [PubMed] [Google Scholar]
- El Zakhem H., Lanoiselle J. L., Lebovka N. I., Nonus M., and Vorobiev E., J. Colloid Interface Sci. 300, 553 (2006). 10.1016/j.jcis.2006.04.055 [DOI] [PubMed] [Google Scholar]
- Oliveira A. A. C., Sousa T. V. S., Amaral P. F. F., Coelho M. A. Z., and Araujo O. Q. F., Chem. Eng. Trans. 20, 133 (2010). 10.3303/CET1020023 [DOI] [Google Scholar]
- Ristenpart W. D., Bird J. C., Belmonte A., Dollar F., and Stone H. A., Nature (London) 461, 377 (2009). 10.1038/nature08294 [DOI] [PubMed] [Google Scholar]