Abstract
Spheroids that are formed from aggregated cells have enhanced biological function compared to individual cells. In particular, hetero-spheroids composed of different types of cells, such as hepatocytes and endothelial cells, express tissue specific functions at a high level, which is advantageous for more precise drug screening and biological research. In this study, we propose rapid formation of size-controlled three-dimensional hetero-cell aggregates consisting of hepatocytes and endothelial cells using micro-rotation flow. Based on previous data, these aggregates are expected to ultimately become hetero-spheroids. The hepatocytes are coated with collagen gel films less than 200 nm thick, which were experimentally verified to increase adhesion strength between hepatocytes and endothelial cells. Gel-coated hepatocytes and endothelial cells are collected in an array by micro-rotational flow, thereby forming hetero-cell aggregates within 2 min. This array allowed the size of the three-dimensional cell aggregates to be hydrodynamically controlled, with standard deviations of less than 19%, by varying the cell density of the medium without altering the device geometry. Endothelial cells were successfully and uniformly dispersed in the aggregates. The proposed microfluidic device, with its capability of rapidly forming size-controlled hetero-cell aggregates, will offer an efficient experimental platform for future hetero-spheroid study that will contribute to drug screening and regenerative medicine.
INTRODUCTION
Cells in the human body aggregate together and interact with neighboring cells and the extracellular matrix. Several cell types, such as hepatocytes,1 osteoblasts,2, 3 and embryonic stem cells,4 form spheroids in vitro that function better than individual cells. For example, a spheroid based on hepatocytes performs about 500 liver-specific functions, such as the expression of new proteins and cell signaling, and increases their metabolic functions. Therefore, a variety of devices have been developed to form three-dimensional spheroids in order to improve the pre-animal and pre-clinical phases of drug candidate testing,5 to perform biological research more precisely, and to establish personalized treatment strategies using tissue biopsies of patients.6
In recent years, several co-culture approaches have also been adopted to more precisely investigate tissue functions and interaction between different cell types.7, 8 In particular, the behavior of spheroids composed of more than one cell type, termed hetero-spheroids (HS),9 as well as their reaction to drug simulation, appears to be closer to those of tissue than spheroids that are composed of one cell type (homo-spheroids).10, 11, 12 Therefore, hetero-spheroids are often used for transplantation assays that are based on grafting of an ex vivo generated spheroid into a mouse13 for evaluation of anti cancer drugs9 and cancer invasion.9 Adhesion between different cell types is not as strong as that between the same cell type,14 and thus, it is difficult to rapidly form a three-dimensional hetero-cell aggregate, which will in turn become a hetero-spheroid. Several techniques of forming mixed cell aggregates have been developed to enhance adhesion between different types of cells, which include the use of a Primaria dish,15 chemical agents,16 micro-channels,2 and hydro gels.17 Abu-Absi et al. reported the formation of a mixed spheroid consisting of hepatocytes and a hepatic stellate cell line using a Primaria dish.15 While this technique was simple to perform and could form multiple spheroids in one test, the spheroid size could not be controlled. Photolithographically manufactured micro channels have been applied to form size-controlled spheroids.2, 4 Three-dimensional hetero-spheroids composed of PC-3 and MC3T3 cells or HUVEC were successfully demonstrated, where their sizes were controlled by the volumes of cell aggregates in a cul-de-sac and on the number of cells. In recent years, techniques involving the seeding of several cell types in hydrogels such as alginate,18, 19 collagen,20 and agarose21 have been developed and result in spheroid formation. The hydrogel beads contain cells at the desired size, leading to an ability to control spheroid size.18 However, these techniques required a long time period for the cell aggregates to reach a desired size.
We previously developed a hepatic spheroid forming chamber that used micro-rotational flow to control the spheroid size.22 In our device, a perfusion medium containing cells was introduced into a microchamber in which a micro-rotational flow was generated. Cells were collected in the center of the microchamber, where they aggregated and formed a spheroid. The developed chamber could create spheroids with diameters in the range of 130–430 μm with a standard deviation of approximately 15%. This chamber, which has an array composition, is superior to other microfluidic devices in that hepatic spheroids can be formed within 120 sec, and spheroid sizes can be controlled by altering the cell density of the medium without the necessity of changing the geometry of the device. Furthermore, this device provides a space for spheroids to grow since the cells are concentrated around the center of the chamber by hydrodynamic forces.
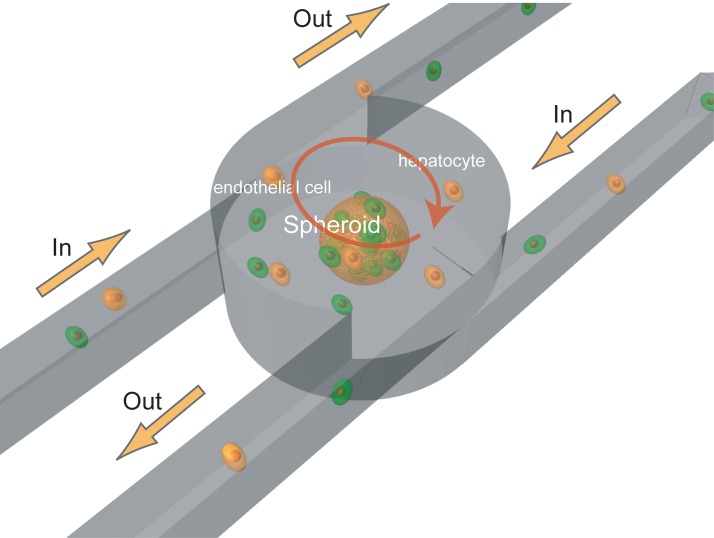
In this study, we demonstrate rapid formation of mixed cell aggregates composed of endothelial cells and hepatocytes, both of which are components of liver, using a modified spheroid formation chamber (Fig. 1). We coated the walls at the base of the spheroid formation chamber with a 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer to prevent endothelial cells from subsiding in the chamber and forming a hetero-aggregates. In order to increase adhesion between hepatocytes and endothelial cells, the hepatocytes were coated with a collagen gel. Without this surface treatment, hepatocytes and endothelial cells did not adhere to each other and did not form hetero-cell aggregates. Thus, collagen coating of cell surfaces, coupled with the cell handling technique using micro-rotation flow, enabled the formation of mixed hetero-cell aggregates of a controlled size within 2 min. Our device can effectively form hetero-cell aggregates and in turn, possibly hetero-spheroids, which will lead to large reduction in the duration of experiments in spheroid research.
Figure 1.
Spheroid formation chamber using micro-rotation flow. The chamber consists of three layers with two inlet channels tangential to the cylinder in the middle layer and two outlet channels tangential to the cylinder in the upper layer.
EXPERIMENTAL METHODS
Array design and fabrication
The array consists of three microchambers; each microchamber is a circular cylinder. Each cylinder has two inlet channels at its base, which are tangential to the cylinder, and two outlet channels at its top (see Fig. 1). Micro-rotational flow is generated by fluids flowing through the two inlet channels. To enhance the throughput of spheroid formation, we developed an array that consists of five sets of chambers coupled in series.23
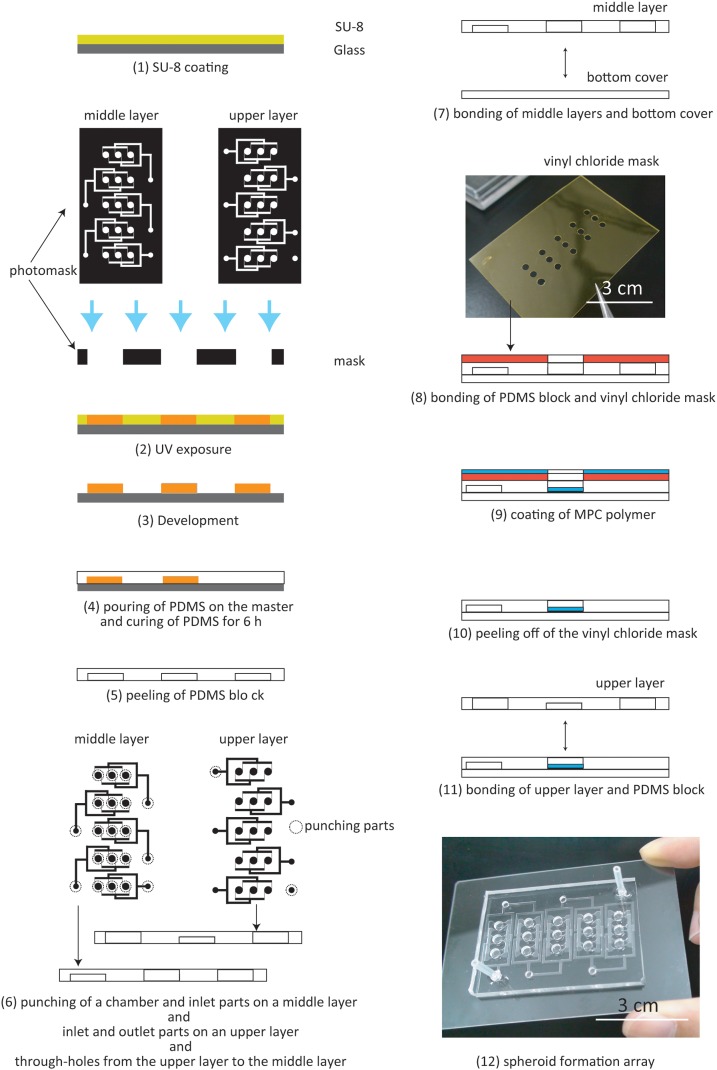
The array is made from polydimethylsiloxane (PDMS) (Silpot 184 W/C, Dow Corning Corp., Japan) and was formed using the following process; First, a negative photoresist SU-8 (SU-8 10, MicroChem Corp, Japan.) was patterned to produce circular cylinders and channels with array geometries on a clean glass slide using photolithography (Fig. 2-1). We used two different geometries for the upper and middle layers (Figs. 2-2, 2-3). Liquid PDMS was poured into the mold and cured on a hotplate at 65 °C for 6 h. Cured PDMS was then peeled from the mold (Figs. 2-4, 2-5). The inlets and outlets in the upper layer, and the inlets, the chamber, and through-holes from the upper layer to the middle layer, were created using a punch (Fig. 2-6). The middle layer and the bottom cover were aligned and bonded by oxygen plasma treatment (Fig. 2-7).
Figure 2.
Fabrication process.
In this study, an MPC polymer (Lipidure-CM5206E, NOF CORPORATION, Japan) which can decrease protein adhesion was coated on the bottom surface of the chamber to prevent cell adhesion to this surface.24 After the bonding process, a vinyl chloride mask, which had through-holes at the same position as the chambers in the array, was aligned with the PDMS block and bonded using pressure (Figs. 2-8, 2-9). The MPC polymer was coated onto the surface via the through-holes and dried. After 1 h, the mask was removed from the PDMS block. Finally, the upper layer and the PDMS block were aligned and bonded by oxygen plasma treatment (Figs. 2-11, 2-12).
The spheroid diameter was designed to be about 180 μm, since spheroids with diameters greater than 180 μm undergo cell necrosis in their cores.25 We, therefore, formed an SU-8 mold with a 3-mm-diameter chamber, which could form spheroids with diameters in the range of 130–240 μm according to our previous work. The channels connecting the chambers were designed to be 100 μm in width and 100 μm in height.
Perfusion system set-up
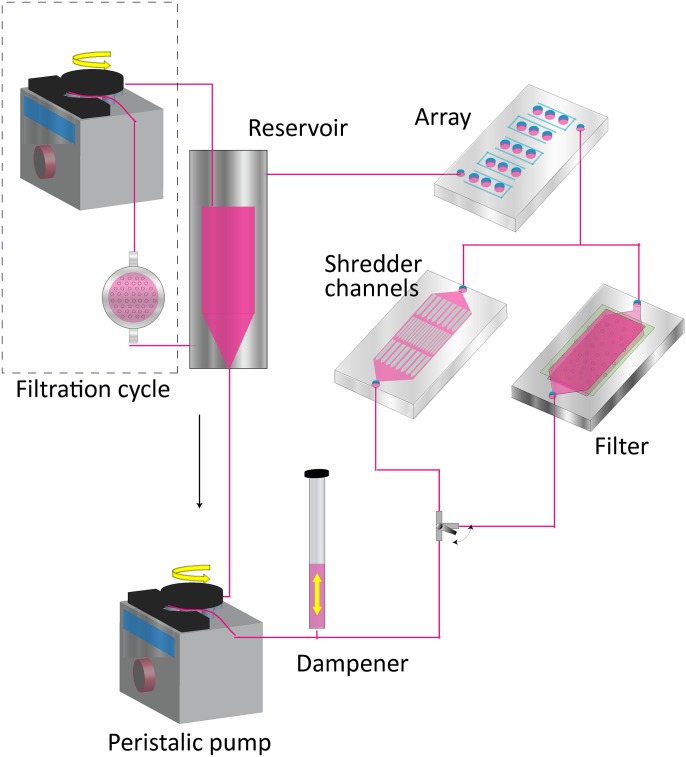
It is also necessary that the cells or spheroids on the spheroid formation platform can be cultured long term, since it takes at least 1 day for spheroids to exert enhanced cellular functions.23 In a previous study, we reported a novel perfusion system consisting of a peristaltic pump, a reservoir, a dampener, shredder channels, and a filtration system to provide a steady flow of cell culture medium containing cells to a rotating spheroid (Fig. 3).23
Figure 3.
Spheroid formation device consisting of an array and a perfusion system with a reservoir, shredder channels, a dampener, a filtration system, a peristaltic pump, and a filter.
Medium containing cells is stored in the reservoir. The medium is circulated in the perfusion system by a peristaltic pump. In order to prevent pulsatile flow generated by the peristaltic pump, we connected a pulsation dampener to the pump. Cells sometimes aggregated in the tubes and the reservoir, thereby clogging the channels of the chamber. We developed shredder channels and a filtration system to prevent undesired cell aggregates entering the chamber and to collect excess cells which were not used for spheroid formation from the medium. Shredder channels break aggregates up by shear stress or, when the bonding force of an aggregate is too strong to break it up, it is trapped in the channels. The filtration system, the Terufusion® Final Filter PS (Terumo, Japan, pore size: 0.2 μm), collects excess cells in the medium and sterilizes the medium by collecting bacteria and debris.
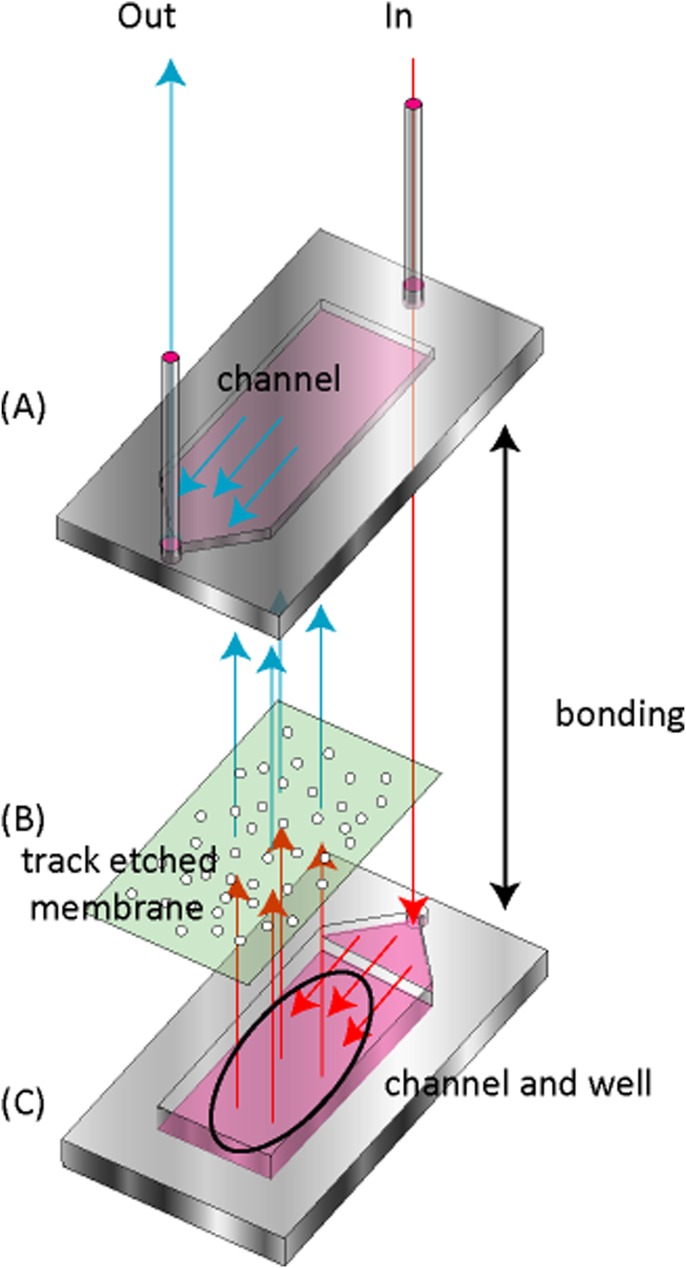
We used shredder channels to prevent large cell aggregates from entering the chamber.22 However, during the experiments, large cell aggregates did get trapped in the shredder channels and clogged the channels of the chamber, resulting in unstable micro-rotation flow after 1 day. We, therefore, developed a new filter to solve this problem (Fig. 4).26 The filter consisted of three parts: a polypropylene lower part, a polypropylene upper part, and a filter (pore size: 30 μm; track etched membrane, It4ip). The upper part has a channel with a depth of 2 mm that is 1 cm in width and 2 cm in height. The lower part has both a channel with a depth of 2 mm that is 1 cm in width and 2 cm in height and a well with a depth of 8 mm that is 1 cm in width and 2 cm in height. These parts are adhered together to form the filter. During the experiments, the medium containing the cells flows along the channel in the lower part and passes through the filter. When cells and cell aggregates (> 30 μm) enter the well in the lower part or pass through the filter, they settle in the well or are trapped in the filter. The developed filter can accumulate cells in the well and trap small cell aggregates with high efficiency. After the aggregates had formed, we exchanged the filter pathway with the shredder channel pathway.
Figure 4.
The filter consisted of three parts: an upper part with a channel (A), a filter (B), and a lower part with a channel and a well (C). Cells and cell aggregates enter the filter device from the inlet and flow into a channel in the lower part (C). When cell aggregates of greater than 30 μm in diameter flow through the pores, they are trapped in the well and the filter.
Collagen gel coating
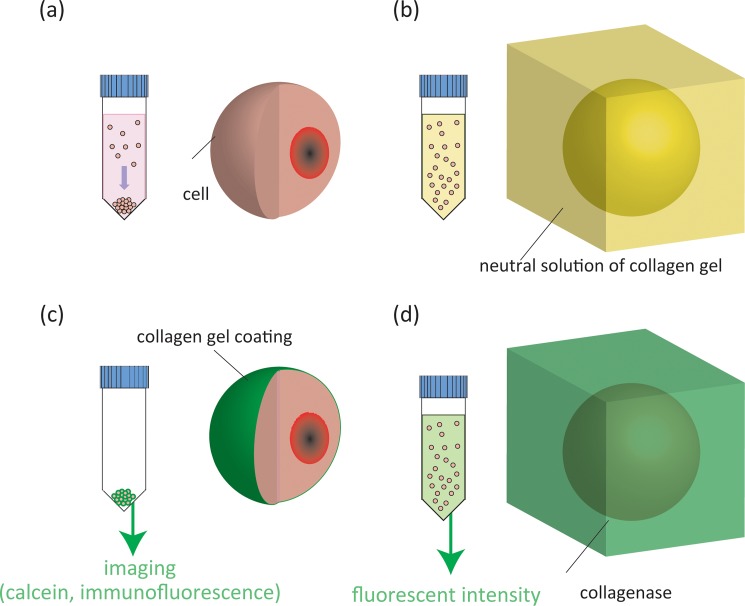
In order to promote adhesion between different cell types, we coated cells with a collagen gel (Atelo cell, KOKEN) (Figs. 5a, 5b). HepG2 cells were cultured and detached using ethylenediaminetetraacetic acid (EDTA) and trypsin (both from DS Pharma Biomedical Co., Ltd, Japan.). Collected cells were incubated in a neutral solution of collagen gel at 20 °C. After 1 h incubation, these cells were centrifuged and collected. Medium at 37 °C was added to the cell suspension, and the cells were incubated at 37 °C for 1 h to reconstitute a collagen gel coating on the surface of the cells.
Figure 5.
Collagen gel coating process. Cells are collected by centrifugation (a) and incubated in a neutral solution of collagen gel (b). Gel-coated cells are collected and observed by adding calcein into the collagen gel followed by immunofluorescent analysis (c). Gels covering cell surfaces are liquefied using collagenase (d).
Imaging of cells coated with a collagen gel
In order to confirm collagen coating on the cells, we added calcein (Sigma, Japan) to a neutral solution of the collagen gel before coating the cells (Fig. 5c). After coating of the gel, we then confirmed the presence of calcein by microscopically monitoring calcein fluorescence. We further visualized collagen on the cells using an immunofluorescent technique. The cells were fixed for 1 h in 4% paraformaldehyde (Wako, Japan) at 4 °C. The cells were then washed twice with PBS, incubated for 60 min with the primary antibody (collagen type 1 antibody, GeneTex, Japan) then washed twice with PBS and labeled for 60 min with the second antibody, Alexa Fluor-conjugated anti-mouse IgG (Cell Signaling, Japan). Fluorescence images of collagen were acquired using microscopy (Nikon TE-2000).
Measurement of collagen gel content
The amount of collagen gel on cell surfaces was determined by liquefaction of the collagen using collagenase (Sigma) treatment followed by assessment of the fluorescent intensity of the incorporated calcein. HepG2 cells were cultured and then detached (Fig. 5d). Collected cells were incubated in a neutral solution of collagen gel containing calcein at 20 °C. After 1 h incubation, these cells were centrifuged and collected. Medium at 37 °C was added to the cell suspension, and the cells were incubated at 37 °C for 1 h to reconstitute a collagen gel coating on the surface of the cells. A suspension of HepG2 cells, coated with collagen plus calcein as described, was treated with collagenase and incubated for 1 h to liquefy the formed collagen gel. The supernatant was collected and analyzed using a spectrofluorometer (ex. 405 nm, em. 620 nm) (RF 1500, Shimadzu, Japan).
Cell experiments
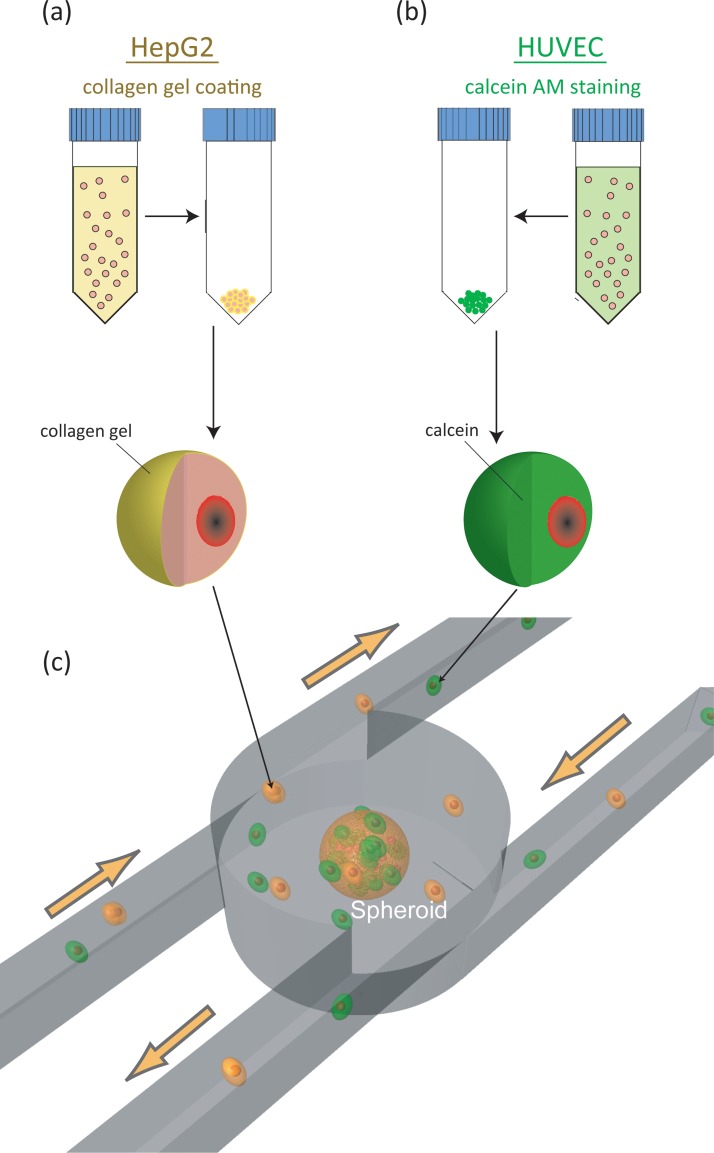
HepG2 cells and HUVEC were cultured and detached using EDTA and trypsin. Collected HepG2 cells were coated with a collagen gel (Fig. 6a), whereas HUVEC were stained with 4 μM calcein acetoxymethyl ester (AM) (Invitrogen, Japan) to detect HUVEC in the cell aggregates (Fig. 6b).
Figure 6.
Schematic illustration of the experimental process. HepG2 are coated with collagen gel (a) and HUVEC are stained with calcein AM (b). The spheroid formation chamber aggregates gel-coated HepG2 cells and stained HUVEC and forms a cell aggregate by micro-rotation flow.
First, the medium containing HepG2 cells and HUVEC was introduced into the array at a flow rate of 3.3 ml/min until stable flow of the cells around the entire array was achieved. Second, we gradually reduced the flow rate to an appropriate flow rate (approximately 1.2 ml/min), when the cells near the center of the array were attracted to the center, enabling a cell aggregate to form.22 After formation of the cell aggregates, the filtration system was activated and the pathway of the filter was opened to prevent undesired cell aggregates from entering the chamber. Stationary and fluorescence two-dimensional images were captured using a CCD camera (Cool SNAP-cf, Nippon Roper Co., Ltd and EOS Kiss X3, Canon). The diameter of the cell aggregates was measured based on these captured images.
RESULTS AND DISCUSSION
Amount of collagen gel coated on cell surfaces
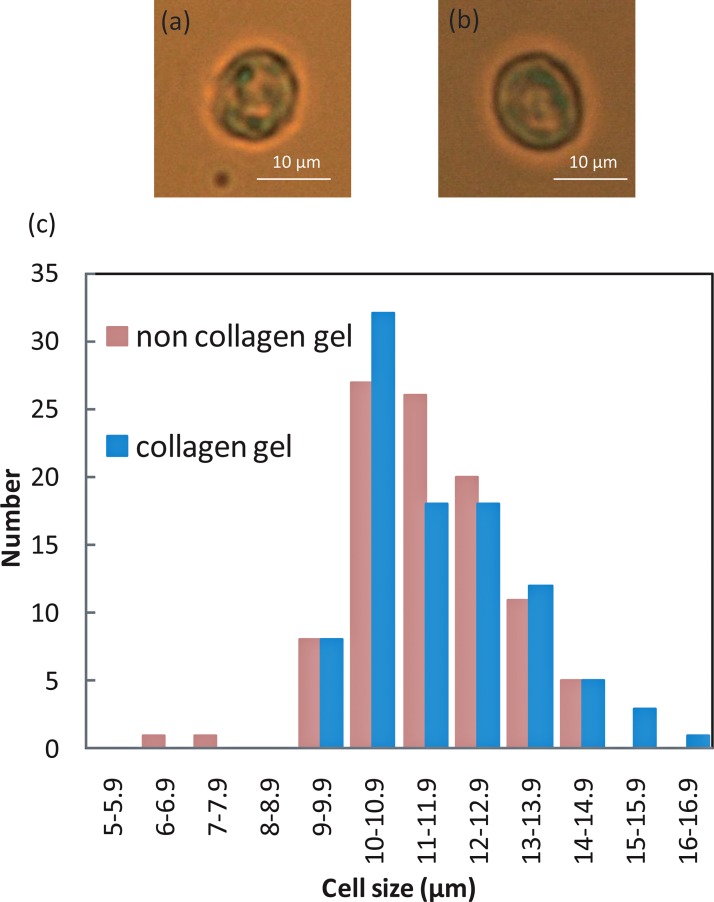
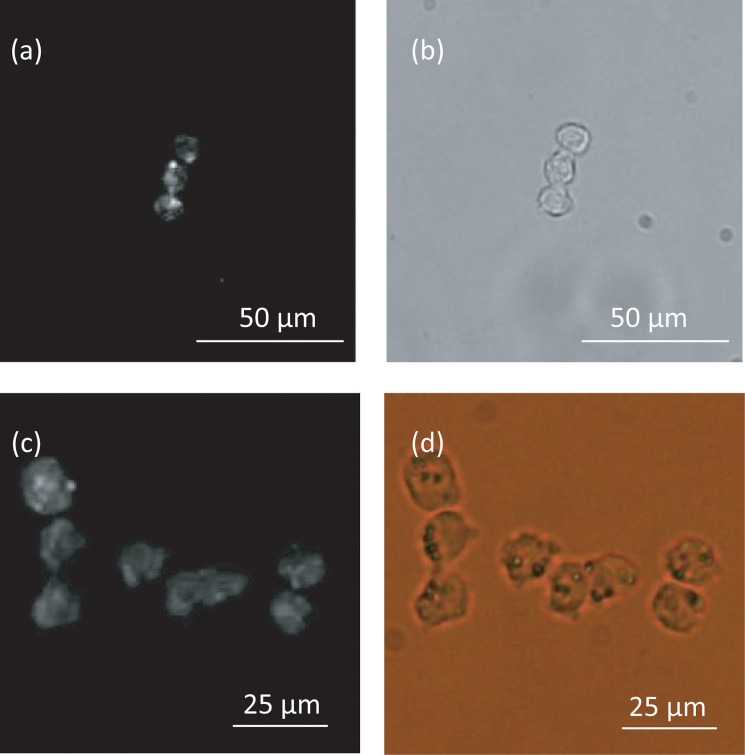
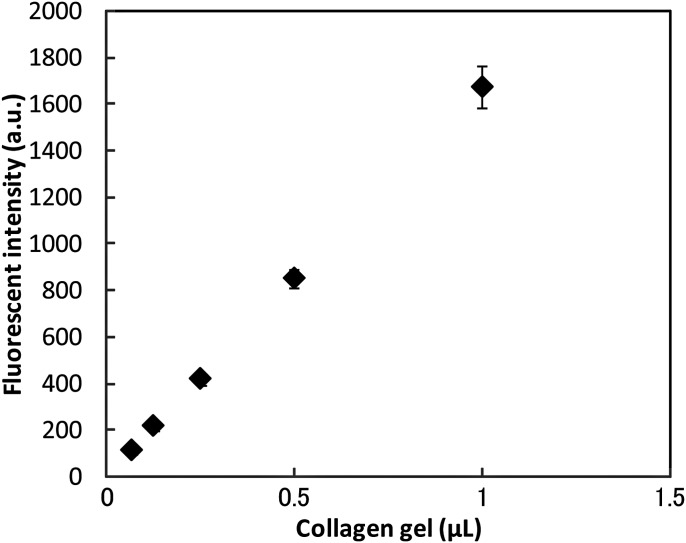
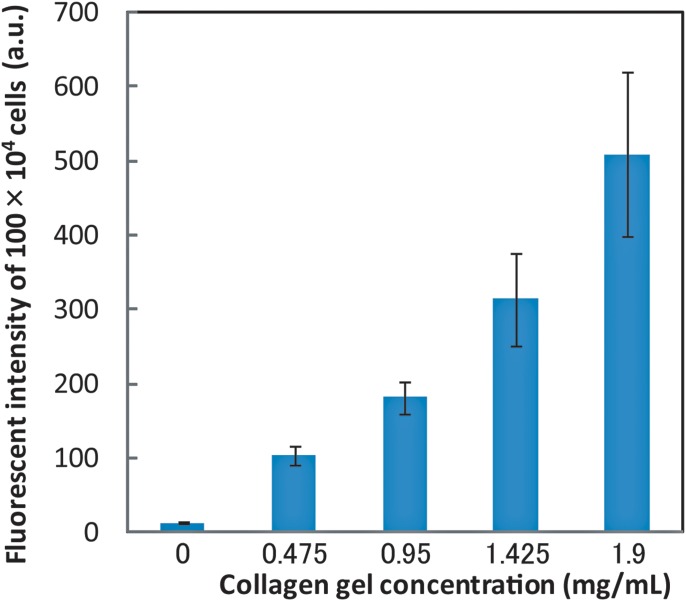
Figure 7 shows the size distribution of collagen gel-coated and non-coated cells. No significant difference in cell size between non-coated and coated cells was observed. Fluorescent images of collagen gels containing calcein and collagen, which were obtained using an immunofluorescent technique, demonstrated that the collagen gel adheres to and covers the cell surface (Fig. 8). We measured the amount of collagen gel that was coated on the cells by liquefying and extracting the collagen coats by collagenase treatment. This experiment showed that the fluorescent intensity of calcein increased as a function of the collagen gel volume (Fig. 9) as well as of the volume of collagen gel that was liquefied by the collagenase (Fig. 10).
Figure 7.
Optical images of non gel-coated (a) and gel-coated (b) cells, and distribution of cell sizes (c) (N = 100).
Figure 8.
HepG2 cells coated with a collagen gel. Fluorescent image (a) and optical image (b), of cells coated with a collagen gel containing calcein. Fluorescence image (c) and optical image (d) of cells coated with collagen using an immunofluorescent technique.
Figure 9.
Fluorescent intensity of calcein in a collagen gel as a function of collagen gel content.
Figure 10.
Content of collagen in the gel extracted from HepG2 cells. Fluorescent intensity of calcein in a collagen gel as a function of collagen gel concentration.
Based on the results of gel extraction and calibration experiments, the collagen gel layer coated on the cells was determined to be 180 nm thick. Thus, using our method, the ratio of the volume of collagen gel coating to cell size is much lower (less than 10%) than that obtained using other hydrogel methods.27, 28 A decreased amount of collagen coating of the cells will lead to decreased inhibition of molecular transportation by the extracellular matrix (ECM). The ECM has been reported to prevent molecules such as nutrients and growth factors from penetrating tissue.29 In addition, our method needs only commercially available products and is simple to perform. Therefore, this cell coating method can be applied for the promotion of adhesion of cell types other than hepatocytes and HUVEC, which adhere to collagen type 1.
Hetero-cell aggregate formation
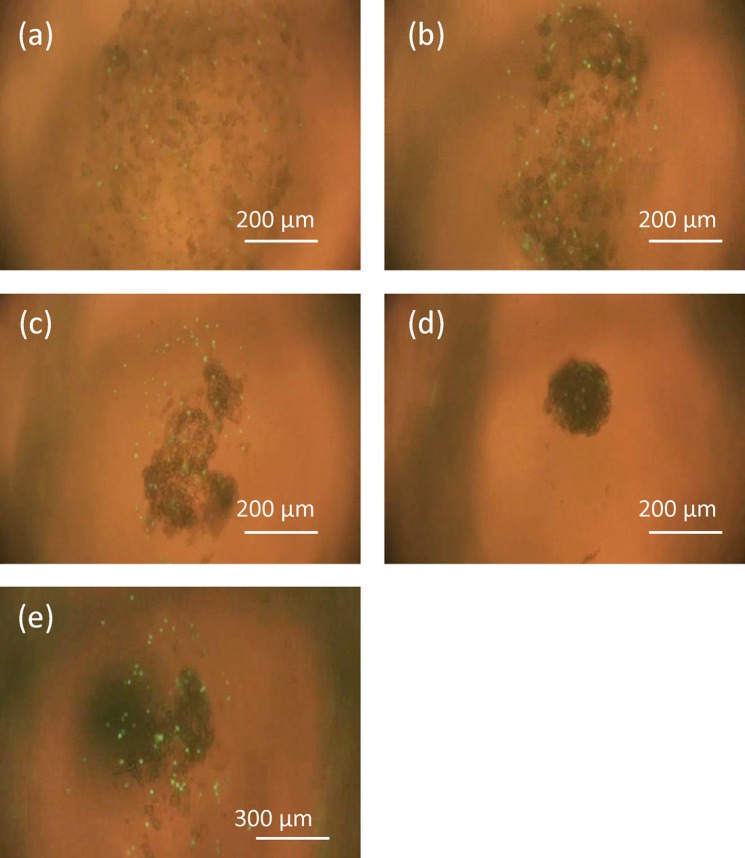
Micro-rotation flow was generated in the entire chamber at a flow rate of 3.3 ml/min. As the flow rate was reduced, the cells gradually aggregated and formed a cell aggregate within 120 sec (Fig. 11). A flow rate of 1.2 ml/min was required for the formation of stable cell aggregates and for their continued rotation. Before performing this experiment, we conducted hetro-cell aggregate formation experiments using non gel-coated HepG2 cells and HUVEC stained with calcein AM. Under these conditions, the cells only adhered to the same cell species and two aggregates, which were composed of HepG2 and HUVEC, were formed. The chamber could not control cell aggregate size, since adhesion between the HepG2 cells and HUVEC was so weak that adhesion of cells of the same cell type was dominant (Fig. 11e). In contrast, gel-coated HepG2 and HUVEC adhered to each other and formed a hetero-cell aggregate in the center of the chamber, which did not break up during culture (Figs. 11a–11d).
Figure 11.
(a)-(d) Process of cell aggregation in the chamber. Cells stably rotated around the entire area of the chamber at a flow rate greater than 3.3 ml/min at (a) 0 sec. When the flow rate was reduced to 1.2 ml/min, they started to aggregate in the center of the chamber and formed a cell aggregate after 120 sec. (b), (c), and (d) show aggregation of the cells after (b) 30 sec (c) 60 sec, and (d) 120 sec, respectively. (e) Process of cell aggregation using non gel-coated HepG2 cells.
In previous work, it took a few hours to create cell aggregates composed of several cell types with designed sizes (Table TABLE I.) since these devices confined cells to a small area but did not actually induce aggregation. In contrast, our proposed device can confine the cells to a hydrodynamically confined region and induce them to aggregate by rotation flow, which enabled rapid formation of hetero-cell aggregates in 120 sec.
TABLE I.
Hetero-aggregate-forming technique.
| Primaria | hydrogel beads | micro-channel | hydrogel well | micro-rotation flow | |
|---|---|---|---|---|---|
| Simple to perform | ○ | × | ○ | ○ | Δ |
| Size controllability | × | ○ | ○ | × | ○ |
| Space for spheroid growth | ○ | Δ | × | ○ | ○ |
| Time scale for formation of aggregation | day | day | day | day | minute |
| Characteristics | can form large spheroids | depends on cell species | |||
| Ref. | 15 | 22 | 2, 4 | 17 | this study |
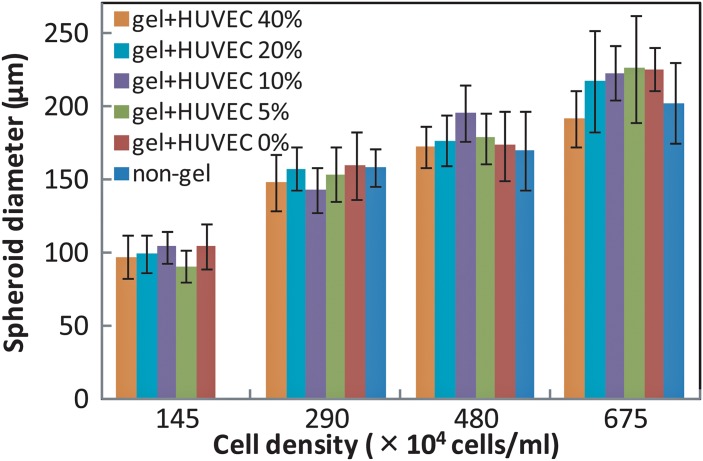
Cell aggregate formation experiments were next performed in an array using 3-mm-diameter chambers and total cell densities of 145 × 104, 290 × 104, 480 × 104, and 675 × 104 cells/ml. In addition, we altered the ratio of HUVEC to HepG2 so that the percentage of HUVEC ranged from 0% to 40%. Since cells other than hepatocytes account for between 30% and 40% of the total number of cells in the human liver, the maximum percentage of HUVEC used in this study was set at 40%.
Figure 12 shows the diameter of the cell aggregates formed as a function of cell density. The cell aggregates became larger as cell density increased. The mean size of cell aggregates in one array ranged from 97 μm at a cell density of 145 × 104/ml to 226 μm at a cell density of 675 × 104/ml, and the standard deviations were 17%, 18.7%, 16.6%, and 16.9%, for cell densities of 145, 290, 480, and 675 × 104/ml, respectively (see Fig. 12). Previously, an array device using only HepG2 cells formed hepatic spheroids with a standard deviation in spheroid size of approximately 18%.22 There was no significant difference in cell aggregate size as a function of the percentage of HUVEC, since size depends on cell numbers present in the confined fluidic region.22 These results demonstrate that this apparatus enables cell aggregate formation with precise control of aggregate size.
Figure 12.
Cell aggregate size as a function of cell density for cell aggregates with different percentages of HUVEC (0, 5%, 10%, 20%, 40%) compared with non gel-coated cells.
In the present study, cell aggregates were formed even when the cell density was as low as 145 × 104 cells/ml when HepG2 were coated with collagen gel. This result means that the adhesion strength of cells was enhanced by using a collagen gel coating, which even helped in homo-aggregate formation.
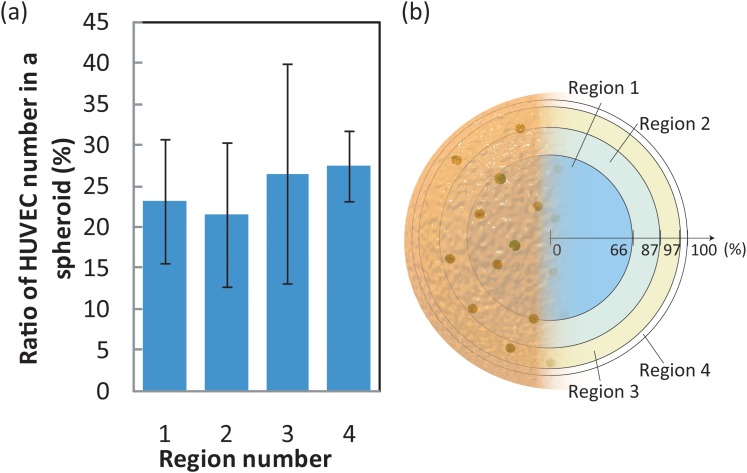
Figure 13 shows the distribution of HUVEC in a cell aggregate. The formed cell aggregate (∼ 200 μm) was so thick that it was difficult to count cell numbers of HUVEC using a confocal microscope. Therefore, we irradiated the cell aggregate with bright-field illumination and exciting light. Optical images prevented fluorescence of HUVEC in the center of the cell aggregates and enabled us to observe fluorescence on the surface of the cell aggregate.
Figure 13.
Distribution of HUVEC in cell aggregates (a). The vertical axis in (a) indicates the percentage of the total HUVEC in one aggregate that is present in each region. Region definitions are shown in Figure 13b. In (b), the outermost ring of an aggregate is defined as 100%.
The number of HUVEC in the cell aggregate was measured using Image J by merging an optical image with an image of calcein fluorescence. First, we determined the center of the cell aggregates using the optical images. Second, the distance of each HUVEC from the center was measured in ten images of one cell aggregate. Finally, each cell aggregate was divided into four regions in a concentric fashion based on distance from the center. Each region shown in Fig. 13 has the same superficial area on a sphere. It was difficult to distinguish individual HUVEC in captured images of cell aggregates composed of more than 10% of HUVEC. We, therefore, used images of cell aggregates consisting of HepG2 cells and 5% HUVEC to investigate HUVEC distribution. Figure 13 shows that HUVEC cells were uniformly dispersed in the cell aggregate. This result indicated that HepG2 and HUVEC mixed and adhered to one another in the confined region in a random manner.
Inamori et al. coated a collagen gel onto the surface of a formed cell aggregate so that HUVEC would adhere to the surface thereby creating HUVEC-covered monolayer spheroids.30 Our microfluidic device can form uniformly mixed cell aggregates containing different cell types. This uniform dispersion of different cells in the aggregate might facilitate networking of cells.
We did not observe significant size variation during one day of culture. Lin et al. reported that HepG2 cell aggregates formed using a hanging-drop culture became compact after one day, which was considered as evidence that the aggregates had turned into a spheroid.31 However, in our prior work, even though, similar to the current work, we did not observe size variation, the HepG2 cells did increase their metabolic function about threefold as assessed by assay of Cytochrome P450, and thus, the cells had turned into a spheroid.26 We, therefore, consider that the aggregates of HepG2 cells and HUVEC that formed in our device will become a spheroid similar to the HepG2 aggregates. The differences between the current and prior work are considered to originate from the culture methods used.
CONCLUSION
In this study, we demonstrated the rapid formation of size-controlled hetero-cell aggregates consisting of HepG2 cells and HUVEC. We found that enhancement of the adhesion between different cell types was critical for successful hetero-cell aggregate formation, which we achieved by exploiting a collagen gel coating of the HepG2 cells. Without this collagen coating, HepG2 cells and HUVEC did not adhere to each other and hetero-cell aggregates were not formed. We developed the collagen gel coating process and further investigated the coating quality. Successful coating was verified using fluorescent imaging, and the collagen gel thickness was experimentally deduced to be approximately 180 nm using collagen immunofluorescence analysis. The ratio of the volume of the collagen gel to cell volume was less than 10%, which was lower than that reported other methods used for adhesion enhancement.
By using the cell aggregate formation array with micro-rotation flow proposed herein it was possible to form hetero-cell aggregates composed of collagen-gel-coated HepG2 cells and HUVEC in only 2 min. The diameters of the spherical cell aggregates were controlled within the range of 97-226 μm, with standard deviations of less than 18%, by altering the cell density of the medium. Aggregate size did not depend on the composition of the cell species. We experimentally verified that HUVEC were uniformly dispersed in the cell aggregates, which we consider should facilitate cell networking.
Rapid and reproducible formation of hetero-cell aggregates of a controlled size using the proposed microfluidic device will greatly contribute to efficient hetero-spheroid study and thus to drug screening, regenerative medicine, and biological research.
ACKNOWLEDGMENTS
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), by a Grant-in-Aid for Scientific Research (S) (21226006) and the Scientific Research of Priority Areas, System Cell Engineering by Multi-scale Manipulation, and, in part, by a Keio University grant, the Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research.
References
- El-Ali J., Sorger P. K., and Jensen K. F., Nature 442(7101), 403 (2006). 10.1038/nature05063 [DOI] [PubMed] [Google Scholar]
- de Barros A. P. D. N., Takiya C. M., Garzoni L. R., Leal-Ferreira M. L., Dutra H. S., Chiarini L. B., Meirelles M. N., Borojevic R., and Rossi M. I., PloS one 5(2), e9093 (2010). 10.1371/journal.pone.0009093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao A. Y., Torisawa Y.-S., Tung Y.-C., Sud S., Taichman R. S., Pienta K. J., and Takayama S., Biomaterials 30(16), 3020 (2009). 10.1016/j.biomaterials.2009.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torisawa Y.-S., Mosadegh B., Luker G. D., Morell M., O’Shea K. S., and Takayama S., Integr. Biol. 1(11-12), 649 (2009). 10.1039/b915965g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott A., Nature 424(6951), 870 (2003). 10.1038/424870a [DOI] [PubMed] [Google Scholar]
- Chang T. T. and Hughes-Fulford M., Tissue Eng. Part A 15(3), 559 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K.-J. and Suh K.-Y., Lab Chip 10(1), 36 (2010). 10.1039/b907515a [DOI] [PubMed] [Google Scholar]
- Kothapalli C. R., van Veen E., de Valence S., Chung S., Zervantonakis I. K., Gertler F. B., and Kamm R. D., Lab Chip 11(3), 497 (2010). 10.1039/c0lc00240b [DOI] [PubMed] [Google Scholar]
- Hirschhaeuser F., Menne H., Dittfeld C., West J., Mueller-Klieser W., and Kunz-Schughart L. A., J. Biotechnol. 148(1), 3 (2010). 10.1016/j.jbiotec.2010.01.012 [DOI] [PubMed] [Google Scholar]
- Kojima R., Yoshimoto K., Takahashi E., Ichino M., Miyoshi H., and Nagasaki Y., Lab Chip 9(14), 1991 (2009). 10.1039/b903388b [DOI] [PubMed] [Google Scholar]
- Karaçali B., Vamvakidou A. P., and Tözeren A., BMC Med. Imaging 7, 7 (2007). 10.1186/1471-2342-7-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann T. K., Schirlau K., Sonkoly E., Brandau S., Lang S., Pivarcsi A., Balz V., Müller A., Homey B., Boelke E., Reichert T., Friebe-Hoffmann U., Greve J., Schuler P., Scheckenbach K., Schipper J., Bas M., Whiteside T. L., and Bier H., Int. J. Cancer 124(11), 2589 (2009). 10.1002/ijc.24269 [DOI] [PubMed] [Google Scholar]
- Laib A. M., Bartol A., Alajati A., Korff T., Weber H., and Augustin H. G., Nat. Protoc. 4(8), 1202 (2009). 10.1038/nprot.2009.96 [DOI] [PubMed] [Google Scholar]
- Kojima N., Miura K., Matsuo T., Nakayama H., Komori K., Takeuchi S., and Sakai Y., J. Rob. Mechatron. 22(5), 619 (2010). [Google Scholar]
- Abu-Absi S.-F., Hansen L. K., and Hu W.-S., Cytotechnology 45(3), 125 (2004). 10.1007/s10616-004-7996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Oizumi S., Kojima N., Niino T., and Sakai Y., Biomaterials 28(26), 3815 (2007). 10.1016/j.biomaterials.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Lee S.-W., Fukuda J., Yang D.-H., and Kunitake T., J. Mater. Sci.: Mater. Med. 17(4), 359 (2006). 10.1007/s10856-006-8237-7 [DOI] [PubMed] [Google Scholar]
- Kim C., Chung S., Kim Y. E., Lee K. S., Lee S. H., Oh K. W., and Kang J. Y., Lab Chip 11(2), 246 (2011). 10.1039/c0lc00036a [DOI] [PubMed] [Google Scholar]
- Lee K. H., No D. Y., Kim S.-H., Ryoo J. H., Wong S. F., and Lee S.-H., Lab Chip 11, 1168 (2011). 10.1039/C0LC00540A [DOI] [PubMed] [Google Scholar]
- Kunz-Schughart L. A., Schroeder J. A., Wondrak M., van Rey F., Lehle K., Hofstaedter F., and Wheatley D. N., Am. J. Physiol.: Cell Physiol. 290(5), C1385 (2006). 10.1152/ajpcell.00248.2005 [DOI] [PubMed] [Google Scholar]
- Inamori M., Mizumoto H., and Kajiwara T., Tissue Eng Part A 15(8), 2029 (2009). 10.1089/ten.tea.2008.0403 [DOI] [PubMed] [Google Scholar]
- Ota H., Yamamoto R., Deguchi K., Tanaka Y., Kazoe Y., Sato Y., and Miki N., Sens. Actuators B 147(1), 359 (2010). 10.1016/j.snb.2009.11.061 [DOI] [Google Scholar]
- Ota H. and Miki N., J. Rob. Mechatron. 22(5), 587 (2010). [Google Scholar]
- Jang K., Xu Y., Tanaka Y., Sato K., Mawatari K., Konno T., Ishihara K., and Kitamori T., Biomicrofluidics 4(3), 32208 (2010). 10.1063/1.3494287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka H., Hirano A., Nagasaki Y., Okano T., Horiike Y., and Kataoka K., ChemBioChem 5(6), 850 (2004). 10.1002/cbic.200300822 [DOI] [PubMed] [Google Scholar]
- Ota H. and Miki N., “Microfluidic experimental platform for producing size-controlled three-dimensional spheroids,” Sens. Actuators A (in press).
- Hou Y.-T., Ijima H., Matsumoto S., Kubo T., Takei T., Sakai S., and Kawakami K., J. Biosci. Bioeng. 110(2), 208 (2010). 10.1016/j.jbiosc.2010.01.016 [DOI] [PubMed] [Google Scholar]
- Loessner D., Stok K. S., Lutolf M. P., Hutmacher D. W., Clements J. A., and Rizzi S. C., Biomaterials 31(32), 8494 (2010). 10.1016/j.biomaterials.2010.07.064 [DOI] [PubMed] [Google Scholar]
- Ramanujan S., Pluen A., McKee T. D., Brown E. B., Boucher Y., and Jain R. K., Biophys. J. 83(3), 1650 (2002). 10.1016/S0006-3495(02)73933-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamori M., Mizumoto H., and Kajiwara T., Tissue Eng. A 15(8), 2029 (2009). 10.1089/ten.tea.2008.0403 [DOI] [PubMed] [Google Scholar]
- Lin R. Z., Chou L. F., Chien C. C., and Chang H. Y., Cell Tissue Res. 324(3), 411 (2006). 10.1007/s00441-005-0148-2 [DOI] [PubMed] [Google Scholar]