Abstract
Treatment of surfaces to change the interaction of fluids with them is a critical step in constructing useful microfluidics devices, especially those used in biological applications. Silanization, the generic term applied to the formation of organosilane monolayers on substrates, is both widely reported in the literature and troublesome in actual application for the uninitiated. These monolayers can be subsequently modified to produce a surface of a specific functionality. Here various organosilane deposition protocols and some application notes are provided as a basis for the novice reader to construct their own silanization procedures, and as a practical resource to a broader range of techniques even for the experienced user.
INTRODUCTION
Self-assembled monolayers (SAMs) are commonly used for the modification of the free chemical group on a surface,1 often to change the physical and chemical properties. For example, SAMs can be used to modify the hydrophobicity of a surface, creating an adhesion layer for subsequent depositions, preparing a surface for protein and biomolecule immobilization2, 3, 4, 5 or preventing unwanted protein adsorption.6, 7, 8 As a result SAMs find many applications in microfluidic systems such as biosensors,9, 10, 11 labs-on-a-chip,12, 13 and droplet microfluidics.14, 15
SAMs are formed by molecules with a strong and specific affinity for a particular surface composition. These molecules usually have a linear morphology with a surface reactive group at one end and a functional group at the other, with the former usually chosen to have a high affinity for the surface that it is being assembled on.1 The latter group is chosen based on the application of the SAM. These molecules often contain a hydrocarbon chain spacer between these two ends. Common SAMs include thiols on metals like gold,1, 16, 17, 18 silver,1, 17 and copper surfaces17, 19, 20; organosilanes on hydroxylated surfaces1, 18, 21; and fatty acids on alumina.1, 22 Detailed reviews on the theory, kinetics and applications of self assembled monolayer formation can be found in the literature.1, 17, 23, 24
Thiol-based SAMs are the most common SAMs used to self-assemble on gold surfaces,19 due to their ease of use and wide variety of functional groups. Upon exposure, the sulphur group strongly chemisorbs to the metal surface. However, they can only assemble on a small subset of the many surfaces that appear in microfluidics, often requiring an extra deposition step. In an actual application this further complicates the fabrication process of a microfluidic device.
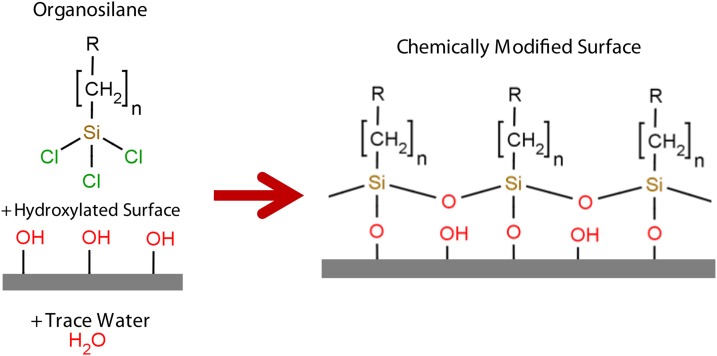
Organosilanes offer tremendous potential as SAMs because they can form monolayers on a wide variety of surfaces.25, 26, 27, 28, 29, 30 Organosilane SAMs form by reacting with trace amounts of water to form intermediate silanol groups. These groups then react with the surface’s free hydroxyl groups to covalently immobilize the organosilane. This basic reaction can be seen in Figure 1. This is very sensitive to the amount of water present in the system.1 While forming a high quality monolayer can be quite difficult, relatively consistent wettablity and functionality can be achieved. Here we outline common methods of the formation of organosilane monolayers, and, furthermore, a comprehensive survey of various deposition protocols are presented.
Figure 1.
When a hydroxylated surface is exposed to an organosilane in the presence of trace amounts of water, the reactive end bonds to the surface while the tail end becomes the dominant surface chemical species.
METHOD
The basic protocol for silanization, whatever the technique, is composed of most of the following steps:
-
1.
Choose the appropriate silanes compatible with your application and process.
-
2.
Clean the substrate and introduce surface hydroxyl groups.
-
3.
Expose substrate to organosilane.
-
4.
Improve SAM quality and remove excess organosilane by (optionally) baking, rinsing, and/or sonicating the sample.
-
5.
Remove or pattern the SAM.
Each step is described in more detail below and in associated multimedia content.32 Note that the intermediate exposure and final quality improvement steps are different depending upon whether a solution or vapor deposition technique is used.
Compatibility and selection of organosilane
There are several different factors that can determine compatibility of a device with the silanization process. This is important because organosilane deposition is normally one of the last steps in the fabrication of lab-on-a-chip system. First and foremost, the device has to be compatible with the cleaning and introduction of surface hydroxyl groups. Brief oxygen plasma or ultraviolet (UV) with ozone cleaning is generally compatible with most structures even though they can oxidize electrodes and may etch some polymers.31, 33 This can ultimately limit the performance of the device. Processing should ideally take place under class 100 clean room conditions.1
Furthermore, the reaction products of the covalent boding of the organosilane are typically either methanol or chloride ions. In the latter case, this can cause high local concentrations of hydrochloric acid which can damage local metal features. This can be circumvented be using an organosilane with CH2O reactive groups instead of Cl reactive groups.34 Lastly, all fabrication steps must be compatible with the solvents used in liquid phase deposition. This limits the use of some polymers as well as lift-off patterning of liquid phase organosilane deposition.
There are many organosilanes available for self assembly, and a few of the more common ones suitable for lab on a chip applications are listed below. Exotic organosilanes can be used for specific applications where the limitations of the process or requirements in use demand it.
3-Aminopropyltriethoxysilane/3-aminopropyltrimethoxysilane (APTES/APTMS)
APTES and APTMS are organosilanes used to introduce surface amine groups. The major difference between these two chemicals is the chemical composition of the reactive end group. This results in the reactive products of ethanol and methanol for APTES and APTMS, respectively. APTES and APTMS are often used to create a charged surface or as a initial step for protein or biomolecule immobilization.3, 35, 36
3-Mercaptopropyltrimethoxysilane (MPTS)
MPTS is suitable for introducing thiol groups to a surface. These thiol groups can then be subsequently used to immobilize molecules with thiol groups by forming a disulphide bond.29, 37, 38 More recently MPTS has been used to act as an adhesion layer for subsequent gold deposition producing ultra low roughness gold.16, 39
Octadecyltrichlorosilane/octadecyltrimethoxysilane (OTS/OTMS)
OTS and OTMS have 18 carbon atom hydrocarbon chains. The primary difference between the two is OTS contains 3 reactive Cl groups while OTMS contains 3 reactive CH3O molecules. This results in OTS being more reactive then OTMS; both are typically used for creating hydrophobic surfaces.15, 40, 41, 42 They can also be used to adsorb proteins43 like bovine serum albumin (BSA), which can be then used for subsequent protein immobilization or to prevent future protein immobilization.7 OTS and OTMS has also been patterned using scanning probe lithography,27 photolithography4 and microcontact printing.44 Furthermore, similar organosilanes exist with the same functional groups but different reactive groups like dimethyloctadecylchorosilane.45
Polytetrafluoroethylene organosilanes (PFS)
PFS typically contain a short hydrocarbon chain followed by a long polyfluoro chain. This produces surface fluoride groups that create hydrophobic surfaces. These organosilanes can be used to make surfaces hydrophobic,41 prevent microcontact printing molds from adhering,46 nanocontact lithography47, 48 and as a resist in electron beam lithography.5 A commonly used PFS is trichloro(1H,1H,2H,2H-perfluorooctyl)silane.
PEG and PEO organosilanes
These are organosilanes with a poly(ethylene glycol) (PEG) and poly(ethylene oxide) (PEO). This creates a relatively hydrophilic surface that does not adsorb proteins or other biological species.8, 49 These organosilanes are often used as blocking agents to reduce or eliminate non-specific binding in biological applications.
Surface cleaning and chemical preparation
In order for successful organosilane monolayer formation, the substrates must be exceptionally clean and present surface hydroxyl groups. Most cleaning methods rely on placing a surface in a strongly oxidizing environment, including piranha cleaning, UV irradiation, UV/Ozone, and oxygen plasma etch. While these cleaning procedures naturally leave the surface full of hydroxyl groups, sometimes surfaces are rinsed in deionized water (ideally ultrapure) to ensure the surface is fully hydroxylated. After cleaning and hydroxylization, organosilane deposition should be performed immediately. Several cleaning methods used are summarized in Tables TABLE I. and TABLE II..
TABLE I.
Several different protocol parameters for liquid phase organosilane deposition.
| Organosilane | Substrate | Surface Preparation | Solvent | Concentration | Time | Notes | Reference |
|---|---|---|---|---|---|---|---|
| APTES/APTMS | Silica gel | NS | Toluene | 10% | 30 min | 52 | |
| Si | Piranha | Ethanol | 100 mM | 24 h | 53 | ||
| Si | Piranha | Unspecified | 2% | 20 min | 20 min bake at 120 °C | 3 | |
| Glass | Unspecified | IPA | 5% | Unspecified | 5% water added, at 60 °C | 54 | |
| Glass | Methanol, HCL, H2SO4, NaOH | Water | 0.40% | 2 h | PH adjusted to 3.0 at 75 °C | 35 | |
| PDMS/glass | Oxygen plasma | Acetone (95%) | 2% | 2 h | 55 | ||
| Si/SiO2 | E-Beam lithography, HF | Ethanol (95%) | 2% | 1 h | Second step in patterning | 5 | |
| ZnO/SiO2 | Sonication in NH4OH:H2O2:H2O (1:1:10) | Ethanol (95%) | 4% | 4-5 h | 36 | ||
| Si | NH3:H2O2:H2O (1:1:5) and H2SO4:H2O2:H2O (1:1:5) | Toluene | 10% | 1 h | 56 | ||
| PDMS/glass | HCL:H2O2:H2O (1:1:5) | Ethanol | 50% | 30 min | In a microfluidic channel | 57 | |
| MPTS | SiO2 | Nitric acid, Piranha | Benzene | 5 mM - 40 mM | 40 min | In N2 atmosphere | 58 |
| ZnO | Ion beam milling | Toluene | 4% | 24 h | 25 | ||
| Si/SiO2 | Piranha, HCL:H2O2:H2O (1:1:6) | Benzene | 1-2 mM | 3 h | 16 | ||
| OTMS | SiO2 | Chromic/sulphuric acid | Toluene | 5% | 1.5 h | n-Butylamine catalyst | 4 |
| Si | Piranha | Toluene | 50 mM | 4 h | 59 | ||
| LiNbO3, Quartz | Oxygen plasma | Hexanes | 1% (24 mM) | 4 h | 34 | ||
| Si | Piranha | Bicyclohexyl | 100 mM | 24 h | 60 | ||
| OTS | Mica | HCL clean, dehydration, controlled rehydration | Cyclohexane | 3 mM | 20 min | Subsequent 2 h bake at 120 °C | 28 |
| Si | Piranha | Bicyclohexyl | 100 mM | 24 h | 60 | ||
| Si | Piranha, HF, UV ozone | Hexane, hexadecane or toluene | 1-50 mM | 30 s | Compared with microcontact printing | 42 | |
| Si | Piranha | Toluene | 0.05-1 mM | Varied | 61 | ||
| Si | Sonocation, HF, Piranha | Bicyclohexyl | 1-10 mM | 24 h | Water added to solvent for some depositions | 62 | |
| SiO2 | Oxygen plasma | Toluene | 0.50% | 16 h | Baked at 120 °C for 30 min | 7 | |
| LiNbO3 | IPA sonication | Toluene | 100 μM | Overnight | Rinsed with toluene and baked (1 h, 110 °C) | 41 | |
| LiNbO3, Quartz | Oxygen plasma | Hexane: Chloroform (4:1) | 0.1% (2.4 mM) | 4 h | 34 | ||
| LiNbO3 | Oxygen plasma | n-Hexane | 1 mM | 1 h | 15 | ||
| Si/SiO2 | Piranha | Unspecified | Unspecified | Unspecified | 43 | ||
| PEO | TaO5 | UV/Ozone | Toluene | 3 mM | 18 h | 0.08% HCl | 8 |
| PEG | PDMS | Oxygen plasma | Toluene | 0.1 M | 30 min | 49 | |
| PFS | Si | Piranha | Iso-octane | 5 mM | 10 min | 44 | |
| LiNbO3 | IPA sonication | Toluene | 100 μM | Overnight | Rinsed with toluene and baked (1 h, 110 °C) | 41 |
APTMS—(3-Aminopropyl)trimethoxysilane, APTES—(3-aminopropyl)triethoxysilane, MPTS—(3-mercaptopropyl)trimethoxysilane, OTS—octadecyltrichlorosilane, OTMS—Octadecyltrimethylsilane, PFS—polytetrafluoroethylene based organosilanes, PEG—poly(ethylene glycol) based organosilane, PEO—poly(ethylene oxide) based organosilane.
TABLE II.
Several different protocol parameters for vapor phase organosilane deposition.
| Organosilane | Substrate | Surface preparation | Temperature | Pressure | Time | Notes | Reference |
|---|---|---|---|---|---|---|---|
| APTMS | Glass | Piranha | Unspecified | 670 Pa | 16 h | 11 | |
| MPTS | SiO2 | Sulphuric acid/sodium peroxydisulphate | 100 °C | Unspecified | Unspecified | Also pumped (pressure unspecified) | 63 |
| Si | Piranha, oxygen Plasma | Unspecified | 1 mTorr | 4 h | 64 | ||
| Silica | Piranha, UV ozone | Unspecified | 200 mBar | 0-120 h | 39 | ||
| OTMS | SiO2 | UV irradiation | 100/150 °C | Unspecified | 1-16 h | Patterned with AFM | 27 |
| OTS | Si | UV irradiation | 150 °C | Unspecified | 0.5-8 h | 26 | |
| PFS | Si | UV irradiation | 150 °C | Unspecified | 0.5-8 h | 26 | |
| Si | NH4O4/H2O2/H2O (1:1:4) | 45 °C | Unspecified | 3 h | In N2 atmosphere | 5 | |
| SiOx | Plasma | Unspecified | 1 Torr | 1 h | 48 | ||
| SU8 | Unspecified (after patterning) | Unspecified | 0.5 bar | Unspecified | 46 |
APTMS—(3-Aminopropyl)trimethoxysilane, MPTS—(3-mercaptopropyl)trimethoxysilane, OTS—octadecyltrichlorosilane, OTMS—octadecyltrimethylsilane, PFS—polytetrafluoroethylene based organosilanes.
All chemical manipulation of organosilanes should be handled under nitrogen because they typically react with trace amounts of water. At the very least, stock solutions should not be exposed to air wherever possible to extend the shelf life of the chemical and improve the quality of subsequent SAMs deposited.
Exposure to organosilane
Solution deposition
Solution deposition involves the submerging of the prepared substrate into a solution of organosilanes. A strong solvent is typically used. Common solvents are toluene, benzene with hexane, acetone, anhydrous ethanol, or mixtures of these. Table TABLE I. shows a list of parameters for the liquid phase deposition of various organosilanes. It is clear that a wide range of cleaning procedures, solvents concentrations and deposition times have been used.
Solution deposition is often used due to the simplicity of the method and low setup cost. However, the monolayer quality is very sensitive to the amount of water in the system. If there is not enough, only a partial monolayer forms, while if there is too much water the organosilanes may polymerize. This can result in inconsistent surface properties. The reader is directed to Ulman’s seminal work1 for a detailed description of complications that can occur.
Vapor deposition
Vapour organosilane deposition is essentially chemical vapor deposition (CVD) of organosilanes to form a monolayer.50 This relies on increasing the percent partial pressure of the organosilane within a closed system, achieved either by heating a closed container or by lowering the base pressure using a vacuum pump with an open source of the liquid organosilane inside. The organosilane is then deposited via chemisorption.
A sample protocol of vapour organosilane deposition is as follows:
-
1.
Clean the substrate with acetone, isopropanol and deionized water.
-
2.
Piranha clean the substrate. Rinse and dry the substrate with nitrogen.
-
3.
Place the substrate and 100 μl of organosilane in a vacuum chamber/dessicator and pump down the system to approximately 100 mTorr.
-
4.
Leave the sample for 30 min to allow the organosilane to chemically adsorb onto the substrate.
-
5.
Vent chamber and dispose of the organosilane appropriately. Store the sample in a clean environment until required.
Table TABLE II. summarizes some process parameters of vapor deposition processes for silanes. Vapor organosilane deposition has several advantages over liquid phase deposition. Monolayers formed are typically of higher order and quality. There is also less likelihood of forming multilayers during the deposition.16 In addition, significantly reduced volumes of reagents are used in the deposition process.
Commercial CVD systems are available for purchase as well. These consist of vacuum chambers that often incorporate the organosilane manipulation. Some of these can also include substrate and chamber cleaning processes which can improve the consistency of the deposition process.51
Improving SAM quality
The final step is the improvement of the silane SAM quality by additional processing. For solution deposition of the silane, the SAM may actually be many layers thick and contain excess polymer. Rinsing and sonication in solvent will help to reduce this material to form a high-quality SAM.
Removal and patterning
Organosilanes can be removed by the oxidation of the tail group. This is best achieved through the use of a dry oxidation process due to its simplicity. Both oxygen plasmas, UV and UV/Ozone cleaning can be used to remove the majority of the SAM.1 There will likely be a residual silicon oxide layer after this process which can be removed through subsequent processing.
UV light alone can slowly oxidize an organosilane surface, but this may require long exposure times. This allows for an organosilane to be patterned by optical lithography without the use of a photoresist.1, 3, 4 However, it is likely that employing a traditional photolithographic or lift of processes with photoresist could provide much faster patterning. After the removal of an organosilane layer the surface will be ready for a subsequent organosilane deposition. Organosilanes have also been used as molecular resists in electron beam lithography5 and scanning probe lithography.27 Patterning can also be achieved through microcontact or nanocontact printing.44, 47, 48
References
- Ulman A., Chem. Rev. 96, 1533 (1996). 10.1021/cr9502357 [DOI] [PubMed] [Google Scholar]
- Hahn C., Leitner C., Weinbrenner T., Schlapak R., Tinazli A., Tampé R., Lackner B., Steindl C., Hinterdorfer P., Gruber H., and Holzl M., Bioconjugate Chem. 18, 247 (2007). 10.1021/bc060292e [DOI] [PubMed] [Google Scholar]
- Koyano T., Saito M., Miyamoto Y., Kaifu K., and Kato M., Biotechnol. Prog. 12, 141 (1996). 10.1021/bp9500573 [DOI] [Google Scholar]
- Mooney J., Hunt A., McIntosh J., Liberko C., Walba B., and Rogers C., Proc. Natl. Acad. Sci. 93, 12287 (1996). 10.1073/pnas.93.22.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. J., Tanii T., Zako T., Hosaka T., Kanari Y., Funatsu T., and Ohdomari I., Small 1, 833 (2005). 10.1002/smll.v1:8/9 [DOI] [PubMed] [Google Scholar]
- Chen S., Zheng J., Li L., and Jiang S., J. Am. Chem. Soc. 127, 14473 (2005). 10.1021/ja054169u [DOI] [PubMed] [Google Scholar]
- Huang T., Sturgis J., Gomez R., Geng T., Bashir R., Bhunia A., Robinson P., and Ladisch M., Biotechnol. Bioeng. 81, 618 (2003). 10.1002/bit.v81:5 [DOI] [PubMed] [Google Scholar]
- Boozer C., Yu Q., Chen S., Lee C.-Y., Homola J., Yee S. S., and Jiang S., Sens. Actuators B 90, 22 (2003). 10.1016/S0925-4005(03)00017-0 [DOI] [Google Scholar]
- Wink T., Zuilen S., Bult A., and Bennekom W., Analyst 122, 43 (1997). 10.1039/a606964i [DOI] [PubMed] [Google Scholar]
- Chaki N. and Vijayamohanan K., Biosens. Bioelectron. 17, 1 (2002). 10.1016/S0956-5663(01)00277-9 [DOI] [PubMed] [Google Scholar]
- Handa H., Gurezynski S., Jackson M., Auner G., Walker J., and Mao G., Surf. Sci. 602, 1392 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurard-Levin Z. A. and Mrksich M., Annu. Rev. Anal. Chem. 1, 767 (2008). 10.1146/annurev.anchem.1.031207.112903 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu H., Qin J., and Lin B., Biomicrofluidics 3, 044105 (2009). 10.1063/1.3259628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz C., Lane J., Chandross M., Stevens M., and Grest G., Langmuir 25, 4535 (2009). 10.1021/la803940b [DOI] [PubMed] [Google Scholar]
- Ducloux O., Galopin E., Zoueshtiagh F., Merlen A., and Thomy V., Biomicrofluidics 4, 011102 (2010). 10.1063/1.3310930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J. and Witten J., J. Phys. Chem. C 112, 19088 (2008). [Google Scholar]
- Love J. C., Estroff L., Kriebel J., Nuzzo R., and Whitesides G., Chem. Rev. 1103 (2005). 10.1021/cr0300789 [DOI] [PubMed] [Google Scholar]
- Senaratne W., Andruzzi L., and Ober C., Biomacromolecules 6, 2427 (2005). 10.1021/bm050180a [DOI] [PubMed] [Google Scholar]
- Love J., Estroff L., Kriebel J., Nuzzo R., and Whitesides G., Chem. Rev. 105, 1103 (2005). 10.1021/cr0300789 [DOI] [PubMed] [Google Scholar]
- Sung M. and Kim Y., Bull. Korean Chem. Soc. 22, 748 (2001). [Google Scholar]
- Toworfe G., Composto R., Shapiro I., and Ducheyne P., Biomaterials 27, 631 (2006). 10.1016/j.biomaterials.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Lim M., Feng K., Chen X., Wu N., Raman A., Nightingale J., Gawalt E., Korakakis D., Hornak L., and Timperman A., Langmuir 23, 2444 (2007). 10.1021/la061914n [DOI] [PubMed] [Google Scholar]
- Gooding J., Mearns F., Yang W., and Liu J., Electroanalysis 15, 81–96 (2003). 10.1002/elan.200390017 [DOI] [Google Scholar]
- Fendler J., Chem. Mater. 8, 1616 (1996). 10.1021/cm960116n [DOI] [Google Scholar]
- Corso C., Dickherber A., and Hunt W., Biosens. Bioelectron. 24, 805 (2008). 10.1016/j.bios.2008.07.011 [DOI] [PubMed] [Google Scholar]
- Sugimura H., Hozumi A., Kameyama T., and Takai O., Surf. Interface Anal. 34, 550 (2002). 10.1002/sia.v34:1 [DOI] [Google Scholar]
- Sugimura H. and Nakagiri N., J. Photopolym. Sci. Technol. 10, 661 (1997). 10.2494/photopolymer.10.661 [DOI] [Google Scholar]
- Carson G. A. and Granick S., J. Mater. Res. Soc. 5, 1746 (1990). 10.1557/JMR.1990.1745 [DOI] [Google Scholar]
- Fischer L. M., “Development of silicon carbonitride nanomechanical resonators for biological detection applications”, M.Sc. Thesis, University of Alberta (Canada), 2007. [Google Scholar]
- Quinton J., Thomsen L., and Dastoor P., Surf. Interface Anal. 25, 931 (1997). [DOI] [Google Scholar]
- Choi K., Eom T.-J., and Lee C., Thin Solid Films 435, 227 (2003). 10.1016/S0040-6090(03)00329-8 [DOI] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.3625605 for complete multimedia demonstration of organic silanisation of a surface.
- Vig J. R., J. Vac. Sci. Technol. 3, 1027 (1985). 10.1116/1.573115 [DOI] [Google Scholar]
- Nihonyanagi S., Eftekhari-Bafrooei A., Hines J., and Borguet E., Langmuir 24, 5161 (2008). 10.1021/la702024x [DOI] [PubMed] [Google Scholar]
- Campbell G. and Mutharasan R., Biosens. Bioelectron. 21, 462 (2005). 10.1016/j.bios.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S., Bei T., Zoumakis E., Chrousos G., and Iliadis A., Biosens. Bioelectron. 22, 707 (2006). 10.1016/j.bios.2006.02.020 [DOI] [PubMed] [Google Scholar]
- Rao S. V., Anderson K. W., and Bachas L. G., Microchim. Acta 128, 127 (1998). 10.1007/BF01243043 [DOI] [Google Scholar]
- Rusmini F., Zhong Z., and Feijen J., Biomacromolecules 8, 1775 (2007). 10.1021/bm061197b [DOI] [PubMed] [Google Scholar]
- Pattier B., Bardeau J.-F., Mathieu Edely A. G., and Delorme N., Langmuir 24, 821 (2008). 10.1021/la702777k [DOI] [PubMed] [Google Scholar]
- Priest C., Biomicrofluidics 4, 032206 (2010). 10.1063/1.3493643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennès J., Ballandras S., and Chérioux F., Appl. Surf. Sci. 255, 1796 (2008). 10.1016/j.apsusc.2008.06.031 [DOI] [Google Scholar]
- Jeon N. L., Finnie K., Branshaw K., and Nuzzo R., Langmuir 13, 3382 (1997). 10.1021/la970166m [DOI] [Google Scholar]
- Schmitt Y., Hähl H., Gilow C., Mantz H., Jacobs K., Leidinger O., Bellion M., and Santen L., Biomicrofluidics 4, 032201 (2010). 10.1063/1.3488672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan F., Chen M., Yang W., Wang J., Zhang R., Yang S., and Xue O., Appl. Surf. Sci. 230, 131 (2004). 10.1016/j.apsusc.2004.02.021 [DOI] [Google Scholar]
- Friend J. and Yeo L., Biomicrofluidics 4, 026502 (2010). 10.1063/1.3259624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wu J., Wang L., Xiao K., and Wen W., Lab Chip 10, 1199 (2010). 10.1039/b923101c [DOI] [PubMed] [Google Scholar]
- Morecroft D., Yang J. K. W., Schuster S., Berggren K. K., Xia Q., Wu W., and Williams R. S., J. Vac. Sci. Technol. B 27, 2837 (2009) 10.1116/1.3264670. [DOI] [Google Scholar]
- Mizuno H. and Buriak J., Appl. Mater. Interfaces 1, 2711 (2009). 10.1021/am900602m [DOI] [PubMed] [Google Scholar]
- Schmolke H., Demming S., Edlich A., Magdanz V., Büttgenbach S., Franco-Lara E., Krull R., and Klages C.-P., Biomicrofluidics 4, 044113 (2010). 10.1063/1.3523059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson U., Olofsson G., Malmqvist M., and Rönnberg I., Thin Solid Films 124, 117 (1985). 10.1016/0040-6090(85)90253-6 [DOI] [Google Scholar]
- I. Yield Engineering Systems (YES), Vapor Deposition Systems—YES-1224 and YES-1224P, Manufacturer Data Sheet (2011).
- Waddell T. G., Leyden D. E., and DeBello M. T., J. Am. Chem. Soc. 103, 5303 (1981). 10.1021/ja00408a005 [DOI] [Google Scholar]
- Mrksich M. and Whitesides G., Trends Biotechnol 13, 228 (1995). 10.1016/S0167-7799(00)88950-7 [DOI] [Google Scholar]
- Ercole C., Gallo M. D., Mosiello L., Baccella S., and Lepidi A., Sens. Actuators B 91, 163 (2003). 10.1016/S0925-4005(03)00083-2 [DOI] [Google Scholar]
- Zhang Z., Crozatier C., Berre M. L., and Chen Y., Microelectron. Eng. 78-79, 556 (2005). 10.1016/j.mee.2004.12.071 [DOI] [Google Scholar]
- Laiwattanapaisal W., Yakovleva J., Bengtsson M., Laurell T., Wiyakrutta S., Meevootisom V., Chailapakul O., and Emnéus J., Biomicrofluidics 3, 014104 (2009). 10.1063/1.3098319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Li C. M., Liu Y., Gao J., Wang W., and Gan Y., Lab on a Chip 9, 1243 (2009). 10.1039/b816018j [DOI] [PubMed] [Google Scholar]
- Hu M., Noda S., Okubo T., Yamaguchi Y. and Komiyama H., Appl. Surf. Sci. 181, 307 (2001). 10.1016/S0169-4332(01)00399-3 [DOI] [Google Scholar]
- Follstaedt S., Last J., Cheung D., Gourley P., and Saski D., Sandia Report, 2000.
- Bierbaum K., Kinzler M., Woll C., Grunze M., Hahner G., Heid S., and Effenberger F., Langmuir 11, 512 (1995). 10.1021/la00002a025 [DOI] [Google Scholar]
- Kulkarni S., Mirji S., Mandale A., Gupta R., and Vijayamohanan K., Mater. Lett. 3890 (2005). 10.1016/j.matlet.2005.07.026 [DOI] [Google Scholar]
- Wang M., Liechti K., Wang Q., and White J., Langmuir 21, 1848 (2005). 10.1021/la048483y [DOI] [PubMed] [Google Scholar]
- Semaltianos N. G., Pastol J., and Doppelt P., Appl. Surf. Sci. 222, 102 (2004). 10.1016/j.apsusc.2003.08.003 [DOI] [Google Scholar]
- Mahapatro A., Scott A., Manning A., and Janes D., Appl. Phys. Lett. 88, 151917 (2006). 10.1063/1.2183820 [DOI] [Google Scholar]