Abstract
A method to easily manufacture and assemble a polydimethylsiloxane (PDMS) based microfluidic device is described. The method uses low cost materials and re-usable laser cut polymethyl methacrylate (PMMA) parts. In addition, the thickness of PDMS layers can be controlled and both PDMS layer surfaces are flat, which allows for multi-layer PDMS structures to be assembled. The use of mechanical clamping to seal the structure allows for easy cleaning and re-use of the manufactured part as it can be taken apart at any time. In this way, selected layers can be re-used or replaced. The process described can be easily adopted and utilised without the need for any costly clean room facilities or equipment such as oxygen bonders, making it ideal for laboratories, universities, and classrooms exploring microfluidics applications.
INTRODUCTION
Microfluidic devices and techniques continue to receive considerable attention, both in the research community and for commercial applications. However, the adoption of this technology appears to be limited to specific niche areas, making it difficult to highlight the possibilities and advantages of utilising such methods to a broader user base. One of the main reasons for this remains the difficulty in designing and manufacturing the devices, since much of the work takes place in a scientific environment, where the necessary engineering skills required are not available. In addition, commercial devices are expensive and difficult to specify for new users. To overcome these issues, researchers have proposed simpler and less costly fabrication methods, either utilising cheaper manufacturing methods or by developing modular microfluidic components for assembly into more complex circuits.
Rapid prototyping of microfluidic systems in PDMS was introduced by Duffy et al.,1 at the same time introducing the use of low cost transparencies as masks. This replaced the vastly more expensive chrome masks typically used in microelectronics processes. Low cost manufacture was also shown with a variety of alternative methods, namely solid object printers,2 photocopying,3 Shrink-film,4 desktop craft cutter,5 and micromilling.6
Modular microfluidic architectures have also been proposed by a number of research groups. Yuen7 introduced a “plug-n-play” LEGO® type concept, utilising a motherboard containing various channels and connectors. Langelier et al.8 introduced a modular system consisting of pre-fabricated microfluidic assembly blocks which could be assembled and used without specialist knowledge. Shaikh et al.9 described an elegant microfluidic breadboard concept consisting of a multifunctional base chip with active elements, onto which a second chip, with passive components, could be mated. Grodzinski et al.10 had previously described a modular approach combining separate microfluidic elements utilising an electronically connected base plate.
Multi-layered microfluidic structures have been shown by a number of groups.11, 12, 13 The multi layered approach allows for complex structures, such as pumps and valves, to be integrated into single components.
PDMS has become the material of choice for rapid prototyping of microfluidic devices.1, 13 Numerous applications have been shown, with PDMS being used to fabricate many new devices.14, 15, 16 One of the most common methods of assembling microfluidic devices made from PDMS is to use oxygen plasma bonding. The surfaces of the PDMS and glass substrates are treated with oxygen plasma. Bonding can then immediately be established between glass-PDMS or PDMS-PDMS by applying pressure. Glass PDMS bonding is permanent, and allows for single layer structures to be manufactured.
PDMS-PDMS bonds have also been realised by partially curing one PDMS surface before bonding the two surfaces, or alternatively by varying the PDMS ratios between base and curing agent. The final curing is done after the two surfaces make contact.11 These methods result in permanent sealing and do not allow for cleaning and re-use of the device.
These approaches are being developed in order to connect the science and engineering aspects of microfluidic technology, but are not yet readily available commercially at costs which would encourage wide spread usage. These approaches require access to expensive microfabrication facilities and utilise bonding methods which result in permanent non-reversible bonds being formed.
This work shows the development of a process for the manufacture and assembly of PDMS-based microfluidic devices which can be disassembled, cleaned, and re-used. This methodology has numerous advantages for laboratories starting in the microfluidic field and wishing to become familiar with the complexities of the technology without requiring expensive manufacturing facilities or commercial products. We show a step-by-step process which is easy to follow, repeatable and requires only a small number of low-cost components. A similar casting process was described by Li et al.,17 but they did not manufacture individual casting areas for each microfluidic component or show a full assembly process. A detailed protocol for using PDMS as a fabrication material is given by Friend and Yeo18 and is not repeated here.
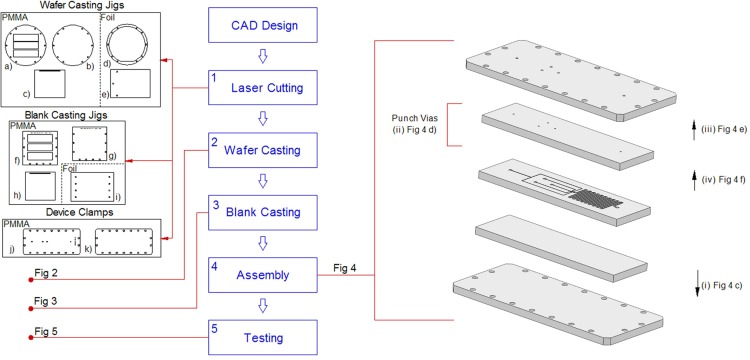
Fig. 1 shows a schematic of the entire process, from initial computer aided design to final device testing.
Figure 1.
Laser cut jigs required for PDMS casting on wafers. a, b, c, f, g, h, j, k are 3 mm PMMA; d, e, i are 100 μm foil. The middle three layers of the assembly are PDMS.
The cost of re-usable jigs (PMMA, foil) is of the order of $3, while the cost of the PDMS and clamping components for the device is $2. This is comparable to other low cost processes such as craft cutting, while being considerably less expensive than once-off plastic and glass assemblies.
METHODS
Mould manufacture
The casting master mould is manufactured with a silicon wafer which has been lithographically patterned with SU-8 photoresist to define the microfluidic structures. The template can also easily be manufactured with other low-cost processes mentioned in Sec. 1.
Laser cutting of parts
Wafer casting jigs (Figs. 1a–1e), blank casting jigs (Figs. 1f–1i), and device clamps (Figs. 1j, 1k) are all laser cut before starting the manufacturing and assembly process. A 35W Epilog CO2 infrared laser system has been utilized for the laser cutting. Process parameters for the different materials are given in Table TABLE I..
TABLE I.
Process parameters for laser cutting of casting jigs.
| Material | Thickness (mm) | Laser power (W) | Speed (mm/s) | Frequency (Hz) |
|---|---|---|---|---|
| PMMA | 3 | 7 | 16 | 5000 |
| Foil | 0.1 | 3 | 36 | 5000 |
Wafer casting
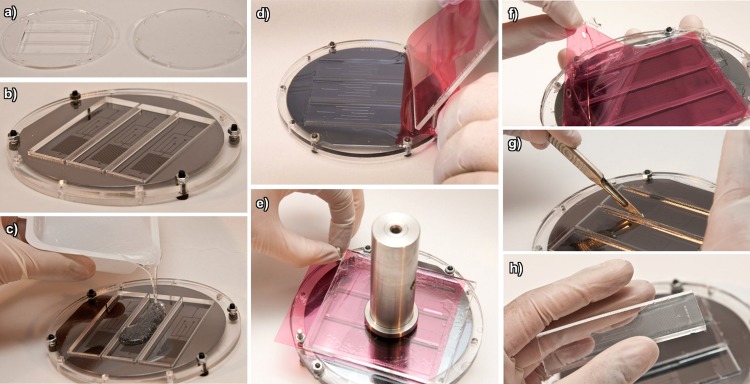
The parts shown in Fig. 2a are used to clamp the wafer. The solid part is behind the wafer, while the 70 × 20 mm slots are positioned over the corresponding SU-8 structures on the wafer. These slot dimensions can be changed to correspond to the particular design. Care should be taken not to tighten the screws beyond finger tight as this can cause cracking of the wafer. To circumvent this, the user can use the foil parts shown in Fig. 1d. These are approximately 100 μm thick, and several can be packed between the two PMMA plates so that the screws cannot be over tightened. In addition, these foils help with the alignment of the wafer. The fully assembled casting jig is shown in Fig. 2b.
Figure 2.
The PDMS wafer casting process (a) laser cut jigs, (b) the assembled jig, (c) PDMS introduction onto the jig, (d) smoothing of PDMS with foil, (e) PDMS and jig ready for curing, (f) lifting of the foil from cured PDMS, (g) cutting of the parts from the jig, and (h) the final PDMS part.
Fig. 2c shows the PDMS being poured into the jig. The slots should be filled so that there is a small amount of excess PDMS which overflows. After pouring, degassing should be done using a dessicator.
Fig 2d shows the foil (Fig. 1e) placed over the two M4 × 20 mm screws which are sticking out of the holes in the jig. Use the smoothing jig (Fig. 1d) to slowly place the foil over the slots, being careful not to introduce bubbles into the PDMS. A small amount of excess PDMS should be evident and will be pushed off the jig.
Once the foil has been completely rolled onto the surface, the smoothing jig (Fig. 1d) should be placed directly onto the foil and a weight (300 g) placed on top of this jig (Fig. 2e). The entire structure can now be placed into the curing oven (2 h @ 60 °C).
After curing, the foil should be carefully peeled off of the PDMS (Fig. 2f). Care should be taken that the PDMS does not lift as this can cause tearing of the PDMS and part wastage.
A sharp scalpel should be used to separate the PDMS edges from the PMMA wall (Fig. 2g). This can be done in 2-3 passes as this will not lead to PDMS damage or scribing of the wafer.
Once the PDMS has been separated, it can be peeled off the silicon wafer substrate (Fig. 2h). It is usually easier to do this while the jig is still on the wafer.
Blank casting
Fig. 3a shows the laser cut PMMA components to be used for casting of PDMS blanks.
Figure 3.
The PDMS blank casting process (a) laser cut jigs, (b) the assembled jig, (c) PDMS and jig ready for curing, (d) cutting of PDMS blanks from the jig, and (e) removal of the PDMS blanks.
The process is similar to the process described for casting on the wafer and will not be repeated in detail. Fig. 3b shows the assembled jig. After pouring and planarising the PDMS with the foil, it can be cured (see Figs. 2c–2f).
Fig. 3d shows the PDMS being cut after curing. Fig. 3e shows the final PDMS blanks being peeled from the jig. If the blanks are not to be used immediately, both surfaces should be covered with Scotch Magic© tape to prevent dust from contaminating the surface.
Assembly of device
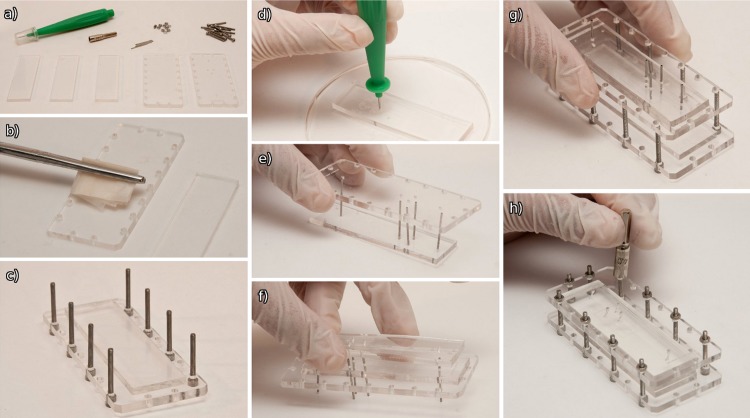
The various components required for assembling the microfluidic device are shown in Fig. 4a. These include the two laser cut PMMA clamping jigs, three PDMS pieces (bottom blank, middle channels, top sealing holes), punches (Harris Unicore 0.75 mm), screws, and alignment tubes (20 gauge). The different parts (both PDMS and PMMA) should be well cleaned with lens tissue (Fig. 4b) and IPA (isopropyl alcohol). Inspection under a light microscope (20 × objective) will help to ensure that the parts have been properly cleaned.
Figure 4.
The device assembly process (a) parts required for assembly, (b) cleaning of the PMMA clamping jigs, (c) assembly of the bottom half, (d) punching of inlet and outlet fluid ports, (e) placement of the top jig onto the PDMS using tubes for alignment, (f) placement of the centre PDMS, (g) positioning of the top and bottom assemblies, and (h) final tightening of the assembly.
The bottom PDMS blank piece should be placed in the centre of the bottom PMMA clamp as shown in Fig. 4c. The M2 × 20 mm screws should be inserted into the bottom half of the assembly. This is the completed bottom half of the assembly.
Place the middle and top PDMS layers on top of each other, and use the alignment marks on the middle layer (indicating fluidic inlets) to punch through both layers (Fig. 4d). After having completed this step for all holes, use a larger punch (1–1.6 mm) to punch through the middle channel layer only (using the smaller holes as reference). The edges of the holes should be checked under a light microscope (20 × objective) to ensure that they are smooth and have not cracked.
Place the tubes into the punched holes of the top layer and lower the top clamping jig onto the tubes, until the PMMA and PDMS are in close contact (Fig. 4e). By slowly rotating the tubes, push them approximately 2 mm out of the PDMS, and then use these protruding tubes as alignment pins for the middle PDMS channel layer (Fig. 4f).
Next, place the top half of the assembly onto the screws which have been placed into the bottom half of the assembly (Fig. 4g).
Finger tighten the nuts as shown in Fig. 4h. By measuring the thickness of the assembly before and after tightening, and knowing the original channel depth, the new channel depth can be estimated.
Testing of device
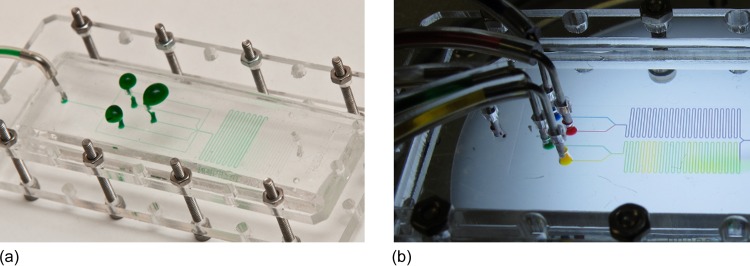
To test the device for leaks or blockages, insert a tube into one of the inlet or outlet holes in the top PMMA surface. Manually inject a fluid (preferably one to be used in the final device) into the channels. An aqueous solution containing food colorant has been used to clearly show the flow through the channels in Fig. 5a. We have tested the device to a pressure of 30 psi without experiencing any leakage problems. This is sufficient for many microfluidic experiments. Where leaking does occur, it is usually first at the ports, and this is caused by incorrect punching of the inlet and outlet holes. Figure 5b shows an actual device, containing mixing elements, being used in an experiment. The entire device can be disassembled and re-assembled in 10–15 min, so that the typical manufacturing time for a PDMS device of a day is greatly reduced.
Figure 5.
(a) Final device leak testing and (b) an experiment utilising a device manufactured with the described method.
CONCLUSION
A low cost, convenient fabrication process for PDMS casting and assembly has been shown. The casting process reduces PDMS usage by casting only the functional part and produces parts which do not require any post-casting processing. The assembly process allows for multilayer devices to be manufactured, while also allowing for the device to be quickly disassembled and cleaned when required. The device has been tested to 30 psi, which is sufficient for many microfluidic experiments.
This method will make the assembly of PDMS structures for testing accessible to a wide audience, with specific relevance to universities and classes being introduced to the field of microfluidics, or where limited manufacturing equipment is available.
The method was developed over a number of iterations and has been successfully utilised in the bench top laboratory environment and for teaching students the manufacture and assembly of microfluidic devices.
ACKNOWLEDGMENTS
The authors wish to thank Louis Fourie for assistance with laser cutting. This work has been supported by the Technology Innovation Agency.
References
- Duffy D. C., Mcdonald J. C., Schueller O. J. A., and Whitesides G. M., Anal. Chem. 70, 4974 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- Mcdonald J. C., Chabinyc M. L., Metallo S. J., Anderson J. R., Stroock A. D., and Whitesides G. M., Anal. Chem. 74, 1537 (2002). 10.1021/ac010938q [DOI] [PubMed] [Google Scholar]
- Tan A., Rodgers K., Murrihy J. P., O’Mathuna C., and Glennon J. D., Lab Chip 1, 7 (2001). 10.1039/b102905n [DOI] [PubMed] [Google Scholar]
- Nguyen D., McLane J., Lew V., Pegan J., and Khine M., Biomicrofluidics 5, 022209, (2011). 10.1063/1.3576930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. K. and Goral V. N., Lab Chip 10, 384 (2010). 10.1039/b918089c [DOI] [PubMed] [Google Scholar]
- Hupert M. L., Jason Guy W., Llopis S. D., Shadpour H., Rani S., Nikitopoulos D. E., and Soper S. A., Microfluid. Nanofluid. 3, 1, (2007). 10.1007/s10404-006-0091-x [DOI] [Google Scholar]
- Yuen P. K., Lab Chip 8, 1374 (2008). 10.1039/b805086d [DOI] [PubMed] [Google Scholar]
- Langelier S. M., Livak-Dahl E., Manzo A. J., Johnson B. N., Walter N. G., and Burns M. A., Lab Chip 11, 1679 (2011). 10.1039/c0lc00517g [DOI] [PubMed] [Google Scholar]
- Shaikh K. A., Ryu K. S., Goluch E. D., Nam J.-M., Liu J., Shad Thaxton C., Chiesl T. N., Barron A. E., Lu Y., Mirkin C. A., and Liu C., Proc. Natl. Acad. Sci. U.S.A. 102, 9745 (2005). 10.1073/pnas.0504082102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski P., Yang J., Liu R. H., and Ward M. D., Biomed. Microdevices 5, 303 (2003). 10.1023/A:1027357713526 [DOI] [Google Scholar]
- Unger M. A., Chou H. P., Thorsen T., Scherer A., and Quake S. R., Science 288, 113 (2000). 10.1126/science.288.5463.113 [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Agirregabiria M., Garcia J., Berganzo J., Tijero M., Arroyo M. T., Ruano J. M., Aramburu I., and Mayora K., J. Micromech. Microeng. 14, 1047 (2004). 10.1088/0960-1317/14/7/027 [DOI] [Google Scholar]
- Anderson J. R., Chiu D. T., Jackman R. J., Cherniavskaya O., Mcdonald J. C., Wu H., Whitesides S. H., and Whitesides G. M., Anal. Chem. 72, 3158 (2000). 10.1021/ac9912294 [DOI] [PubMed] [Google Scholar]
- Liu L., Cao W., Wu J., Wen W., Chang D., and Sheng P., Biomicrofluidics 2, 034103, (2008). 10.1063/1.2966453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanapalli S., Duits M., and Mugele F., Biomicrofluidics 3, 012006, (2009). 10.1063/1.3067820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Cao W., Wen W., Chang D., and Sheng P., Biomicrofluidics 3, 012005, (2009). 10.1063/1.3058587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Chen Q., and Zhao J., Lab Chip, Chips and Tips (2009), http://www.rsc.org/Publishing/Journals/lc/Chips_and_Tips/casting_holder.asp.
- Friend J. and Yeo L., Biomicrofluidics 4, 026502 (2010). 10.1063/1.3259624.1 [DOI] [PMC free article] [PubMed] [Google Scholar]