Abstract
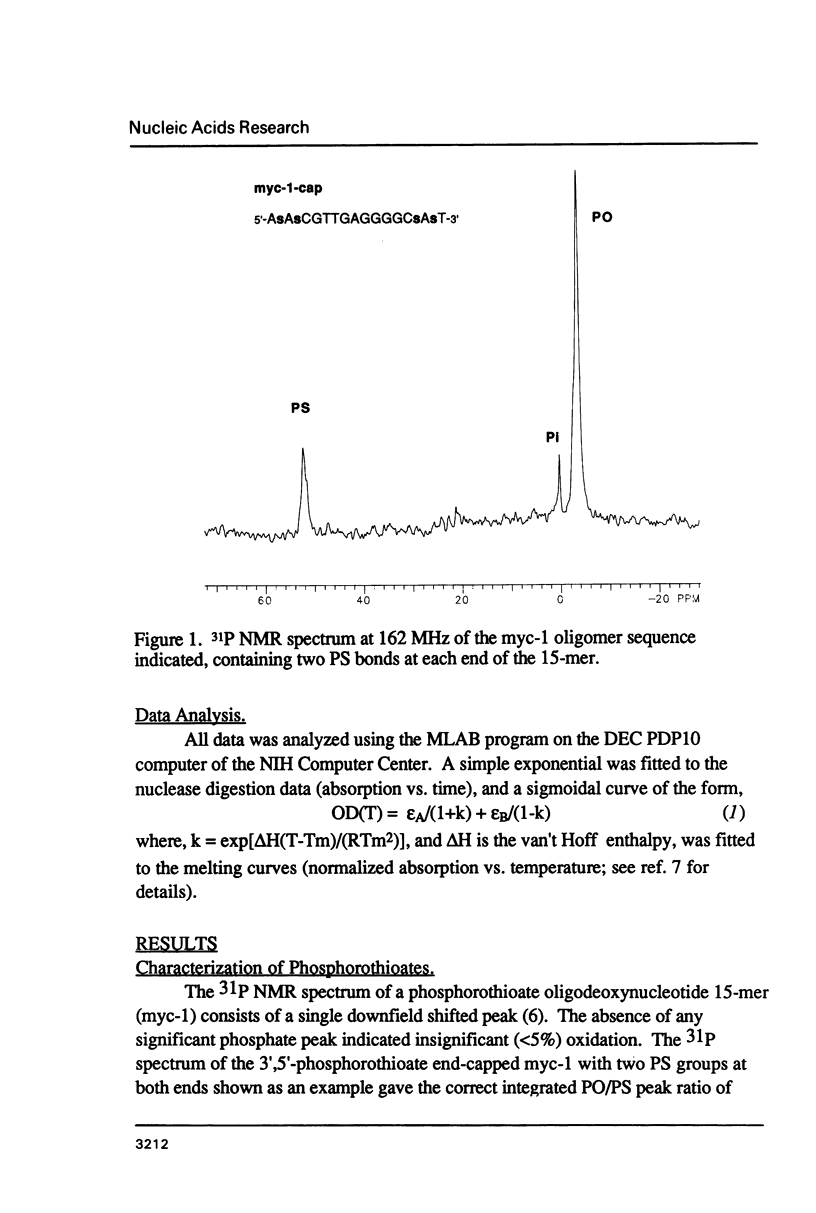
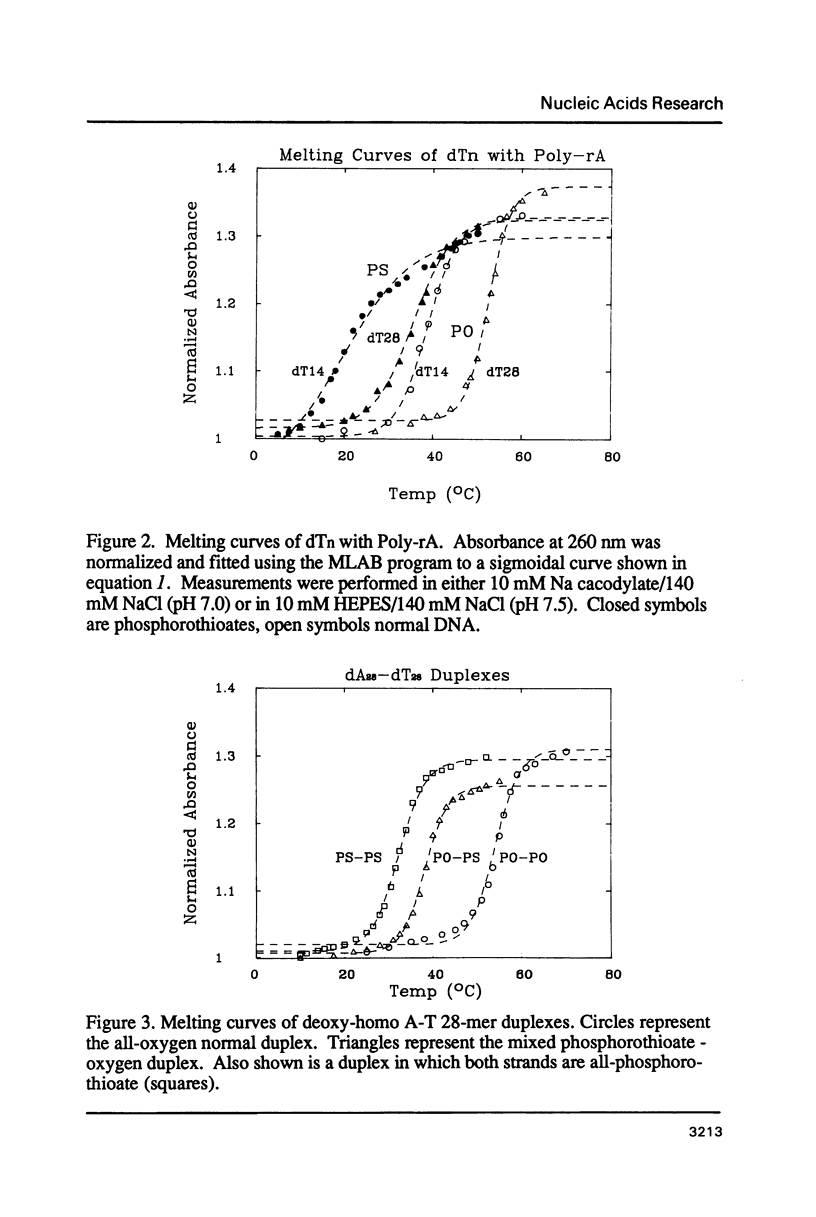
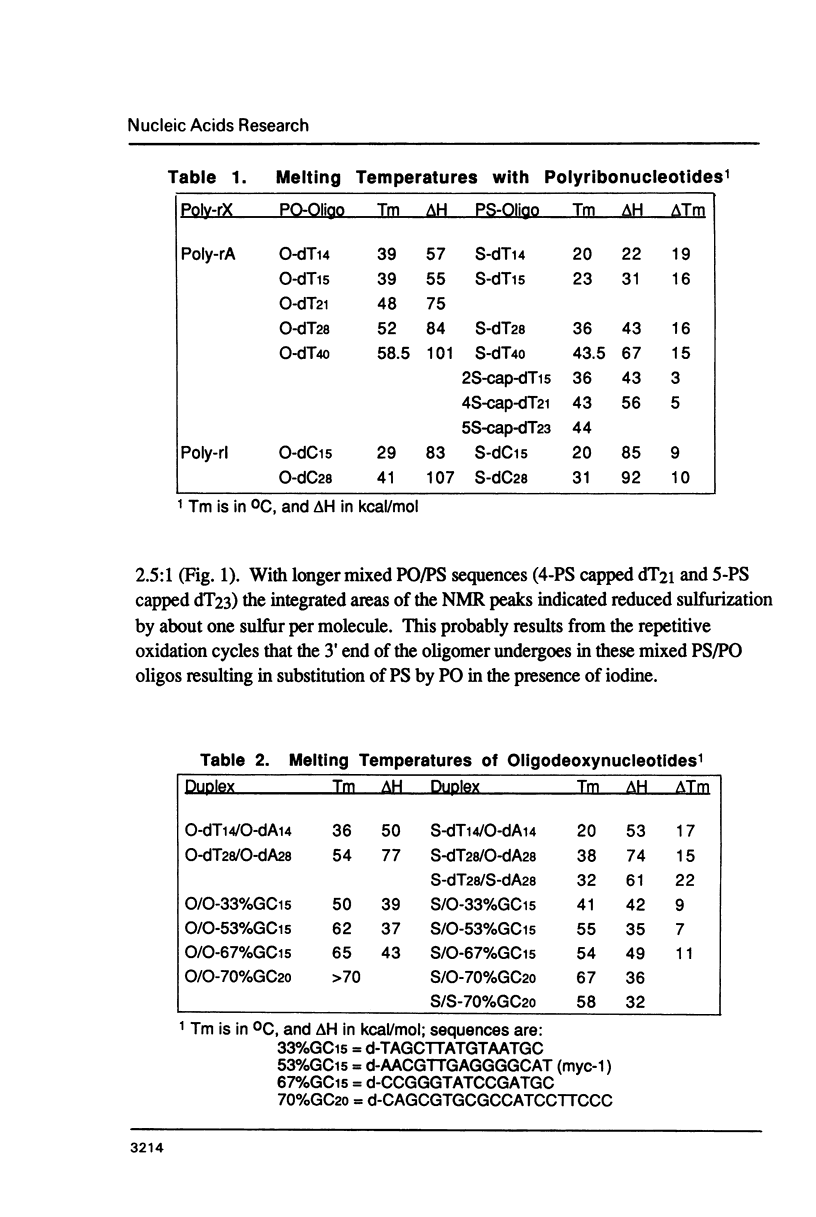
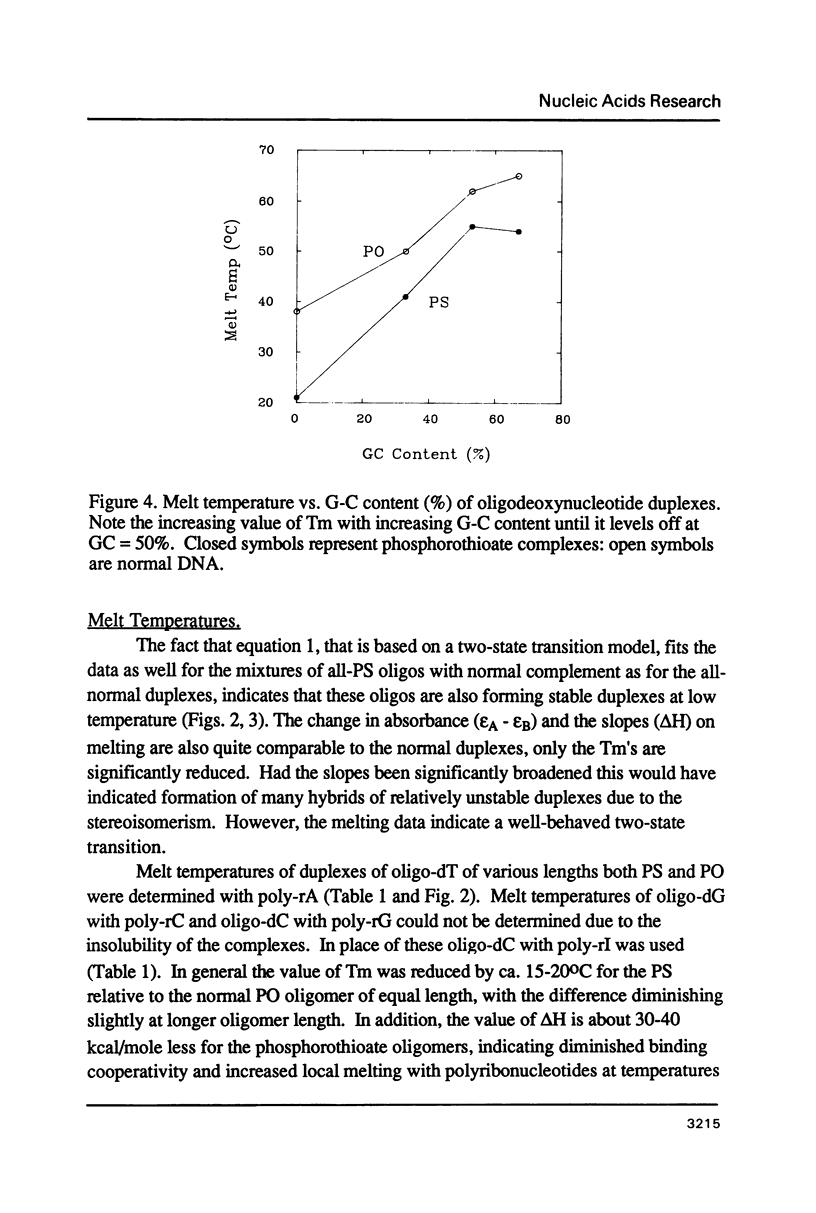
We have recently shown that phosphorothioate (PS) oligodeoxynucleotide (ODN) analogs, unlike their normal congeners, exhibit significant anti-HIV activity (Matsukura et al., (1987) Proc. Natl. Acad. Sci. USA 84, 7706-7710). We now report the syntheses, melting temperatures (Tm), and nuclease susceptibilities of a series of phosphorothioate ODN analogs. These include all-PS duplexes, duplexes with one normal chain and the other chain either all-PS, or end-capped with several PS groups at both 3' and 5' ends. The DNase susceptibilities of the S-ODNs are much less than the normal phosphodiesters, but by contrast duplexes of poly-rA with S-dT40 are much more susceptible to RNase H digestion. The Tm's for AT base pairs of S-ODNs are significantly depressed relative to normals, while GC base pairs show much less Tm depression. The Tm's of S-dT oligomers with poly-rA are reduced relative to the duplexes with normal dA oligomers. These results have significance for the biological properties of these analogs as anti-message inhibitors of gene expression, and provide a rational basis for the S-dC/G sequences as potential effective anti-AIDS agents.
Full text
PDF


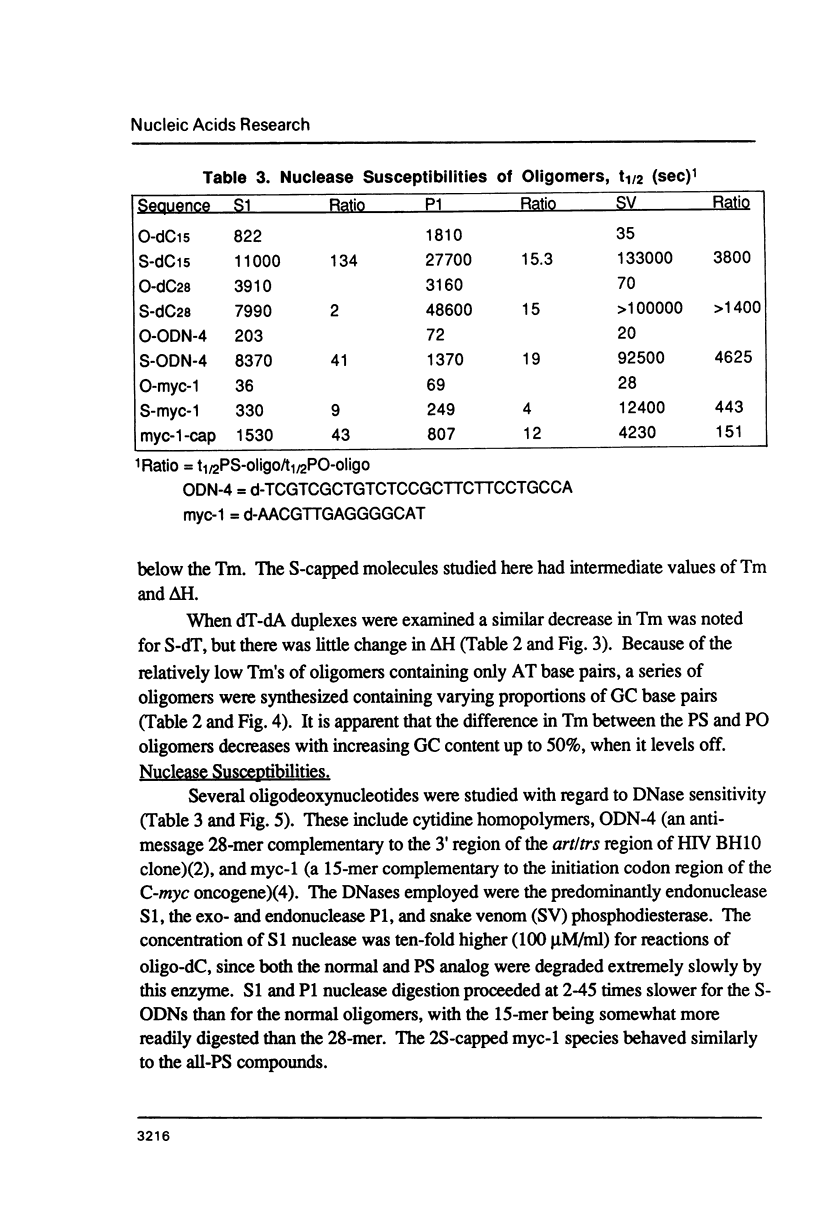
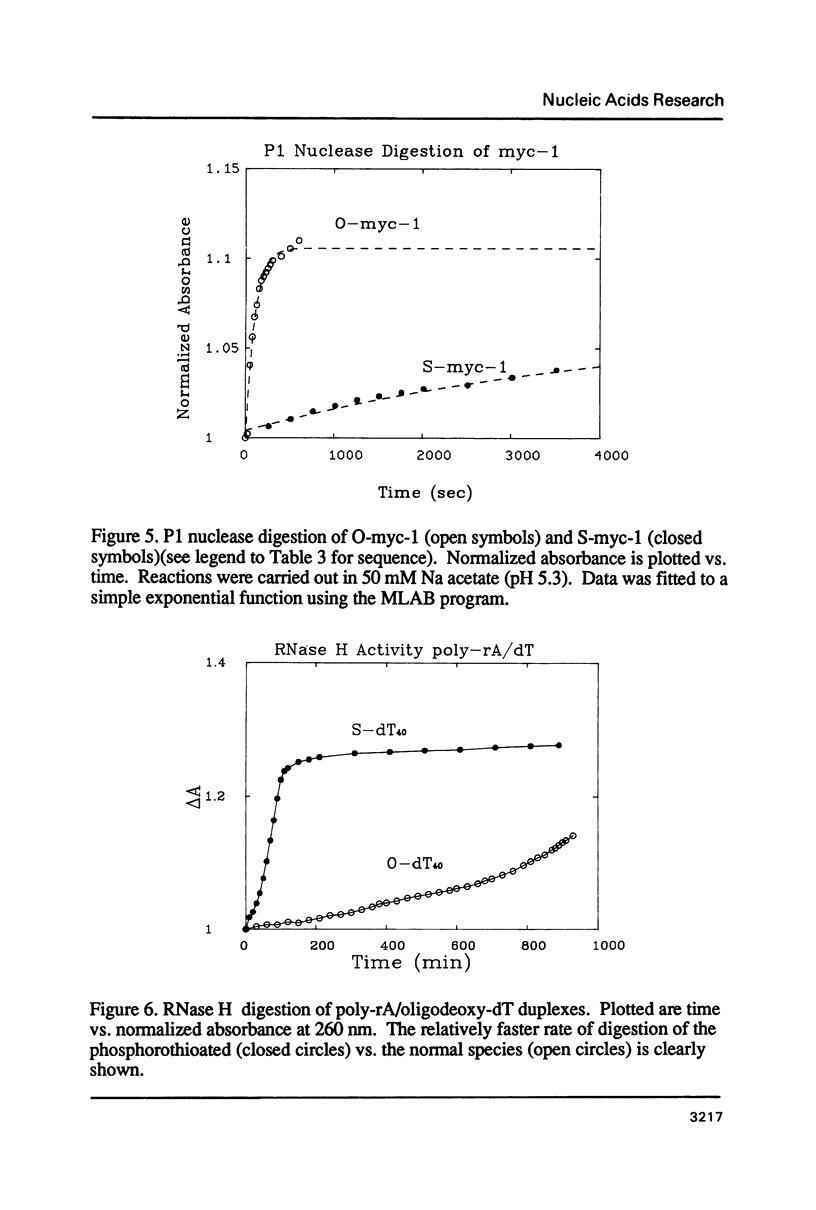




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake K. R., Murakami A., Miller P. S. Inhibition of rabbit globin mRNA translation by sequence-specific oligodeoxyribonucleotides. Biochemistry. 1985 Oct 22;24(22):6132–6138. doi: 10.1021/bi00343a015. [DOI] [PubMed] [Google Scholar]
- Bryant F. R., Benkovic S. J. Stereochemical course of the reaction catalyzed by 5'-nucleotide phosphodiesterase from snake venom. Biochemistry. 1979 Jun 26;18(13):2825–2828. doi: 10.1021/bi00580a022. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F., Hunneman D. H. Stereochemistry of hydrolysis by snake venom phosphodiesterase. J Biol Chem. 1979 Aug 25;254(16):7476–7478. [PubMed] [Google Scholar]
- Burgers P. M., Sathyanarayana B. K., Saenger W., Eckstein F. Crystal and molecular structure of adenosine 5'-O-phosphorothioate O-p-nitrophenyl ester (Sp diastereomer). Substrate stereospecificity of snake venom phosphodiesterase. Eur J Biochem. 1979 Oct 15;100(2):585–591. doi: 10.1111/j.1432-1033.1979.tb04205.x. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- LaPlanche L. A., James T. L., Powell C., Wilson W. D., Uznanski B., Stec W. J., Summers M. F., Zon G. Phosphorothioate-modified oligodeoxyribonucleotides. III. NMR and UV spectroscopic studies of the Rp-Rp, Sp-Sp, and Rp-Sp duplexes, [d(GGSAATTCC)]2, derived from diastereomeric O-ethyl phosphorothioates. Nucleic Acids Res. 1986 Nov 25;14(22):9081–9093. doi: 10.1093/nar/14.22.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Kirchhoff W., Schwarz F., Appella E., Chiu Y. Y., Cohen J. S., Sussman J. L. Conformational transitions of synthetic DNA sequences with inserted bases, related to the dodecamer d(CGCGAATTCGCG). Nucleic Acids Res. 1987 May 11;15(9):3877–3890. doi: 10.1093/nar/15.9.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., McParland K. B., Jayaraman K., Ts'o P. O. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry. 1981 Mar 31;20(7):1874–1880. doi: 10.1021/bi00510a024. [DOI] [PubMed] [Google Scholar]
- Murakami A., Blake K. R., Miller P. S. Characterization of sequence-specific oligodeoxyribonucleoside methylphosphonates and their interaction with rabbit globin mRNA. Biochemistry. 1985 Jul 16;24(15):4041–4046. doi: 10.1021/bi00336a036. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Connolly B. A., Eckstein F. Synthesis and configurational analysis of a dinucleoside phosphate isotopically chiral at phosphorus. Stereochemical course of Penicillium citrum nuclease P1 reaction. Biochemistry. 1983 Mar 15;22(6):1369–1377. doi: 10.1021/bi00275a008. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Romaniuk P. J., Eckstein F. Stereochemical course of DNA hydrolysis by nuclease S1. J Biol Chem. 1983 Feb 10;258(3):1758–1760. [PubMed] [Google Scholar]



