Abstract
Background
The rice roots are highly salt-sensitive organ and primary root growth is rapidly suppressed by salt stress. Sucrose nonfermenting 1-related protein kinase2 (SnRK2) family is one of the key regulator of hyper-osmotic stress signalling in various plant cells. To understand early salt response of rice roots and identify SnRK2 signaling components, proteome changes of transgenic rice roots over-expressing OSRK1, a rice SnRK2 kinase were investigated.
Results
Proteomes were analyzed by two-dimensional electrophoresis and protein spots were identified by LC-MS/MS from wild type and OSRK1 transgenic rice roots exposed to 150 mM NaCl for either 3 h or 7 h. Fifty two early salt -responsive protein spots were identified from wild type rice roots. The major up-regulated proteins were enzymes related to energy regulation, amino acid metabolism, methylglyoxal detoxification, redox regulation and protein turnover. It is noted that enzymes known to be involved in GA-induced root growth such as fructose bisphosphate aldolase and methylmalonate semialdehyde dehydrogenase were clearly down-regulated. In contrast to wild type rice roots, only a few proteins were changed by salt stress in OSRK1 transgenic rice roots. A comparative quantitative analysis of the proteome level indicated that forty three early salt-responsive proteins were magnified in transgenic rice roots at unstressed condition. These proteins contain single or multiple potential SnRK2 recognition motives. In vitro kinase assay revealed that one of the identified proteome, calreticulin is a good substrate of OSRK1.
Conclusions
Our present data implicate that rice roots rapidly changed broad spectrum of energy metabolism upon challenging salt stress, and suppression of GA signaling by salt stress may be responsible for the rapid arrest of root growth and development. The broad spectrum of functional categories of proteins affected by over-expression of OSRK1 indicates that OSRK1 is an upstream regulator of stress signaling in rice roots. Enzymes involved in glycolysis, branched amino acid catabolism, dnaK-type molecular chaperone, calcium binding protein, Sal T and glyoxalase are potential targets of OSRK1 in rice roots under salt stress that need to be further investigated.
Background
Salinity is a major constraint to crop productivity. Plant salt tolerance involves diverse mechanisms such as osmolyte accumulation, ion homeostasis, cellular protection from damage by reactive oxygen species, growth regulation, and signal perception and transduction [1-4]. As plant roots are primary site of perception and highly sensitive organ to salt stress, the understanding for roots to salt response can contribute to increasing the crop productivity. So far, numerous salt-regulated genes and proteins were identified by microarray and proteomic studies in roots of various plants such as tomato, Arabidopsis, tobacco and rice [5-9]. The identified salt-responsive proteins are involved in diverse cellular functions such as regulation of carbohydrate, nitrogen and energy metabolism, ROS scavenging, detoxification, signal transduction, RNA and protein processing and cytoskeleton. On the other hands, large numbers of salt responsive genes reported in the literature have not been identified so far by proteomic approaches, and mRNA level was not correlated well with the protein level. Therefore, characterization of post-transcriptional and post-translational regulatory systems is crucial for the deeper understanding of the molecular mechanisms governing plant adaptation to salt stress. Phosphorylation is one of the best known post-translational protein modifications affecting conformation, activity, localization and stability of target proteins [10]. Under salt stress, protein phosphorylation cascades are activated and play a critical role in rice salt tolerance [11-15]. However, phosphoproteome or proteome changes related to specific protein kinase signaling under salt stress have not been well characterized.
Protein kinases function as key regulators of salt stress and ABA signaling in plants. Diverse protein kinase families such as mitogen-activated protein kinases (MAPK), calcium-dependent protein kinases (CDPK), SNF1-related protein kinases (SnRK) and receptor like kinases (RLKs) were found to be activated by ABA and diverse stress signals [13,16-20]. Arabidopsis SOS2 (salt overly sensitive2), the calcium sensor-associated SnRK3 family, regulates sodium ion homeostasis and is required for salt tolerance [21]. This SOS salt tolerant pathway is likely to be conserved in cereals such as rice [14]. Transgenic plants over-expressing rice OsCIPK3, OsCIPK12, and OsCIPK15 showed significantly improved stress tolerance to cold, drought, and salt stress, respectively [22].
Osmotic stress or ABA treatment rapidly activates 42-48 kDa protein kinases, known as SnRK2 family later [17,19,23-26]. Biochemical studies indicated that all rice and Arabidopsis SnRK2 kinases except AtSnRK2.9 are activated by salt treatment [15,27], implicating that they may have important function in plant salt responses. On the other hand, several SnRK2 members of Arabidopsis (2.2, 2.3, 2.6, 2.7 and 2.8) and rice (SAPK8, SAPK9 and SAPK10) were activated by ABA as well [15,27]. It has been appeared that these ABA-activated SnRK2 subfamily members are key regulators of ABA signaling pathway function in control of seed development, dormancy, seed germination, seedling growth as well as stomata regulation in drought response [28-30]. Recent progress showed that Arabidopsis SnRK2.6/OST1 is a core component of ABA signal transduction pathway leading to activation of an ion channel SLAC1 in guard cells [31] and ABF-induced gene expression in plant cell [32]. In spite of the recent progress in those limited number of SnRK2 signaling pathway in ABA responses of Arabidopsis, little is known about the function of other SnRK2 kinase members, in particularly, associated with salt stress signaling. There is a report showing that over-expression of a rice SnRK2 kinase SAPK4 regulates salt stress response [33].
OSRK1/SAPK6 is a rice SnRK2 kinase function in ABA and hyperosmotic stress signaling [15,34]. In this study, proteome analysis was conducted in the transgenic rice over-expressing OSRK1 to identify OSRK1-regulated proteome changes associated with early salt stress responses of rice roots. Two-DE based comparable proteomic analysis and LC-MS/MS were applied to identify protein changes during the early response of roots to salt stress. Proteomic analysis indicates that OSRK1 is an upstream regulator of the salt stress responses associated with regulation of carbon, nitrogen and energy metabolism, and detoxifying enzymes in rice roots.
Results and discussion
Phosphorylation activity in roots of OSRK1 transgenic rice
OSRK1/SAPK6 showed strong kinase activity in vitro and rapidly activated by salt and osmotic stress when transiently expressed in rice suspension cells [15,34]. In an attempt to identify OSRK1 signaling pathway, we have generated transgenic rice over-expressing OSRK1 under the control of CaMV 35S promoter. Insertion and expression of the transgene was confirmed by genomic PCR and Northern blot analysis (Figure 1A). Compared to wild type, seedling growth of the transgenic rice was retarded (Figure 1B), and plant height was significantly decreased under paddy field growth condition (data not shown). We often observed root growth of the transgenic rice was impaired depending on the growth condition (data not shown). It was found that primary root elongation of OSRK1 transgenic rice was more sensitive to NaCl stress (Figure 1C).
Figure 1.
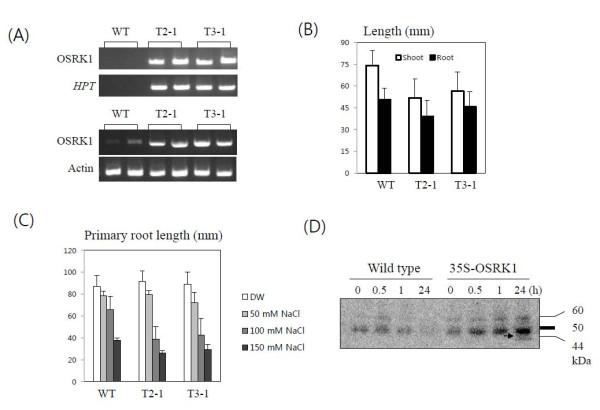
Phenotypes of OSRK1 transgenic rice. (A) PCR analysis of OSRK1 transgenic rice. Insertion and expression of the transgene was confirmed by genomic PCR (upper) and RT-PCR analysis (bottom). The PCR primers for OSRK1 were designed to specifically detect transgene or mRNA. RT-PCR analysis indicated that transcript level of OSRK1 was greatly increased in the transgenic rice roots. (B) Comparison of shoot and root growth of OSRK1 transgenic rice. Root and shoot length was measured after 9 days-growth on 1/2 MS agar media. (C) Effect of NaCl on the primary root growth of wild type and transgenic rice roots. Germinating seedlings with equally growing primary roots were selected and primary root length was measured 5 days after NaCl treatment at indicated concentrations. (D) In-gel kinase assay. Total soluble proteins were extracted from the roots of wild type or OSRK1 transgenic rice seedlings after 150 mM NaCl treatment for the indicated time period. Histone was used as a substrate.
Phosphorylation activity in wild type and transgenic rice roots in response to NaCl was compared. In gel kinase assay with histone as a substrate detected two phosphorylation bands approximately at 52 kDa and 61 kDa, and these kinase activities were transiently increased by NaCl treatment in wild type roots (Figure 1D). In transgenic rice roots, the signal intensity of these bands was much stronger and remained for longer time (Figure 1D). Our data indicates that protein phosphorylation activity was significantly increased by over-expression of OSRK1 in rice roots. Whether the 52-60 kDa salt-responsive kinases detected in our in gel kinase assay are targets of OSRK1 remains to be elucidated.
Proteome analysis of early salt-responsive proteins in OSRK1 transgenic rice roots
Rice roots are very sensitive organ to sense salt stress, and could be a suitable system to investigate OSRK1-dependent targets related to salt stress response. As we found that over-expression of OSRK1 resulted in high phosphorylation activity and salt-sensitive root growth (Figure 1C and 1D), proteomic analysis was carried out to monitor the salt response of OSRK1 transgenic rice roots at protein level. Protein kinase activities of many plant SnRK2 family members were known to be rapidly activated by salt stress [15,17,19,23-27]. We therefore focussed on the initial changes of protein profiles in order to identify OSRK1-mediated salt stress signalling components. Two-DE based comparative proteomic analysis was performed in wild type and OSRK1 transgenic rice roots with 3 h or 7 h of salt treatment (Figure 2 and 3).
Figure 2.
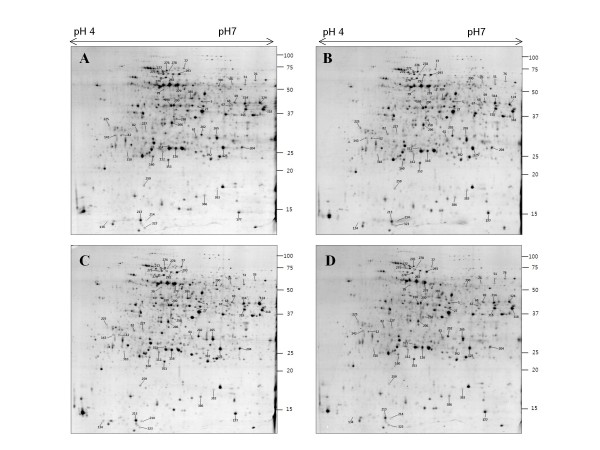
Protein expression profiles in response to a salt treatment in OSRK1 transgenic rice roots. Proteins were extracted from transgenic rice roots after treatment with 150 mM NaCl (B, D) or control medium (A, C) for 3 h (A, B) and 7 h (C, D). Spot numbers indicated the identified protein spots describe in Table 1, that their abundance was increased or decreased following NaCl treatment at 3 h or 7 h.
Figure 3.
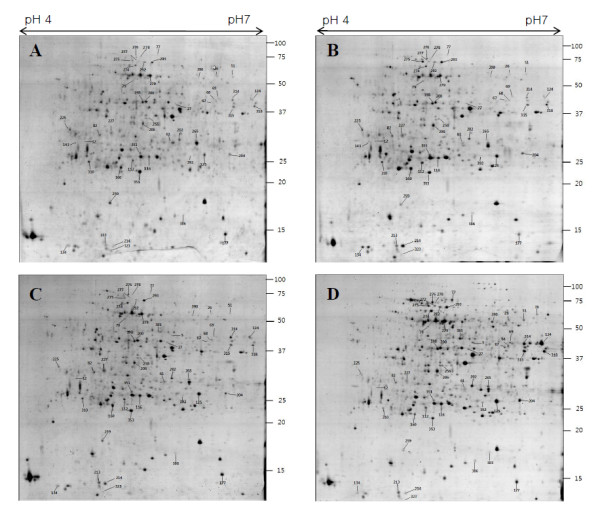
Protein expression profiles in response to a salt treatment in WT rice roots. Proteins were extracted from WT rice roots after treatment with 150 mM NaCl (B, D) or control medium (A, C) for 3 h (A, B) and 7 h (C, D). Spot numbers indicated the identified protein spots described in Table 1, that their abundance was increased or decreased following NaCl treatment at 3 h or 7 h.
Previous reports have presented diverse proteome profiles changed by salt stress in plant roots [5,8,9,35-37]. Contrary to our expectation, however, only seven salt-responsive protein spots were detected as an significant different (P = 0.05) from control group in the transgenic rice roots either by 3 h or 7 h salt stress (Figure 2 and Table 1). They are two translation elongation factors (spot 5 and 12), dihydrolipoamide dehydrogenase (spot 76), glutathione S-transferase, proteasome subunit beta type 2 and/or chaperonin21 precursor (spot 112), ascorbate peroxidase (spot 160), 15 kda Organ-specific salt-induced protein and/or inorganic pyrophosphatase (spot 227), and glyoxalase II (spot 265). With less significant difference (P = 0.05 ~ 0.07), dnaK-type molecular chaperone precursor (spot 276, 278) and enolase (spot 279) is changed by salt treatment (Figure 2 and Table 1). Most of these proteins were salt-responsive in wild type rice roots as well (Table 1), and proteins specific to the transgenic rice were not found.
Table 1.
Identification and quantitative analysis of early salt-responsive proteins in wild type and OSRK1 transgenic rice roots
| Spota | Protein Identification | Matched peptide |
Sequence Coverage (%) |
Mrb (Ex/Tr) |
NCBI Accession Number |
Fold changec | Protein ratiod (OSRK1/WT) |
Express Graphe |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | OSRK1 | ||||||||||
| 3 h | 7 h | 3 h | 7 h | ||||||||
| Translation and transcription | |||||||||||
| 5i | Elongation factor Tu | 9 | 26.3 | 43.7/48.4 | gi|21685576 | ndf | 7.32 | 2.47 | 1.19 | Ig | 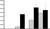 |
| 12i | Putative translation elongation factor eEF-1 beta chain |
5 | 35.3 | 30.0/23.8 | gi|113612079 | 0.97 | 2.00 | 1.92 | 0.55 | 1.25 | 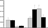 |
| 323 | Glycine-rich RNA-binding protein | 6 | 74.0 | 13.1/15.5 | gi|115441831 | 1.02 | 0.38 | 1.14 | 0.85 | 0.93 | 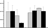 |
| 383 | Translation initiation factor 5A | 2 | 25.0 | 17.7/17.4 | gi|113611710 | nd | 2.84 | 1.04 | 0.62 | Ig | 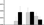 |
| Amino acid and purine metabolism | |||||||||||
| 26 | Putative inosine monophosphate dehydrogenase | 2 | 4.70 | 57.4/51.9 | gi|215694434 | 0.38 | 1.25 | 0.89 | 0.88 | 1.05 | 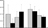 |
| 27 | Aspartate-semialdehyde dehydrogenase | 3 | 10.9 | 40.1/40.1 | gi|113549818 | 2.11 | 3.05 | 1.15 | 0.80 | 5.46 | 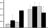 |
| 67 | Aspartate aminotransferase | 4 | 13.0 | 40.6/44.8 | gi|29468084 | 1.48 | 4.51 | 0.87 | 0.97 | 3.64 | 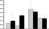 |
| 68 | Putative isovaleryl-CoA dehydrogenase | 1 | 2.70 | 41.5/44.5 | gi|113578072 | 0.88 | 1.89 | 0.91 | 0.89 | 2.53 | 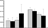 |
| 200 | Glutamine synthetase | 4 | 10.4 | 41.4/39.2 | gi|124052115 | 1.51 | 1.37 | 0.98 | 1.02 | 1.72 | 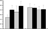 |
| 259 | Methylmalonate semi-aldehyde dehydrogenase | 5 | 10.5 | 18.1/57.2 | gi|113610618 | 0.46 | 0.60 | 1.22 | 0.67 | 0.27 | 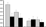 |
| 314 | Aspartate aminotransferase | 6 | 18.8 | 42.2/44.9 | gi|215768565 | 1.13 | 3.14 | 0.87 | 1.06 | 3.08 | 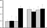 |
| 314 | Glutamate dehydrogenase | 7 | 21.4 | 42.2/44.3 | gi|33242905 | 1.13 | 3.14 | 0.87 | 1.06 | 3.08 | |
| Detoxyfying enzymes | |||||||||||
| 51 | Glutathione reductase, cytosolic | 11 | 24.8 | 55.6/53.4 | gi|113538016 | 0.63 | 2.80 | 0.89 | 1.01 | 1.82 | 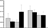 |
| 112i | Gutathione S-transferase | 4 | 25.6/25.6 | gi|31433227 | 1.67 | 0.72 | 1.35 | 1.51 | 0.28 | 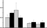 |
|
| 116 | Ascorbate peroxidase | 5 | 33.6 | 25.8/27.1 | gi|50920595 | 1.79 | 1.13 | 0.80 | 1.10 | 2.40 | 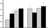 |
| 125 | Glutathione S-transferase II | 6 | 32.1 | 26.0/24.0 | gi|3746581 | 2.05 | 1.42 | 0.94 | 0.90 | 2.63 | 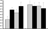 |
| 160i | Ascorbate peroxidase | 10 | 46.0 | 24.0/27.1 | gi|1321661 | 1.35 | 0.38 | 1.51 | 0.71 | 0.41 | 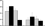 |
| 198 | Quercetin 3-O-methyltransferase | 2 | 6.8 | 41.4/39.7 | gi|113623000 | 1.57 | 1.19 | 1.06 | 0.84 | 1.83 | 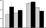 |
| 200 | Peroxidase | 3 | 8.6 | 41.4/37.8 | gi|257657027 | 1.51 | 1.37 | 0.98 | 1.02 | 1.72 | 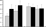 |
| 202 | Similar to glyoxalase II | 6 | 28.2 | 29.9/33.1 | gi|113533338 | 1.07 | 1.98 | 0.94 | 0.90 | 1.47 | 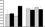 |
| 206 | Glyoxalase I | 10 | 51.5 | 33.9/32.5 | gi|113623141 | 3.57 | 1.73 | 0.84 | 1.00 | 4.46 | 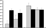 |
| 213 | Thioredoxin Type H | 7 | 50.8 | 13.6/13.9 | gi|82407383 | 1.94 | 0.87 | 1.01 | 0.98 | 3.20 | 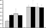 |
| 265i | Similar to glyoxalase II | 7 | 32.5 | 32.5/33.2 | gi|113533338 | 0.59 | 1.52 | 0.68 | 0.74 | 1.90 | 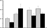 |
| 310 | Ascorbate peroxidase | 5 | 38.2 | 25.6/27.1 | gi|257707656 | 0.79 | 0.34 | 1.85 | 4.40 | 0.10 | 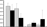 |
| 351 | Ascorbate peroxidase | 4 | 24.8 | 26.7/27.1 | gi|1321661 | 1.98 | 0.65 | 1.00 | 0.97 | 1.35 | 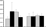 |
| 390 | Aldehyde dehydrogenase | 3 | 8.0 | 54.6/59.2 | gi|8163730 | 3.16 | 3.10 | 0.85 | 1.25 | 10.85 | 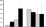 |
| Glycolysis and other carbohydrate metabolism related proteins | |||||||||||
| 61 | Fructose -bisphosphate aldolase | 3 | 9.8 | 29.3/38.8 | gi|790970 | 0.26 | 0.94 | 0.69 | 0.81 | 0.82 | 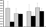 |
| 67 | Phosphoglycerate kinase | 3 | 11.0 | 40.6/42.3 | gi|113596357 | 1.48 | 4.51 | 0.87 | 0.97 | 3.64 | 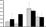 |
| 76i | Dihydrolipoamide dehydrogenase precursor | 5 | 13.5 | 60.6/52.6 | gi|113532449 | nd | 16.87 | 0.87 | 1.54 | Ig | 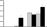 |
| 77 | Transketolase | 7 | 10.6 | 76.8/80.0 | gi|227468492 | 2.14 | 15.09 | 1.02 | 0.98 | 18.68 | 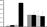 |
| 79 | Similar to enolase | 6 | 15.9 | 52.0/50.7 | gi|115478881 | 1.90 | 0.78 | 0.80 | 0.74 | 3.14 | 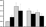 |
| 116 | Triose phosphate isomerase | 13 | 79.1 | 25.8/27.6 | gi|553107 | 1.79 | 1.13 | 0.80 | 1.10 | 2.40 | 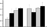 |
| 275 | 2,3-Bisphosphoglycerate-independent phosphoglycerate mutase | 8 | 27.2 | 70.8/60.8 | gi|257353836 | 1.45 | 2.56 | 0.80 | 0.84 | 2.07 | 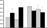 |
| 277 | 2,3-Bisphosphoglycerate-independent phosphoglycerate mutase | 3 | 12.2 | 70.8/60.8 | gi|257353836 | 1.26 | 2.83 | 0.86 | 0.88 | 2.33 | 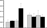 |
| 279i | Enolase | 4 | 14.4 | 57.0/47.9 | gi|113548027 | 1. 03 | 1.70 | 0.72 | 0.80 | 1.41 | 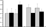 |
| 292 | Phosphoglyceromutase | 9 | 20.8 | 69.9/60.7 | gi|257353838 | 1.03 | 2.13 | 0.93 | 1.04 | 2.66 | 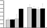 |
| 293 | Phosphoglyceromutase | 12 | 28.1 | 69.9/60.7 | gi|257353838 | 1.50 | 1.79 | 0.87 | 1.02 | 1.90 | 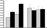 |
| 315 | Glyceralde-3-phosphate dehydrogenase | 4 | 16.3 | 39.1/36.5 | gi|968996 | 0.99 | 2.14 | 0.91 | 1.10 | 2.15 | 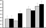 |
| 318 | Glyceralde-3-phosphate dehydrogenase | 4 | 16.3 | 38.7/36.4 | gi|968996 | 1.17 | 2.68 | 1.03 | 1.01 | 3.06 | 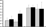 |
| 353 | Triose phosphate isomerise | 4 | 20.9 | 22.0/27.0 | gi|169821 | 0.83 | 0.62 | 1.16 | 0.67 | 0.32 | 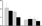 |
| 385 | Phosphoglycerate kinase | 5 | 16.7 | 45.4/42.2 | gi|113596357 | nd | 4.57 | 1.03 | 1.10 | Ig | 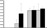 |
| Proteolytic enzymes | |||||||||||
| 112i | Proteasome subunit beta type 2 | 5 | 26.4 | 25.6/23.4 | gi|17380213 | 1.67 | 0.72 | 1.35 | 1.51 | 0.28 | 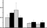 |
| 134 | Oryzacystatin | 3 | 23.5 | 13.3/11.4 | gi|1280613 | 2.10 | 0.46 | 0.87 | 1.00 | 0.22 | 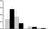 |
| 204 | Proteasome subunit alpha type 2 | 7 | 34.6 | 26.2/27.6 | gi|259443357 | 0.59 | 1.88 | 0.89 | 1.15 | 1.22 | 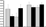 |
| 351 | Proteasome subunit alpha type 2 | 4 | 16.6 | 26.7/25.8 | gi|259443327 | 1.98 | 0.65 | 1.00 | 0.97 | 1.35 | 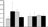 |
| Heat shock proteins | |||||||||||
| 112i | Putative chaperonin21 precursor | 5 | 26.2 | 25.6/26.3 | gi|51090748 | 1.67 | 0.72 | 1.35 | 1.51 | 0.28 | 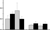 |
| 274 | Chaperonin CPN60-1, mitochondrial precursor | 10 | 17.5 | 64.1/61.0 | gi|113547409 | 1.17 | 2.77 | 0.88 | 1.12 | 1.49 | 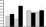 |
| 276i | DnaK-type molecular chaperone precursor | 2 | 4.6 | 75.0/70.4 | gi|257307253 | 1.26 | 3.35 | 0.76 | 1.00 | 2.78 | 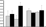 |
| 278i | DnaK-type molecular chaperone precursor | 6 | 14.5 | 72.9/70.4 | gi|257307253 | 2.35 | 2.38 | 0.66 | 1.47 | 4.47 | 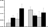 |
| Lipid biosynthesis | |||||||||||
| 258 | Putative enoyl-ACP reductase | 3 | 15.7 | 35.4/39.1 | gi|113623526 | 1.32 | 2.41 | 1.13 | 1.01 | 2.92 | 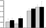 |
| 69 | Putative acetyl-CoA C-acetyltransferase | 6 | 19.2 | 43.1/44.1 | gi|113630918 | 1.28 | 3.46 | 0.80 | 0.99 | 6.40 | 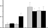 |
| 82 | Putative inorganic pyrophosphatase | 5 | 21.4 | 31.8/33.0 | gi|113537770 | 0.88 | 0.41 | 0.97 | 0.99 | 0.42 | 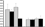 |
| 177 | Nucleoside diphosphate kinase from rice | 2 | 16.6 | 14.5/16.8 | gi|113639936 | 0.39 | 0.81 | 0.88 | 0.83 | 1.14 | 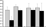 |
| 227i | Putative inorganic pyrophosphatase | 5 | 13.9 | 32.1/33.0 | gi|113537770 | 1.70 | 0.41 | 0.92 | 1.30 | 0.44 | 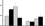 |
| Stress related proteins | |||||||||||
| 124 | Formate dehydrogenase, mitochondrial precursor | 13 | 47.2 | 42.4/41.2 | gi|21263611 | 0.64 | 7.24 | 0.78 | 0.90 | 4.13 | 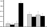 |
| 134 | 15 kda Organ-specific salt-induced protein | 2 | 23.4 | 13.3/15.2 | gi|256638 | 2.10 | 0.46 | 0.87 | 1.00 | 0.22 | 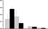 |
| 214 | 15 kda Organ-specific salt-induced protein | 7 | 77.2 | 13.4/15.2 | gi|256638 | 4.05 | 0.33 | 0.87 | 1.12 | 0.48 | 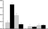 |
| 227i | 15 kda Organ-specific salt-induced protein | 6 | 64.8 | 32.1/15.2 | gi|256638 | 1.70 | 0.41 | 0.92 | 1.30 | 0.44 | 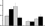 |
| 386 | Pathogen-related protein | 4 | 21.3 | 16.5/17.2 | gi|16589076 | 4.29 | 2.28 | 1.30 | 0.38 | 6.70 | 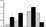 |
| Signal transduction related proteins | |||||||||||
| 225 | Calreticulin | 3 | 5.9 | 33.4/47.9 | gi|6682833 | 0.75 | 0.43 | 1.47 | 1.26 | 0.26 | 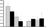 |
| Unkwown proteins | |||||||||||
| 392 | Hypothetical protein | 3 | 10.7 | 25.8/27.4 | gi|14192878 | 5.65 | 4.00 | 0.79 | 0.96 | 18.95 | 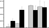 |
aSpot numbers correspond to the spots in Figure 2 and Figure 3
bMolecular weight (kDa) of protein spots estimated from gel analysis (Ex) and theoretical molecular weight of identified protein (Tr)
cFold change of each spot after NaCl treatment for 3 h or 7 h, calculated from the mean spot volumes in control and NaCl treated gel groups
dSpot-volume ratio of WT to OSRK1, calculated from the mean spot volumes in 3 h control of WT and OSRK1 groups
eRelative expression graphs of protein spots after NaCl treatment in WT and OSRK1. Spot volumes are analyzed by Progenesis software. The left four bars indicate spot volume of WT root and right four bars indicate spot volumes of OSRK1. From left to right, each bar indicate spot volume of 3 h control, 3 h NaCl treated, 7 h control and 7 h NaCl treated gel groups. Values are means ± SE. Error bars from three spots in three independent gels
fnd: not detected
g I: spots were not detected in WT groups, but appeared in OSRK1 groups
hProteins not identified from the database
i Salt-responsive proteome identified from OSRK1 transgenic rice roots
Proteome analysis of early salt-responsive proteins in wild type rice roots
Because the salt-responsive proteome changes in transgenic rice were unexpectedly low, proteome changes in wild type and transgenic rice roots were compared. Figure 3 shows the 2-DE gel images of proteins extracted from control and NaCl-treated WT roots. We identified 52 spots which were changed their abundance (vol %) more than 1.5 fold by either 3 h or 7 h salt treatment (Table 1). Of the 52 spots identified, 8 spots (15.4% of identified proteins) were found to have more than two unrelated proteins, indicating the presence of multiple proteins in one spot. Some proteins were found in multiple spots. These proteins were aspartate aminotransferase (spot 67 and 314), inorganic pyrophosphatase (spot 82 and 227), ascorbate peroxidase (spot 310 and 353), 15 kDa organ-specific salt-induced protein (spot 134, 214 and 227), glyoxalase II (spot 202 and 265), proteasome subunit alpha (spot 204 and 351), 2,3-bisphosphoglycerate-independent phosphoglycerate mutase (spot 275 and 277), dnaK-type molecular chaperone (spot 276 and 278), phosphoglyceromutase (spot 292 and 293), glyceralde-3-phosphate dehydrogenase (spot 315 and 318), phosphoglycerate kinase (spot 67 and 385) and triose phosphate isomerase (spot 116 and 353). These multiple spots on 2-DE gel are presumably due to post-transcriptional modification, or expression of differential isoforms derived from different genes, or proteolytic degradation of proteins in vivo and in vitro. Of the 52 salt-responsive protein spots identified, 6 spots were down-regulated and 16 were up-regulated more than 1.2 fold by 3 h and 7 h of salt treatment. Others are transiently up or down-regulated at 3 h or changed only after 7 h. The identified early salt-responsive proteins were classified into 11 functional categories (Table 1). Similar to the previous reports on the salt-responsive proteins of Arabidopsis and rice [5,8,9,36,37], enzymes related to energy metabolism, primary metabolism and detoxification were major protein families rapidly changed by salt stress. In addition, proteins related to translation, proteolytic enzymes and heat shock proteins were significantly changed by salt stress. Other proteins related to lipid biosynthesis, RNA process, stress-related and signal transduction were identified as well.
Our current proteome data indicated that rice roots rapidly changed broad spectrum of energy metabolism upon challenging salt stress. The major up-regulated proteomes were metabolic enzymes related to glycolysis, pentose phosphate pathway, ammonium assimilation, aspartate pathway, branched amino acid breakdown, lipid synthesis, methylglyoxal detoxification and redox regulation. Change in the anerobic respiratory metabolism is a key component of early salt response of rice roots and glycolysis may play central role. It is noteworthy that several GA-responsive proteomes [38-40] were clearly down-regulated in rice roots by salt stress. They are fructose-bisphosphate aldolase (spot 61), inorganic pyrophosphatase (spot 82 and 227), oryzasystatin (spot 134), 15 kDa organ-specific salt induced protein (spot 134, 214 and 227), calreticulin (spot 225) and methylmalonate semi-aldehyde dehydrogenase (MMADH, spot 259). Among these, fructose bisphosphate aldolase and calreticulin were also identified as proteins down-regulated by ABA, a GA antagonistic hormone [41-43]. GA is a key regulator of root cell elongation and development of rice. It was reported that aldolase and MMADH function in GA-induced root growth in rice [38,40]. Suppression of GA signaling by salt stress may responsible for arrest of rice root growth and development under salinity condition.
Glycolysis and carbohydrate metabolism
Salt stress is known to induce a decrease in the oxygen uptake in several plants [44]. At the oxygen limiting circumstances, glycolytic pathway is activated to maintain cellular homeostasis and energy production [45]. Coordinate regulation of the expression of different glycolytic enzymes contribute to maintaining homeostasis in rice cells under oxygen deprivation [46]. Glycolytic gene expression was increased in rice shoots and roots under various abiotic stresses condition including salt stress [45]. Previous rice proteome analysis revealed that glycolytic enzymes were up-regulated by long-term salt stress [8,36]. Our proteome analysis further confirmed that glycolytic pathway is rapidly activated at early phase of salt response of rice roots. Five glycolytic enzymes such as triosephosphate isomerase (spot 116), glyceralde-3-phosphate dehydrogenase (spot 315 and 318), phosphoglycerate kinase (spot 67 and 385), 2,3-bisphosphoglycerate-independent phosphoglycerate mutase (spot 275 and 277), and enolase (spot 79 and 279) were increased at either 3 h or 7 h salt treatment (Table 2). In addition to these enzymes, dramatic increase of transketolase (spot 77) and formate dehydrogenase (spot 124) was observed (Table 2). Transketolase is known as the key enzyme regulating flux between pentose phosphate pathway and glycolysis in E. coli. In plants, transketolase is an essential enzyme of the Calvin cycle. When the transketolase was repressed in tobacco, dramatic changes in photosynthesis and phenylpropanoid metabolism were induced, indicating the central role of transketolases in the primary metabolism [47]. Formate dehydrogenase is an enzyme of anaerobic metabolism and the protein was increased in stressed potato [48,49]. Glycolysis may play an important role in formate synthesis [48]. Taken together, these data indicate that anaerobic metabolism is a key component of early salt response of rice roots and glycolysis play an important role. The cytosolic network of glycolytic enzymes may provide an essential metabolic flexibility that facilitates plant developments and acclimation to environmental stress [49]. Glycolysis also has a role in the production of a wide range of metabolites including amino acids, lipids and related compounds [45,49].
Table 2.
The potential SnRK2 phosphorylation sites found in protein spots showing more than 1.5 fold change in OSRK1 transgenic rice roots at unstressed condition
| Spota | Protein Identification | NCBI Accession # | Potential recognition motifs (SnRK2)b | Sc | T/Wd |
|---|---|---|---|---|---|
| 5 | Elongation factor Tu | gi|21685576 | RRILS, RGIT | U | I |
| 27 | Aspartate-semialdehyde dehydrogenase | gi|113549818 | RRPSS, FLRVIS | U | U |
| 61 | Fructose-bisphosphate aldolase | gi|790970 | RFAS | D | D |
| 67 | Aspartate aminotransferase | gi|29468084 | RVAT, RVKS | U | U |
| 68 | Putative isovaleryl-CoA dehydrogenase | gi|113578072 | RRLYS, LVRHGS | U | U |
| 69 | Putative acetyl-CoA C-acetyltransferase | gi|113630918 | RSSS, RKGS | U | U |
| 76 | Dihydrolipoamide dehydrogenase precursor | gi|113532449 | RLGS, RFMT | U | I |
| 77 | Transketolase | gi|227468492 | RNLS, RVVS | U | U |
| 79 | Simillar to enolase | gi|115478881 | RAAT, RNQS, RMGS | U | U |
| 82, 227 | Putative inorganic pyrophosphatase | gi|113537770 | RRRCSLRTNS, RKVS | D | D |
| 112 | Glutathione S-transferase | gi|31433227 | Not found | U | D |
| 112 | Proteasome subunit beta type 2 | gi|17380213 | RRAYT | U | D |
| 112 | Putative chaperonin21 precursor | gi|51090748 | RVCS, RRPS | U | D |
| 116 | Triose phosphate isomerise | gi|553107 | Not found | U | U |
| 124 | Formate dehydrogenase, mitochondrial precursor | gi|21263611 | Not found | U | U |
| 125 | Glutathione S-transferase II | gi|3746581 | MARPSS | U | U |
| 134 | Oryzacystatin | gi|1280613 | Not found | D | D |
| 134, 214, 227 | 15 kda organ-specific salt-induced protein | gi|256638 | FGRSGT | D | D |
| 160 | Ascorbate peroxidise | gi|1321661 | Not found | D | D |
| 200 | Peroxidase | gi|257657027 | IDRAKS, RNDS | U | U |
| 200 | Glutamine synthetase | gi|124052115 | RTLS, RRLT, RHET, RGAS, RPAS | U | U |
| 202, 265 | Putative glyoxalase II | gi|113533338 | RARPIS | U | U |
| 206 | Glyoxalase I | gi|113623141 | IQRGPT | U | U |
| 213 | Thioredoxin type H | gi|82407383 | Not found | U | U |
| 225 | Calreticulin | gi|6682833 | RARSSS | D | D |
| 258 | Putative enoyl-ACP reductase | gi|113623526 | IGRALS, RAMS, RVNT | U | U |
| 259 | Methylmalonate semi-aldehyde dehydrogenase | gi|113610618 | LLRSGS, RVQS, RDAT, VKRASS | D | D |
| 275,277 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | gi|257353836 | RSET, VKRNKS | U | U |
| 276, 278 | DnaK-type molecular chaperone precursor | gi|257307253 | FARTFS, RQAT, INRNTT | U | U |
| 292, 293 | Phosphoglyceromutase | gi|257353838 | RIAS, RAET, VKRNKS | U | U |
| 314 | Glutamate dehydrogenase | gi|33242905 | LTRVFT | U | U |
| 314 | Aspartate aminotransferase | gi|215768565 | RLPT | U | U |
| 315, 318 | Glyceralde-3-phosphate dehydrogenase | gi|968996 | RAAS, RVPT | U | U |
| 385, 67 | Phosphoglycerate_kinase | gi|113596357 | Not found | U | U |
| 383 | Translation initiation factor 5A | gi|113611710 | RLPT | U | I |
| 386 | Pathogen-related protein | gi|16589076 | Not found | U | U |
| 390 | Aldehyde dehydrogenase | gi|8163730 | RRGSS, LQRFST, RVGT | U | U |
| 392 | Hypothetical protein | gi|14192878 | VLRLRS, RYAT | U | U |
aSpot numbers correspond to the spots in Figure 2
bPotential phosphorylation motifs for SnRK2 kinase, RXX(S/T) were analyzed. Motifs with favouring amino acids (L, I, V, M, F, R) at -5 position for CDPK/SnRK2 were marked as bold characters according to Vlad et al. (2008). Among these, the highly preferred motifs, (L/I)XRXX(S/T) were underlined
cUp (U) or down (D)-regulated protein spots in wild type rice roots after NaCl treatment for 3 h or 7 h
dUp (U) or down (D)-regulated or induced (I) protein spots in OSRK1 transgenic rice roots compared to wild type roots at 3 h or 7 h control
ROS regulation and detoxifying enzymes
Reactive oxygen species (ROS) are regarded as the main source of cell damage under abiotic stresses including salt stress [50]. Plants have developed protective mechanisms to eliminate or reduce ROS, and the enzymatic antioxidant system is one of the protective mechanisms [51]. We identified several ROS removing and redox regulating proteins up-regulated by salt treatment in rice roots (Table 1). The rapid increase of an ascorbate peroxidase (APX, spot 116 and 351) and glutathione reductase (GR, spot 51) by salt stress is consistent with the previous report that the activity and transcript levels of these proteins were increased within 4 h or 0.5 h in salt treated rice roots [51-53]. Increase in activity of antioxidant enzymes, such as ascorbate peroxidase, catalase and glutathione reductase, can contribute to salt tolerance of potato [54]. On the other hand, another APX spot (spot 310) was found to be decreased by salt stress (Table 1). This differential response of APXs by salt stress was observed in Arabidopsis roots and may reflect sub-functionalization of APX gene families at the regulatory and catalytic levels [55].
Glyoxalase I (GlyI, spot 206) and glyoxalase II (Gly2, spot 202 and 265) were increased by salt stress. These two enzymes are related to glyoxalase pathway which is required for glutathione-based detoxification of methylglyoxal (MG). The glyoxlase transcript level is rapidly induced by salt stress and closely correlated to salt tolerance of tomato [56,57]. Over-expression of glyoxalase pathway genes in transgenic plants has been found to keep a check on the MG level under stress conditions, regulate glutathione homeostasis, and the transgenic plants are able to survive and grow under various abiotic stresses [58]. In rice roots, glyoxalase I was identified as a salt-induced proteome [36]. Recent report showed that MG detoxification system (enzyme activity of glyoxalase I and II) was significantly higher in salt tolerant Pokkali rice [59]. Transgenic rice over-expression of glyoxalse II showed enhanced tolerance to toxic concentration of MG and NaCl, implicating functional importance of this enzyme in salt tolerance of rice [58].
Thioredoxin H-type protein (spot 213) and aldehyde dehydrogenase (spot 390) were identified as salt up-regulated proteins. Thioredoxin H is a protein involved in redox regulation by reducing disulfide bridges [60]. It has been known that thioredoxin H is induced under oxidative stress condition [61]. Recently, thioredoxin H-type protein was identified as a salt-responsive apoplastic protein in rice roots [62]. Aldehydes are intermediates in several fundamental metabolism pathways for carbohydrates, vitamins, steroids, amino acids, and lipids [63]. They are also phytotoxic metabolites produced by stresses that disturb metabolism, including salinity [64,65]. By catalyzing the irreversible oxidation of a wide range of reactive aldehydes to their corresponding carboxylic acids, aldehyde dehydrogenases protect cells against stress induced damage [37,64].
Amino acids and other metabolism
Our present study revealed that diverse proteins related to nitrogen and amino acids metabolism rapidly respond to salt treatment in rice roots. Enzymes related to ammonium assimilation, aspartate pathway and branched amino acids pathway were up-regulated. They are glutamate dehydrogenase (GDH, spot 314), glutamine synthetase (GS, spot 200), aspartate-semialdehyde dehydrogenase (ASDH, spot 27), aspartate aminotransferase (AAT, spot 67 and 314) and isovaleryl-CoA dehydrogenase (IVDH, spot 68). This is the first report that ASDH and IVDH proteins were up-regulated by salts in rice roots.
Previous reports indicated that GDH and GS are salt stress responsive proteins in rice and Arabidopis roots [5,8,66]. GS catalyze the combination of ammonia and glutamate into glutamine [67]. GDH work as a link between carbon and nitrogen metabolism or deaminate glutamate into ammonium and 2-oxoglutarate. Because glutamate is a precursor of proline, activation of these two enzymes may contribute proline synthesis under salt stress condition [67,68]. Proline is well-known osmo-protectant involved in stress resistant mechanisms in plants. Proline is also known to act as a free radical scavenger [69]. It is noted that two aspartate pathway enzymes, ASDH (spot 27) and AAT (Spot 67 and 314), are increased by salt stress in our data. The aspartate pathway is responsible for the biosynthesis of lysine, threonine, isoleucine, and methionine in most plants and microorganisms. ASDH produce the branch point intermediate between the lysine and threonine/methionine pathways [70]. AAT is an important enzyme involved in carbon and nitrogen metabolism [71]. Li et al. (2010) reported that AAT was induced in rice shoots by combined treatment of ABA and salt [43]. Function of these two aspartate pathway enzymes in plant salt stress is unknown. IVDH (spot 68) was increased by salt treatment. IVDH involved in the catabolism of branched chain amino acids such as leucine and valine [72,73]. Recent report showed that Arabidopsis IVDH mutant increased in 12 of 20 free proteogenic amino acids in seeds, indicating that catabolism plays an important role in regulating levels of branched chain amino acids. In tomato roots, IVDH was reported as aluminum induced proteome [74].
Methylmalonate semi-aldehyde dehydrogenase (MMSDH, spot 259) was down-regulated by salt. MMSDH is a CoA-dependent aldehyde dehydrogenase. It is involved in the distal part of the valine and pyrimidine catabolic pathway and regulation of the long chain fatty acylation in animals and microorganisms. In plant, there is a report that MMSDH is a GA responsive protein and is involved in GA signaling. The rice MMSDH gene and protein was expressed in roots and may play important role in GA induced root cell growth and development [40]. The rapid down-regulation of MMSDH level by salt (Table 1) might be closely correlated with root growth arrest by salt stress. Inorganic phosphatase (spot 82 and 227) was identified as a down-regulated protein by salt. Inorganic phosphatase hydrolyzes PPi and involved in oxidative phosphorylation. Previous reports indicated that it is induced in roots under phosphate starvation condition or GA treatment [75]. It was also identified as a salt-responsive protein in Arabidopsis roots, and as a phosphoprotein in AtSnRK2.8 transgenic plant [5,76]. Over-expression of H+PPase has been known to enhance salt tolerance, drought tolerance and phosphorous nutrition in several plants [77,78].
Enoyl-ACP reductase (ENR, spot 258), a fatty acid synthetase, is up-regulated by salt. High expression of ENR is associated with increasing oil concentration of maize [79]. Deficiency in Arabidopsis ENR (MOD1) caused premature cell death in multiple organs [80]. Bacterial ENR homologous transcripts were known to be induced by phosphate stress [81]. However, their function in plant stress response is mostly unknown.
Signaling molecules and translation related proteins
A calcium binding protein of calreticulin family (spot 225) was identified as a down-regulated spot by salt (Table 1). Calreticulin (CRT) is multifunctional Ca2+-binding protein mainly resident in the endoplasmic reticulum (ER) where it serves as a calcium modulator and chaperone to newly synthesized glycoproteins. Plant CRTs have function in plant growth and development as well as biotic and abiotic stress responses [82]. Previous proteomic reports indicated that CRT protein was up-regulated by salt stress in potato leaves [83]. Jiang et al. (2007) reported that two CRTs were rapidly down-regulated by salt stress in Arabidopsis roots [5]. In rice, CRT protein was induced by GA treatment, but decreased by wound in leaf sheath [84,85]. It is also known that CRT is phosphorylated by CK2 or CDPK [86,87] and dephosphorylated by ABA treatment [88]. There is a report that a wheat CRT was involved in drought stress response [89]. However, function of CRT proteins in plant stress response is mostly unknown.
Salt treatment markedly increased the abundance of two translation elongation factors (Tu and eEF-1 beta, spot 5 and 12) and a translation initiation factor 5A (Spot 383). Ndimba et al. (2005) showed that salt stress affected protein de novo synthesis and several translation initiation factors are up-regulated by hyperosmotic stress [37]. Regulation of the translational machinery is considered to be an important component of cellular stress response [90]. Recently it appeared that Arabidopsis eIF5A3 was involved in supporting growth and to play a regulatory role in the response of plants to sub-lethal osmotic and nutrient stress [91].
Comparative proteome analysis of wild type and OSRK1 transgenic rice roots
As described, the salt-responsive proteome changes of OSRK1 transgenic rice roots were very tiny compared to that of wild type plants (Table 1). We therefore examined basal expression level of protein spots at unstressed conditions. Interestingly, most of the protein spots identified as early salt-responsive proteins from wild type rice roots were up or down-regulated in transgenic rice to the level of stressed conditions of WT rice even at unstressed condition (see protein ratio in Table 1). Among 52 salt-responsive proteins identified in wild type roots, 38 spots were up-regulated and 10 spots were down-regulated in transgenic rice roots at unstressed condition compared to wild type. Therefore, the protein levels of those spots in unstressed transgenic rice roots were similar to those in salt-treated wild type roots, and salt treatment could not make further differences in transgenic plants. This strongly suggests that salt stress responsive pathway is constitutively turned on by over-expression of OSRK1 in rice roots. In addition, the broad spectrum of functional categories of proteins affected by over-expression of OSRK1 indicates that OSRK1 may act as an upstream regulator of the salt stress response. These include enzymes involved in glycolysis, detoxification and branched amino acid catabolism, GA-responsive proteins, signaling and protein turn over.
In order to know if these protein changes are correlated with gene expression profiles, a comparative microarray analysis were conducted in wild type and transgenic rice roots at 0 h, 3 h or 7 h of salt treatment. NSF rice 45 K oligonucleotide chip was used in this experiment. The microarray analysis revealed 148 up-regulated and 75 down-regulated genes in the transgenic rice roots compared to wild type roots (data not shown). Transcript level of a few genes such as thioredoxin type H (Os07g018600) or DnaK-type molecular chaperone precursor (Os03g0113700) were significantly higher in OSRK1 transgenic rice roots. However, unexpectedly, expression patterns of most identified genes were not positively correlated with our proteome data (Additional file 1: Table S1). This implicates that many changes in proteomes of OSRK1 transgenic rice roots may be attributed to posttranscriptional regulations.
Recently, plant phosphoproteomes were extensively identified in different tissues and in response to various signals. Large-scale comparative phosphoproteomics identified thousands of phosphoproteins from rice and Arabidopsis [92]. It should be noted that many early salt-responsive proteins identified in our proteome analysis were previously known as phosphoproteins in rice. Fructose-bisphosphate aldolase (spot 61), proteasome subunit beta type 2 (spot 112), glutathione S-transferase (spot 112), triose phosphate isomerase (spot 116), ascorbate peroxidase (spot 116, 160, 310 and 351), salt-induced protein (spot 134, 214, and 227), glutamine synthetase (spot 200), methylmalonate-semialdehyde dehydrogenase (spot 259), dnaK-type molecular chaperone (spot 276 and 278) and glyceralde-3-phosphate dehydrogenase (315 and 318) were identical to previous reported phosphoproteoms from rice roots and shoots [36,92-94]. Glyoxalase I (spot 206) and glyceraldehyde 3-phosphate dehydrogenase (spot 315 and 318) were identified as GA-regulated phosphoproteins in rice leaf sheath [94]. Glutamine synthetase (spot 200), fructose bisphosphate aldolase (spot 61) and triosephosphate isomerase (spot 116) were ABA responsive phosphoptorteins in rice leaves [93]. Calreticulin (spot 225) was identified as a phosphoprotein in rice and tobacco cells and phosphorylated by CK2 or CDPK [86,87,95]. In addition to these rice phosphoptroteins, Shin et al. (2007) have identified phosphoproteins from transgenic Arabidopsis over-expressing SnRK2.8 [76]. Among these potential targets of SnRK2.8, ten proteins shared similarity to protein spots up or down-regulated in the OSRK1 transgenic rice roots at unstressed condition (Table 2). They were elongation factor Tu (spot 5), phosphoglycerate kinase (spot 67 and 385), enolase (spot 79), triose phosphate isomerase (spot 116), glutamine synthetase (spot 200), glyoxlase I (glyI, spot 206), glyoxalase II (spot 202 and 265), phosphoglyceromutase (spot 275, 277, 292 and 293), heat shock protein 70 (spot 276 and 278) and glyceraldehyde 3-phosphate dehydrogenase (spot 315 and 318). Taken together, these previous reports suggest that many proteins magnified in OSRK1 transgenic rice roots at unstressed condition are likely to be phosphoproteins.
Potential SnRK2 phosphorylation sites in the early salt-responsive proteomes
In order to know if these proteins were potential targets of OSRK1, presence of potential phosphorylation sites for SnRK2 kinase were investigated for 38 proteins 1.5 fold up or down regulated in the OSRK1 transgenic rice roots (Table 2). It has been known that plant SnRK2 kinase recognize RXXS/T motif found in ABF/AREB/ABI5 bZIP transcription factors [34,96]. Vlad et al. (2008) suggested LXRXX(S/T) as a recognition motif for Arabidopsis SnRK2.10 using semi-degenerate peptide array [97]. They further found strong phosphorylation motif preference of arginine at -3 position and leucine/isoleucine at -5 position. Table 2 showed that 30 proteins were found to have single or multiple RXX(S/T) motives. The five protein spots known as GA-responsive, i.e., fructose-bisphosphate aldolase (spot 61), inorganic pyrophosphatase (spot 82 and 227), 15 kD organ-specific salt-induced protein (spot 134, 214 and 227), calreticulin (spot 225) and methylmalonate-semialdehyde dehydrogenase (spot 259) contain potential SnRK2 phosphorylation sites. Among 30 proteins, 8 proteins contain (L/I)/XRXXS/T motif, which were isovaleryl-CoA dehydrogenase (spot 68), peroxidase (spot 200), glyoxalase I (spot 206), enoyl-ACP- reductase (spot 258), methylmalonate-semialdehyde dehydrogenase (spot 259), dnaK-type molecular chaperone (spot 276 and 278) and glutamate dehydrogenase (spot 314). It is noted that some of these proteins were found as multiple spots, supporting their post-translational modifications. They were inorganic pyrophosphatase (spot 82 and 227), 15 kDa organ-specific salt-induced protein (spot 134, 214 and 227), 2,3-bisphosphoglycerate-independent phosphoglycerate mutase (spot 275 and 277), dnaK-type molecular chaperone (spot 276 and 278), phosphoglyceromutase (spot 292 and 293) and glyceralde-3-phosphate dehydrogenase (spot 315 and 318). In addition, the potential SnRK2 recognition sites for fructose bisphosphate aldolase (spot 61), proteasome subunit beta type 2 (spot 112) and glyceraldehyde 3-phosphate dehydrogenase (spot 315 and 318) were identical to the phosphopeptides identified from large scale phosphoproteome study [92]. They are potential targets of OSRK1 that need to be further investigated.
Among these potential targets of OSRK1, phosphorylation of a calcium binding protein, calreticulin (spot 225) was further investigated, because the calreticulin gene (Os07g0246200) was isolated as an OSRK1-interacting clone from yeast two hybrid screening. After affinity purification of GST-fused recombinant protein in E. coli, two major bands were appeared (Figure 4) and the 31 kDa lower band (Calreticulin-S) was likely to be a degradation form of calreticulin. The potential SnRK2 phosphorylation site of calreticulin is located at the proximal N-terminus end. Indeed, our in vitro kinase assay data showed that OSRK1 could phosphorylate both bands, indicating that calreticulin is a good substrate for OSRK1 kinase (Figure 4).
Figure 4.

OSRK1 phosphorylates a rice calreticulin protein. In vitro phosphorylation of GST-OsCRT1 by GST-OSRK1 kinase was assayed in the presence MnCl2 at indicated concentrations.
Our current data implicate that OSRK1 is possibly involved in the diverse metabolic regulation and signaling pathway under salt stress condition by phosphorylation of multiple target proteins. As OSRK1 is involved in ABA signalling pathway, it is likely that these OSRK1-induced proteome/phosphoproteome changes is not only specific to salt stress response, but also involved in multiple stress responses as well. Diverse function of SnRK2 kinase in regulation of glycolysis, detoxification, GA signaling and cell elongation, protein turnover and amino acid catabolism under stress conditions remains to be elucidated.
Conclusions
Our current proteome data indicated that rice roots rapidly changed broad spectrum of energy metabolism upon challenging salt stress. Anaerobic metabolism is a key component of early salt response of rice roots and glycolysis may play central role. It is noted that several GA-responsive proteomes were clearly down-regulated in rice roots by salt stress. GA is a key regulator of root cell elongation and development of rice. Suppression of GA signaling by salt stress may responsible to the arrest of root growth and developmental under salt condition.
Plant SnRK2 kinase family is a core component of ABA signal transduction pathway and hyperosmotic stress responses. We pursued SnRK2 kinase function in salt response of rice roots by comparative proteomic analysis of OSRK1 transgenic rice. Our proteome data indicated that salt stress responsive diverse metabolic pathways were constitutively activated by over-expression of OSRK1 in rice roots. Most of these proteome changes were not correlated with transcriptional changes. Post-translational regulation, especially phosphorylation is expected to be involved. Many proteins differentially expressed in OSRK1 transgenic rice shared homology to the previously identified phosphoproteins, and contain consensus SnRK2 phosphorylation sites, (L/I)XRXXS/T. One of these potential targets, calreticulin was found to be a good substrate for OSRK1. These results provide new insight for further investigation of SnRK2 function in regulation of metabolism of rice roots under stress condition.
Methods
Rice transformation and growth
Rice (Oryza sativa cv Nagdong) callus were transformed with Agrobacterium fumefaciens LBA4404 carrying the pCAM35S-OSRK1 vector [34]. Transgenic callus were selected and shoots were regenerated in the presence of 30 μg•mL-1 hygromycin. The regenerated plants were transferred to soil and grown in a green house. Homozygous T2 lines were selected based on the hygromycin resistance. Insertion and expression of the transgene was verified by genomic PCR and RT-PCR analysis. For in gel kinase assay, proteome analysis and microarray analysis, 14-day-old seedlings of wild type and OSRK1 transgenic rice grown on 1/2 MS agar medium were transferred to a solution containing 150 mM NaCl. Roots were harvested, frozen in liquid nitrogen, and stored at -80°C until use. For primary root growth analysis, germinated seeds with equally growing primary roots were selected and arrayed on a sheet of pre-wet caligraphy paper. Then the sheets were rolled up and put in a 100 ml beaker containing water or NaCl solution and incubated at 28°C for five days.
Genomic PCR and RT-PCR analysis
Genomic DNAs and total RNAs were isolated from roots of 14-day-old wild type and transgenic rice seedlings with plant genomic DNA kit (Inclone biotech) or plant RNA kit (Qiagen). For confirmation of transgene insertion, genomic PCR was performed with primers specific to OSRK1 cDNA or hygromycin phosphotransferase (hpt) gene. For RT-PCR analysis of transgene expression, first cDNA was synthesized from 5 μg total RNA with oligo (dT) primer using Superscript II reverse transcriptase (Invitrogen). PCR was run for 30 cycles with primers specific to OSRK1 cDNA. As an internal control, transcript level of a rice actin gene (OSJNBa0078A17.12) was monitored. The PCR primers used in these experiments were OSRK1F (5'-atggagaagtacgagctgctc-3'), OSRK1R (5'-tcagctcttctgcaagtcac-3'), HPT5 (5'-agcctgacctattgcatctcc-3'), HPT3 (5'-tgtccgtcaggacattgttgg-3'), ACTIN5 (5'-atcaccattggtgctgag-3') and ACTIN3 (5'-tcctgtgcacaatggatgg-3').
In gel kinase assay
In-gel kinase assays were performed according to the modified protocol of Ichimura et al. [98]. Proteins were extracted from wild type and transgenic rice roots with extraction buffer (2 mM EDTA, 2 mM EGTA, 2 mM DTT, 25 mM NaF, 0.1 mM Na3VO4, 50 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail (Sigma-Aldrich), and 20 mM Tris-HCl, pH 7.5). Proteins (40 μg per lane) were separated on a 10% SDS-PAGE gel containing 0.25 mgmL-1 histone (Sigma H4524, Type III-SS) as a substrate. The gel was washed three times for 30 min with washing buffer (0.5 mM DTT, 5 mM NaF, 0.1 mM Na3VO4, 0.5 mgmL-1 BSA, 0.1% Triton X-100, and 25 mM Tris-HCl, pH 7.5). For protein renaturation, the gel was washed twice for 30 min with renaturation buffer (1 mM DTT, 5 mM NaF, 0.1 mM Na3VO4, and 25 mM Tris-HCl, pH 7.5) and further incubated for 16 h at 4°C. The gel was incubated in a reaction buffer (0.1 mM EGTA, 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, and 25 mM Tris-HCl, pH 7.5, 50 μCi of [γ-32P]ATP and 20 μM cold ATP) for 90 min at room temperature. The gel was washed with 5% TCA and 1% sodium pyrophosphate more than five times for 30 min each, incubated with 10% glycerol, dried and analyzed with a phosphoimage analyzer (Personal Molecular Imager FX system, Bio-Rad, USA).
Protein extraction and 2-DE analysis
The protein extraction procedure was based on those of Kamo et al. [99] with some modifications. Roots (1.5 g) were ground in liquid nitrogen and precipitated with 10% TCA in acetone with 0.07% mercaptoethanol at -20°C for 1 h, followed by centrifugation for 15 min at 10,000 g. The protein pellet was washed with ice-cold acetone containing 0.07% mercaptoethanol at least three times in order to remove contaminants, and lyophilized before 2-DE analysis.
For isoelectric focusing in the first dimension, dried protein samples (1.0 mg) were resolved in rehydration buffer (8 M urea, 2.0% CHAPS, 60 mM DTT, 0.5% IPG buffer) and loaded on immobilized linear gradient strips (pH 4-7, 18 cm). Focusing was performed using the following three steps: 500 V for 1 hr, 1000 V for 1 h, and 8000 V for 8 h. The gel strips were equilibrated for 20 min in equilibration buffer (50 mM Tris-HCl, pH 6.8, 6 M Urea, 30% glycerol, 2% SDS, 1% DTT, and 0.002% (w/v) bromophenol blue. The second dimension was run on a 12% polyacrylamide SDS gel using an Ethan Dalt electrophoresis kit (Amersham Biosciences, Sweden). Gels were stained with Coomassie brilliant blue (CBB).
Image analysis
CBB-stained gels were scanned using a PowerLook III image scanner (UMAX data system). Image treatment, spot detection, and protein quantification were carried out using Progenesis PG240 version 2006 software (Nonlinear dynamics, UK). Spot volumes were determined from at least three gels on which proteins were extracted in triplicate.
Proteolytic digestion
The stained protein spots excised from the gel were detained with 25 mM ammonium bicarbonate and 50% acetonitrile prior to digestion, and digested with trypsin (Promega, Madison, WI, USA). Gel pieces were swollen in digestion buffer containing 40 mM ammonium bicarbonate and 2 μg trypsin, and incubated at 37°C for 16 h. The peptides were recovered by stepwise extraction with 50 mM ammonium bicarbonate in 50% acetonitrile and 100% acetonitrile. The resulting peptide extracts were pooled and lyophilized in a vacuum centrifuge and stored at -20°C.
Protein identification by nano-LC-ESI-MS/MS and data analysis
All MS/MS experiments for peptide identification were performed using a nano LC/MS system consisting of an HPLC system (Thermo Scientific, CA, USA) and ESI-quadrupole ion trap MS (LCQ Deca XP-Plus, Thermo Scientific) equipped with a nano-ESI source. Ten μL of sample was loaded by the autosampler onto a C18 trap column (I.D. μm, length 5 mm, particle size 5 μm; LC Packings, Amsterdam, Netherlands) for desalting and concentration at a flow rate of 20 μL•min-1. The trapped peptides were then back-flushed and separated on a homemade microcapillary column (150 mm in length) packed with C18 resin (particle size 5 μm) in 75 μm silica tubing (8 μm id orifice). The mobile phases, A and B, were composed of 0% and 90% acetonitrile, respectively, each containing 0.02% formic acid and 0.5% acetic acid. The gradient began at 5% of mobile phase B for 15 min and was ramped to 20% for 3 min, 50% for 32 min, 60% for 5 min, 100% for 5 min and finally held at 100% B for 8 min. The column was equilibrated with 5% mobile phase B for 10 min before the next run. MS and MS/MS spectra were obtained at a heated capillary temperature of 220°C, an ESI voltage of 2.5 kV, and a collision energy setting of 35%. Data-dependent peak selection of the three most abundant MS ions from MS was used. Dynamic exclusion was enabled with a repeat count of 2, a repeat duration of 0.5 min, and 3 min exclusion duration. Mass spectrometer scan functions and HPLC solvent gradients were controlled by the Xcalibur data system (Thermo Scientific). MS/MS mass peak lists were analyzed for b and y ions using SEQUEST (version 3.3.1, Thermo Scientific) software. SEQUEST was used to match MS/MS spectra to peptides in rice database from the National Center for Biotechnology Information (NCBI: the entry number was 405933) in March 2010. Searches for peptide were first performed with following parameters: a mass tolerance of 2.0 Da on the parent ion and 1.0 Da on the MS/MS, one missed cleavage per peptide was allowed, and modifications of proteins were not taken into account. The validity of peptide/spectrum matches was hence assessed using the SEQUEST defined parameters, cross-correlation score (XCorr), and normalized difference in cross-correlation scores (ΔCn). Matched peptide had to pass the following filters for provisional identification: 1) ΔCn was at least 0.1 and 2) minimum XCorr of 1.9, 2.2, and 3.75 for charge states +1, +2, and +3, respectively. SEQUEST automatically saves search results. An SRF file including merging of proteins, filter and sort settings, ratios and protein area/height values was used to select and sort peptide/spectrum matches passing this set of criteria. Proteins were considered detected if they were identified by more than two peptides per spot.
Purification of GST-fusion protein in E. coli
The ORF of calreticulin gene (Os07g0246200) was PCR-amplified from pAD57, a yeast two hybrid interacting clone of OSRK1, and cloned in frame into pGEX 4T-1 (Amersham). E. coli cells (BL21 plysisS, Novagen) carrying the pGEX-fused gene construct were cultured at 37°C until the A600 reached 0.5. GST fusion proteins were induced by adding 0.1 mM isopropyl thio-β-D-galactoside (IPTG) and cultures were incubated for 4 h at 37°C. E. coli cells were harvested by centrifugation, resuspended in ice-cold TBS (Tris, NaCl) buffer, and lysed by freeze-thaw method. The cell lysates were centrifuged at 20,000 g for 30 min at 4°C and the supernatant was applied to glutathione-Agarose (Peptron, Korea) column. After washing the column with TBS buffer, GST-fusion proteins were eluted with 5 mM glutathione in TBS buffer and used for the kinase assay.
In vitro kinase assay
In vitro kinase assay with OSRK1 protein was performed according to Chae et al. (2007). Briefly, aliquots of GST-fused OSRK1 kinase and calreticulin were incubated in a kinase assay buffer (10 μCi γ-32P-ATP, 20 mM Tris-HCl pH 7.0, MnCl2) for 30 min at 30°C. The reaction was stopped by adding 5X SDS sample buffer, boiled immediately for 5 min, and products were analyzed by SDS-PAGE. Gels were stained with Coomasie Brilliant Blue R-250, dried, and analyzed with a phosphoimage analyzer (Personal Molecular Imager FX system, Bio-Rad, USA).
Microarray analysis
Total RNAs from rice roots were isolated with plant RNA kit (Qiagen). RNA length distribution and integrity were assessed by capillary electrophoresis with fluorescence detection (Agilent Bioanalyzer 2100) using the Agilent Total RNA Nano chip assay for presence of 28S and 18S rRNA bands. Fluorescent-labeled cRNA for Oligo micorarray analysis was prepared by amplification of total RNA in the presence of aminoallyl-UTP followed by the coupling of Cy3 or Cy5 dye (AmershamPharmacia, Uppsala, Sweden). NSF 45 K Oligo Microarray kit was hybridized with the fluorescently labeled cRNA at 42°C for 16 h and then washed. DNA chips were scanned using GenePix 4000B (Axon Instruments, Union City, CA). Scanned images were analyzed with GenePix Pro 3.0 software (Axon Instruments, Union City, CA) to obtain gene expression ratios. Transformed data were normalized using the Lowess procedure.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MHN performed proteomic analysis including its design, coordination, analysis of the data, and drafted the manuscript. KMK performed 2-DE and gel image analysis. JBS and KC conceived the LC-MS analysis and analysed the proteome data. DYK and BGK performed rice transformation. SMH and WJP performed transgenic rice analysis and in gel kinase assay. ISY conceived of overall experimental design and manuscript preparation. All authors read and approved the final manuscript.
Supplementary Material
Table S1. Fold changes of transcript level of genes correspond to protein spots showing more than 1.5 fold change in OSRK1 transgenic rice roots at unstressed condition.
Contributor Information
Myung Hee Nam, Email: nammh@kbsi.re.kr.
Sun Mi Huh, Email: sunmihuh@gmail.com.
Kyung Mi Kim, Email: viridian67@hanmail.net.
Woong June Park, Email: parkwj@dku.edu.
Jong Bok Seo, Email: sjb@kbsi.re.kr.
Kun Cho, Email: chokun@kbsi.re.kr.
Dool Yi Kim, Email: dykim22@korea.kr.
Beom Gi Kim, Email: bgkimpeace@korea.kr.
In Sun Yoon, Email: isyoon@korea.kr.
Acknowledgements
This work was supported by grants from the Next-Generation BioGreen 21 program (SSAC, PJ00817305 to ISY) and the National Academy of Agricultural Science (PJ906968 to ISY) of the Rural Development Administration, Republic of Korea.
References
- Zhu JK. Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol. 2001;4(5):401–406. doi: 10.1016/S1369-5266(00)00192-8. [DOI] [PubMed] [Google Scholar]
- Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6(2):66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Rus AM, Bressan RA, Hasegawa PM. Unraveling salt tolerance in crops. Nat Genet. 2005;37(10):1029–1030. doi: 10.1038/ng1005-1029. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Yang B, Harris NS, Deyholos MK. Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. J Exp Bot. 2007;58(13):3591–3607. doi: 10.1093/jxb/erm207. [DOI] [PubMed] [Google Scholar]
- Walia H, Wilson C, Ismail AM, Close TJ, Cui X. Comparing genomic expression patterns across plant species reveals highly diverged transcriptional dynamics in response to salt stress. BMC Genomics. 2009;10:398. doi: 10.1186/1471-2164-10-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Kathiresan A, Bennett J, Takabe T. Comparative transcriptome analyses of barley and rice under salt stress. Theor Appl Genet. 2006;112(7):1286–1294. doi: 10.1007/s00122-006-0231-4. [DOI] [PubMed] [Google Scholar]
- Yan S, Tang Z, Su W, Sun W. Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics. 2005;5(1):235–244. doi: 10.1002/pmic.200400853. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Qi Y, Zhu Q, Chen X, Wang N, Zhao X, Chen H, Cui X, Xu L, Zhang W. New changes in the plasma-membrane-associated proteome of rice roots under salt stress. Proteomics. 2009;9(11):3100–3114. doi: 10.1002/pmic.200800340. [DOI] [PubMed] [Google Scholar]
- Mazzucotelli E, Mastrangelo AM, Crosatti C, Guerra D, Stanca AM, Cattivelli L. Abiotic stress response in plants: when post-transcriptional and post-translational regulations control transcription. Plant Sci. 2008;174:420–431. doi: 10.1016/j.plantsci.2008.02.005. [DOI] [Google Scholar]
- Asano T, Hakata M, Nakamura H, Aoki N, Komatsu S, Ichikawa H, Hirochika H, Ohsugi R. Functional characterisation of OsCPK21, a calcium-dependent protein kinase that confers salt tolerance in rice. Plant Mol Biol. 2010;75(1-2):179–191. doi: 10.1007/s11103-010-9717-1. [DOI] [PubMed] [Google Scholar]
- Xiong L, Yang Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell. 2003;15(3):745–759. doi: 10.1105/tpc.008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang SQ, Liu YF, Liu P, Lei G, He SJ, Ma B, Zhang WK, Zhang JS, Chen SY. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J. 2010;62(2):316–329. doi: 10.1111/j.1365-313X.2010.04146.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007;143(2):1001–1012. doi: 10.1104/pp.106.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T. Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell. 2004;16(5):1163–1177. doi: 10.1105/tpc.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knetsch M, Wang M, Snaar-Jagalska BE, Heimovaara-Dijkstra S. Abscisic acid induces mitogen-activated protein Kinase activation in Barley aleurone protoplasts. Plant Cell. 1996;8(6):1061–1067. doi: 10.1105/tpc.8.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Assmann SM. An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell. 1996;8(12):2359–2368. doi: 10.1105/tpc.8.12.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Ca2 + -dependent protein kinases and stress signal transduction in plants. Science. 1996;274(5294):1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002;43(12):1473–1483. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- Jossier M, Bouly JP, Meimoun P, Arjmand A, Lessard P, Hawley S, Grahame Hardie D, Thomas M. SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J. 2009;59(2):316–328. doi: 10.1111/j.1365-313X.2009.03871.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Ishitani M, Halfter U, Kim CS, Zhu JK. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA. 2000;97(7):3730–3734. doi: 10.1073/pnas.060034197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Huang Y, Xiong L. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol. 2007;144(3):1416–1428. doi: 10.1104/pp.107.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HW, Kim MC, Shin PG, Kim JS, Kim CY, Lee SY, Hwang I, Bahk JD, Hong JC, Han C. et al. Differential expression of two functional serine/threonine protein kinases from soybean that have an unusual acidic domain at the carboxy terminus. Mol Gen Genet. 1997;255(4):359–371. doi: 10.1007/s004380050507. [DOI] [PubMed] [Google Scholar]
- Hoyos ME, Zhang S. Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol. 2000;122(4):1355–1363. doi: 10.1104/pp.122.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell. 2000;12(1):165–178. [PMC free article] [PubMed] [Google Scholar]
- Monks DE, Aghoram K, Courtney PD, DeWald DB, Dewey RE. Hyperosmotic stress induces the rapid phosphorylation of a soybean phosphatidylinositol transfer protein homolog through activation of the protein kinases SPK1 and SPK2. Plant Cell. 2001;13(5):1205–1219. doi: 10.1105/tpc.13.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Barbier-Brygoo H, Lauriere C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem. 2004;279(40):41758–41766. doi: 10.1074/jbc.M405259200. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Nakashima K, Yoshida T, Katagiri T, Kidokoro S, Kanamori N, Umezawa T, Fujita M, Maruyama K, Ishiyama K. et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 2009;50(12):2123–2132. doi: 10.1093/pcp/pcp147. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Fujita Y, Kanamori N, Katagiri T, Umezawa T, Kidokoro S, Maruyama K, Yoshida T, Ishiyama K, Kobayashi M. et al. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 2009;50(7):1345–1363. doi: 10.1093/pcp/pcp083. [DOI] [PubMed] [Google Scholar]
- Fujii H, Zhu JK. An autophosphorylation site of the protein kinase SOS2 is important for salt tolerance in Arabidopsis. Mol Plant. 2009;2(1):183–190. doi: 10.1093/mp/ssn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA. 2009;106(50):21419–21424. doi: 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462(7273):660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedhiou CJ, Popova OV, Dietz KJ, Golldack D. The SNF1-type serine-threonine protein kinase SAPK4 regulates stress-responsive gene expression in rice. BMC Plant Biol. 2008;8:49. doi: 10.1186/1471-2229-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae MJ, Lee JS, Nam MH, Cho K, Hong JY, Yi SA, Suh SC, Yoon IS. A rice dehydration-inducible SNF1-related protein kinase 2 phosphorylates an abscisic acid responsive element-binding factor and associates with ABA signaling. Plant Mol Biol. 2007;63(2):151–169. doi: 10.1007/s11103-006-9079-x. [DOI] [PubMed] [Google Scholar]
- Chen C, Plant A. Salt-induced protein synthesis in tomato roots: the role of ABA. J Exp Bot. 1999;50:677–687. doi: 10.1093/jexbot/50.334.677. [DOI] [Google Scholar]
- Chitteti BR, Peng Z. Proteome and phosphoproteome differential expression under salinity stress in rice (Oryza sativa) roots. J Proteome Res. 2007;6(5):1718–1727. doi: 10.1021/pr060678z. [DOI] [PubMed] [Google Scholar]
- Ndimba BK, Chivasa S, Simon WJ, Slabas AR. Identification of Arabidopsis salt and osmotic stress responsive proteins using two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics. 2005;5(16):4185–4196. doi: 10.1002/pmic.200401282. [DOI] [PubMed] [Google Scholar]
- Komatsu S, Konishi H. Proteome analysis of rice root proteins regulated by gibberellin. Genomics Proteomics Bioinformatics. 2005;3(3):132–142. doi: 10.1016/S1672-0229(05)03019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Zang X, Tanaka N. Comparison of two proteomics techniques used to identify proteins regulated by gibberellin in rice. J Proteome Res. 2006;5(2):270–276. doi: 10.1021/pr0502929. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Takahashi H, Kitano H, Matsuoka M, Akao S, Uchimiya H, Komatsu S. Proteome approach to characterize the methylmalonate-semialdehyde dehydrogenase that is regulated by gibberellin. J Proteome Res. 2005;4(5):1575–1582. doi: 10.1021/pr050114f. [DOI] [PubMed] [Google Scholar]
- Rakwal R, Komatsu S. Abscisic acid promoted changes in the protein profiles of rice seedling by proteome analysis. Mol Biol Rep. 2004;31(4):217–230. doi: 10.1007/s11033-005-2710-0. [DOI] [PubMed] [Google Scholar]
- Alvarez S, Hicks LM, Pandey S. ABA-dependent and -independent G-protein signaling in Arabidopsis roots revealed through an iTRAQ proteomics approach. J Proteome Res. 2011;10(7):3107–3122. doi: 10.1021/pr2001786. [DOI] [PubMed] [Google Scholar]
- Li XJ, Yang MF, Chen H, Qu LQ, Chen F, Shen SH. Abscisic acid pretreatment enhances salt tolerance of rice seedlings: proteomic evidence. Biochim Biophys Acta. 2010;1804(4):929–940. doi: 10.1016/j.bbapap.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Serraj R, Roy G, Drevon JJ. Salt stress induces a decrease in the oxygen uptake of soybean nodules and in their permeability to oxygen diffusion. Physiol Plant. 1994;91:161–168. doi: 10.1111/j.1399-3054.1994.tb00414.x. [DOI] [Google Scholar]
- Minhas D, Grover A. Transcript levels of genes encoding various glycolytic and fermentation enzymes change in response to abiotic stresses. Plant Sci. 1999;146:41–51. doi: 10.1016/S0168-9452(99)00092-8. [DOI] [Google Scholar]
- Umeda M, Uchimiya H. Differential transcript levels of genes associated with glycolysis and alcohol fermentation in rice plants (Oryza sativa L.) under submergence stress. Plant Physiol. 1994;106(3):1015–1022. doi: 10.1104/pp.106.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkes S, Sonnewald U, Badur R, Flachmann R, Stitt M. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell. 2001;13(3):535–551. doi: 10.1105/tpc.13.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourton-Cabassa C, Ambard-Bretteville F, Moreau F, De Davy Virville J, Remy R, Francs-Small CC. Stress induction of mitochondrial formate dehydrogenase in potato leaves. Plant Physiol. 1998;116(2):627–635. doi: 10.1104/pp.116.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton WC. The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9(10):490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan H, Sivakumar1 P, Chakrabarty R, Thomas G. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativaL.)--differential response in salt-tolerant and sensitive varieties. Plant Sci. 2003;165:1411–1418. doi: 10.1016/j.plantsci.2003.08.005. [DOI] [Google Scholar]
- Tsai YC, Hong CY, Liu LF, Kao CH. Expression of ascorbate peroxidase and glutathione reductase in roots of rice seedlings in response to NaCl and H2O2. J Plant Physiol. 2005;162(3):291–299. doi: 10.1016/j.jplph.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Hong CY, Chao YY, Yang MY, Cheng SY, Cho CC, Kao CH. NaCl-induced expression of glutathione reductase in roots of rice (Oryza sativa L.) seedlings is mediated through hydrogen peroxide but not abscisic acid. Plant Soil. 2009;320:103–115. doi: 10.1007/s11104-008-9874-z. [DOI] [Google Scholar]
- Aghaei K, Ehsanpour AA, Komatsu S. Potato responds to salt stress by increased activity of antioxidant enzymes. J Integr Plant Biol. 2009;51(12):1095–1103. doi: 10.1111/j.1744-7909.2009.00886.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Deyholos MK. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006;6:25. doi: 10.1186/1471-2229-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xu XN, Zhu HS, Liu AH, Liu L, Li JM, Hua XJ. Comparative transcriptomic profiling of a salt-tolerant wild tomato species and a salt-sensitive tomato cultivar. Plant Cell Physiol. 2010;51(6):997–1006. doi: 10.1093/pcp/pcq056. [DOI] [PubMed] [Google Scholar]
- Espartero J, Sanchez-Aguayo I, Pardo JM. Molecular characterization of glyoxalase-I from a higher plant; upregulation by stress. Plant Mol Biol. 1995;29(6):1223–1233. doi: 10.1007/BF00020464. [DOI] [PubMed] [Google Scholar]
- Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK. Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res. 2008;17(2):171–180. doi: 10.1007/s11248-007-9082-2. [DOI] [PubMed] [Google Scholar]
- El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma. 2010;245(1-4):85–96. doi: 10.1007/s00709-010-0144-6. [DOI] [PubMed] [Google Scholar]
- Gelhaye E, Rouhier N, Jacquot JP. The thioredoxin h system of higher plants. Plant Physiol Biochem. 2004;42(4):265–271. doi: 10.1016/j.plaphy.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Serrato AJ, Cejudo FJ. Type-h thioredoxins accumulate in the nucleus of developing wheat seed tissues suffering oxidative stress. Planta. 2003;217(3):392–399. doi: 10.1007/s00425-003-1009-4. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tian LH, Zhao JF, Song Y, Zhang CJ, Guo Y. Identification of an apoplastic protein involved in the initial phase of salt stress response in rice root by two-dimensional electrophoresis. Plant Physiol. 2009;149(2):916–928. doi: 10.1104/pp.108.131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Rzhetsky A, Hsu LC, Chang C. Human aldehyde dehydrogenase gene family. Eur J Biochem. 1998;251(3):549–557. doi: 10.1046/j.1432-1327.1998.2510549.x. [DOI] [PubMed] [Google Scholar]
- Kotchoni SO, Bartels D. Water stress induces the up-regulation of specific set of genes in plant: aldehy dehydrogenases as an example. BULG J Plant Physiol. 2003;special issue:37–51. [Google Scholar]
- Bartels D. Targeting detoxification pathways: an efficient approach to obtain plants with multiple stress tolerance? Trends Plant Sci. 2001;6(7):284–286. doi: 10.1016/S1360-1385(01)01983-5. [DOI] [PubMed] [Google Scholar]
- Zhou W, Sun Q-J, Zhang C-F, Yong-Ze Yuan, Zhang J, Lu B-B. Effect of salt stress on ammonium assimilation enzymes of the roots of rice (Oryza sativa) cultivars differing in salinity resistance. Acta Botanica Sinica. 2004;46(8):921–927. [Google Scholar]
- Skopelitis DS, Paranychianakis NV, Paschalidis KA, Pliakonis ED, Delis ID, Yakoumakis DI, Kouvarakis A, Papadakis AK, Stephanou EG, Roubelakis-Angelakis KA. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell. 2006;18(10):2767–2781. doi: 10.1105/tpc.105.038323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Yuan YZ, Ou JQ, Lin QH, Zhang CF. Glutamine synthetase and glutamate dehydrogenase contribute differentially to proline accumulation in leaves of wheat (Triticum aestivum) seedlings exposed to different salinity. J Plant Physiol. 2007;164(6):695–701. doi: 10.1016/j.jplph.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Lutts S, Majerus V, Kinet J-M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol Plant. 1999;05(3):450–458. [Google Scholar]
- Viola RE. The central enzymes of the aspartate family of amino acid biosynthesis. Acc Chem Res. 2001;34(5):339–349. doi: 10.1021/ar000057q. [DOI] [PubMed] [Google Scholar]
- Miesak BH, Coruzzi GM. Molecular and physiological analysis of Arabidopsis mutants defective in cytosolic or chloroplastic aspartate aminotransferase. Plant Physiol. 2002;129(2):650–660. doi: 10.1104/pp.005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Nitschke SE, Couee I, Vermel M, Grienenberger JM, Gualberto JM. Purification, characterization and cloning of isovaleryl-CoA dehydrogenase from higher plant mitochondria. Eur J Biochem. 2001;268(5):1332–1339. doi: 10.1046/j.1432-1327.2001.01999.x. [DOI] [PubMed] [Google Scholar]
- Daschner K, Couee I, Binder S. The mitochondrial isovaleryl-coenzyme a dehydrogenase of arabidopsis oxidizes intermediates of leucine and valine catabolism. Plant Physiol. 2001;126(2):601–612. doi: 10.1104/pp.126.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Sauve R, Thannhauser TW. Proteome changes induced by aluminium stress in tomato roots. J Exp Bot. 2009;60(6):1849–1857. doi: 10.1093/jxb/erp065. [DOI] [PubMed] [Google Scholar]
- Konishi H, Maeshima M, Komatsu S. Characterization of vacuolar membrane proteins changed in rice root treated with gibberellin. J Proteome Res. 2005;4(5):1775–1780. doi: 10.1021/pr050079c. [DOI] [PubMed] [Google Scholar]
- Shin R, Alvarez S, Burch AY, Jez JM, Schachtman DP. Phosphoproteomic identification of targets of the Arabidopsis sucrose nonfermenting-like kinase SnRK2.8 reveals a connection to metabolic processes. Proc Natl Acad Sci USA. 2007;104(15):6460–6465. doi: 10.1073/pnas.0610208104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv SL, Lian LJ, Tao PL, Li ZX, Zhang KW, Zhang JR. Overexpression of Thellungiella halophila H+-PPase (TsVP) in cotton enhances drought stress resistance of plants. Planta. 2009;229(4):899–910. doi: 10.1007/s00425-008-0880-4. [DOI] [PubMed] [Google Scholar]
- Li Z, Baldwin CM, Hu Q, Liu H, Luo H. Heterologous expression of Arabidopsis H + -pyrophosphatase enhances salt tolerance in transgenic creeping bentgrass (Agrostis stolonifera L.) Plant Cell Environ. 2010;33(2):272–289. doi: 10.1111/j.1365-3040.2009.02080.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yang X, Fu Y, Zhang Y, Yan J, Song T, Rocheford T, Li J. Proteomic analysis of early germs with high-oil and normal inbred lines in maize. Mol Biol Rep. 2009;36(4):813–821. doi: 10.1007/s11033-008-9250-3. [DOI] [PubMed] [Google Scholar]
- Mou Z, He Y, Dai Y, Liu X, Li J. Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell. 2000;12(3):405–418. doi: 10.1105/tpc.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers ML, Denton MC, McDermott TR. Genes coding for phosphotransacetylase and acetate kinase in Sinorhizobium meliloti are in an operon that is inducible by phosphate stress and controlled by phoB. J Bacteriol. 1999;181(7):2217–2224. doi: 10.1128/jb.181.7.2217-2224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XY, He LH, Jing RL, Li RZ. Calreticulin: conserved protein and diverse functions in plants. Physiol Plant. 2009;136(2):127–138. doi: 10.1111/j.1399-3054.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- Aghaei K, Ehsanpour AA, Komatsu S. Proteome analysis of potato under salt stress. J Proteome Res. 2008;7(11):4858–4868. doi: 10.1021/pr800460y. [DOI] [PubMed] [Google Scholar]
- Shen S, Sharma A, Komatsu S. Characterization of proteins responsive to gibberellin in the leaf-sheath of rice (Oryza sativa L.) seedling using proteome analysis. Biol Pharm Bull. 2003;26(2):129–136. doi: 10.1248/bpb.26.129. [DOI] [PubMed] [Google Scholar]
- Shen S, Jing Y, Kuang T. Proteomics approach to identify wound-response related proteins from rice leaf sheath. Proteomics. 2003;3(4):527–535. doi: 10.1002/pmic.200390066. [DOI] [PubMed] [Google Scholar]
- Baldan B, Navazio L, Friso A, Mariani P, Meggio F. Plant calreticulin is specifically and efficiently phosphorylated by protein kinase CK2. Biochem Biophys Res Commun. 1996;221(3):498–502. doi: 10.1006/bbrc.1996.0625. [DOI] [PubMed] [Google Scholar]
- Li Z, Onodera H, Ugaki M, Tanaka H, Komatsu S. Characterization of calreticulin as a phosphoprotein interacting with cold-induced protein kinase in rice. Biol Pharm Bull. 2003;26(2):256–261. doi: 10.1248/bpb.26.256. [DOI] [PubMed] [Google Scholar]
- Kline KG, Barrett-Wilt GA, Sussman MR. In planta changes in protein phosphorylation induced by the plant hormone abscisic acid. Proc Natl Acad Sci USA. 2010;107(36):15986–15991. doi: 10.1073/pnas.1007879107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XY, Xu CY, Jing RL, Li RZ, Mao XG, Wang JP, Chang XP. Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought-stressed responses. J Exp Bot. 2008;59(4):739–751. doi: 10.1093/jxb/erm369. [DOI] [PubMed] [Google Scholar]
- Sahi C, Singh A, Blumwald E, Grover A. Beyond osmolytes and transporters: novel plant salt-stress tolerance-related genes from transcriptional profiling data. Physiol Plant. 2006;127:1–9. doi: 10.1111/j.1399-3054.2005.00610.x. [DOI] [Google Scholar]
- Ma F, Liu Z, Wang TW, Hopkins MT, Peterson CA, Thompson JE. Arabidopsis eIF5A3 influences growth and the response to osmotic and nutrient stress. Plant Cell Environ. 2010;33(10):1682–96. doi: 10.1111/j.1365-3040.2010.02173.x. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Sugiyama N, Mochida K, Daudi A, Yoshida Y, Toyoda T, Tomita M, Ishihama Y, Shirasu K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010;153(3):1161–1174. doi: 10.1104/pp.110.157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Li J. Proteomic analysis of phosphoproteins regulated by abscisic acid in rice leaves. Biochem Biophys Res Commun. 2008;371(4):883–888. doi: 10.1016/j.bbrc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Khan MM, Jan A, Karibe H, Komatsu S. Identification of phosphoproteins regulated by gibberellin in rice leaf sheath. Plant Mol Biol. 2005;58(1):27–40. doi: 10.1007/s11103-005-4013-1. [DOI] [PubMed] [Google Scholar]
- Droillard MJ, Guclu J, Le Caer JP, Mathieu Y, Guern J, Lauriere C. Identification of calreticulin-like protein as one of the phosphoproteins modulated in response to oligogalacturonides in tobacco cells. Planta. 1997;202(3):341–348. doi: 10.1007/s004250050136. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Murata M, Minami H, Yamamoto S, Kagaya Y, Hobo T, Yamamoto A, Hattori T. Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 2005;44(6):939–949. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- Vlad F, Turk BE, Peynot P, Leung J, Merlot S. A versatile strategy to define the phosphorylation preferences of plant protein kinases and screen for putative substrates. Plant J. 2008;55(1):104–117. doi: 10.1111/j.1365-313X.2008.03488.x. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24(5):655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- Kamo M, Kawakami T, Miyatake N, Tsugita A. Separation and characterization of Arabidopsis thaliana proteins by two-dimensional gel electrophoresis. Electrophoresis. 1995;16(3):423–430. doi: 10.1002/elps.1150160169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Fold changes of transcript level of genes correspond to protein spots showing more than 1.5 fold change in OSRK1 transgenic rice roots at unstressed condition.