ABSTRACT
Prenatal testosterone exposure impacts postnatal reproductive and endocrine function, leading to alterations in sex steroid levels. Because gonadal steroids are key regulators of cardiovascular function, it is possible that alteration in sex steroid hormones may contribute to development of hypertension in prenatally testosterone-exposed adults. The objectives of this study were to evaluate whether prenatal testosterone exposure leads to development of hypertension in adult males and females and to assess the influence of gonadal hormones on arterial pressure in these animals. Offspring of pregnant rats treated with testosterone propionate or its vehicle (controls) were examined. Subsets of male and female offspring were gonadectomized at 7 wk of age, and some offspring from age 7 to 24 wk received hormone replacement, while others did not. Testosterone exposure during prenatal life significantly increased arterial pressure in both male and female adult offspring; however, the effect was greater in males. Prenatal androgen-exposed males and females had more circulating testosterone during adult life, with no change in estradiol levels. Gonadectomy prevented hyperandrogenism and also reversed hypertension in these rats. Testosterone replacement in orchiectomized males restored hypertension, while estradiol replacement in ovariectomized females was without effect. Steroidal changes were associated with defective expression of gonadal steroidogenic genes, with Star, Sf1, and Hsd17b1 upregulation in testes. In ovaries, Star and Cyp11a1 genes were upregulated, while Cyp19 was downregulated. This study showed that prenatal testosterone exposure led to development of gonad-dependent hypertension during adult life. Defective steroidogenesis may contribute in part to the observed steroidal changes.
Keywords: gonadectomy, hypertension, prenatal testosterone, sex steroids, steroidogenesis
Elevated maternal testosterone leads to hypertension that is associated with hyperandrogenism and defective gonadal steroidogenesis in adult offspring; peripubertal gonadectomy prevents hyperandrogenism and reverses hypertension in these animals.
INTRODUCTION
Exposure to excess androgen during fetal life causes intrauterine growth retardation and low birth weight [1–3] and disrupts several metabolic, reproductive, and cardiovascular parameters in adult life [3–6]. Exposure to excess androgen can occur in fetuses of pregnant women with pre-eclampsia or polycystic ovary syndrome (PCOS) who show elevated levels of plasma testosterone during the last third of pregnancy [7–11]. Studies of endocrine disruptors have shown the existence of environmental substances with androgenic actions, such as 17-β-trenbolone and triclocarban, which are a potential cause of abnormal fetal outcomes [12–14]. Alternatively, it has been proposed that the placenta [15] or the fetal gonads [16] can by themselves be a source of androgen due to abnormalities in genes controlling steroidogenesis. Because hormonal perturbations during this critical developmental window may have adverse effects that persist into adulthood, it is important to study the consequences of androgen exposure in utero.
Experimental studies show that prenatal androgen exposure leads to development of adult life cardiovascular dysfunctions, as shown by enlargement of the left cardiac ventricle and kidneys [17, 18], and a moderate increase in blood pressure [3, 4]. Moreover, epidemiological studies show that offspring born to PCOS mothers or offspring of pre-eclampsia pregnancies are more likely to have increased blood pressure during postnatal life [19–22]. We have recently shown that hypertension in adult female offspring exposed prenatally to testosterone is associated with reduced endothelium-dependent vascular relaxation involving decreased endothelial nitric oxide synthase (eNOS) expression and reduced nitric oxide production from endothelial cells [3]. The cardiovascular consequences of prenatal testosterone exposure in adult males have not been studied.
In addition to cardiovascular dysfunctions, prenatal androgen exposure has been shown to alter sex steroid hormone production during postnatal life. Prenatal exposure of a female fetus to androgens results in the development of features characteristic of PCOS in nonhuman primates, rats, and sheep [5, 23, 24]. The females exposed to prenatal testosterone have disruptions in the regulation of the hypothalamic-pituitary-gonadal (HPG) axis with desensitization of gonadotropin-releasing hormone (GnRH) neurons to steroid feedback, elevated luteinizing hormone (LH) levels [25–28], and irregular reproductive cycles [29, 30]. Male fetuses prenatally exposed to androgens also have altered gonadal responsiveness to GnRH [31], increased volume of sexually dimorphic nuclei in the brain [32], and increased expression of follicle-stimulating hormone receptor (FSHR) in testes [6]. Both males and females exposed to testosterone during fetal life tend to produce increased testosterone during adult life, with little or no change in estradiol levels [30, 31, 33, 34].
Studies of spontaneous and secondary types of hypertension have provided abundant evidence that manipulation of sex steroid hormones through gonadectomy alters the course of hypertension [35–39]. Sex differences in blood pressure have been attributed to protection of the female by estrogens [40] or the prohypertensive activities of testosterone [40, 41]. Ovariectomy led to hypertension in aging female Dahl salt-sensitive and female mRen2.Lewis rat models, in which intact female rats are normotensive while their male counterparts are hypertensive [42, 43]. Castration of male spontaneously hypertensive rats (SHR) or Dahl salt-sensitive rats at a young age (3–5 wk) delayed development of hypertension, and testosterone treatment of castrated males exacerbated their hypertension [35, 44, 45]. These studies suggest that sex steroids in both males and females may contribute to the development and progression of hypertension.
Because gonadal steroids are key regulators of cardiovascular function, we hypothesized that prenatal testosterone exposure leads to defective gonadal steroidogenesis, generating alterations in plasma steroid hormone levels during adult life, contributing to development of hypertension. Thus, the objectives of this study were to evaluate whether prenatal testosterone exposure leads to development of hypertension in adult males and females and to assess the influence of gonadal hormones on arterial pressure in these animals. To mimic the gestational excess androgen associated with PCOS, pre-eclampsia, and other inappropriate androgen exposure, our laboratory developed a rat model treated with testosterone propionate late in gestation [1, 3]. Previously, we described cardiovascular abnormalities in the female offspring, using this model [3].
MATERIALS AND METHODS
Animals
All experimental procedures were carried out in accordance with National Institutes of Health guidelines (NIH publication no. 85–23 [revised 1996]) with approval by the Animal Care and Use Committee at the University of Texas Medical Branch. Rats were housed in a temperature-controlled room (23°C), with a 12L:12D cycle, and with food and water available ad libitum. Timed pregnant Sprague-Dawley rats were purchased from Harlan Laboratories, Inc. (Houston, TX). At Day 14 of gestation, rats were divided into two groups. Group I received daily injections of testosterone propionate (TP) subcutaneously from Gestational Days 15 to 19 with 0.5 mg/kg/day (n = 8), and Group II received only vehicle (sesame oil; n = 8). All dams in both groups were allowed to deliver at term, and birth weights of pups were recorded within 12 h of birth. At that time, the number of pups in the control and TP litters was adjusted to 10 pups per dam to ensure equal nutrient access for all offspring (pups with weights at each extreme were euthanized). The male:female pup ratio remained equivalent after culling, when possible. Pups were weaned at 3 wk of age. At 7 wk of age, control and TP rats underwent sham surgery or were gonadectomized or received replacement therapy with estradiol benzoate, TP, or sesame oil vehicle up to 24 wk of age, as described below. Changes in arterial pressure were measured in conscious, free-moving rats at 24 wk of age. Animals were euthanized by CO2 inhalation, and blood was collected by cardiac puncture to determine plasma estradiol and testosterone levels. Testes and ovaries were collected and snap frozen in liquid nitrogen and stored at −80°C for assessment of genes controlling steroidogenesis. Measurements of all experimental end points were performed when rats reached 24 wk of age. Care was taken to use only one male and female per litter for any parameter.
Ovariectomy and Estrogen Supplementation
Females of both control and TP litters underwent ovariectomy as described previously at 7 wk of age [46]. In those rats receiving estradiol supplementation, 17-β-estradiol (5 μg/kg in 100 μl of sesame oil [Sigma, St. Louis, MO]) was injected subcutaneously once every 4 days immediately after ovariectomy and up to 24 wk of age. Rats have an estrous cycle of 4 days; hence, this regimen of estradiol was chosen to closely mimic physiological changes in 17-β-estradiol levels across the rat estrous cycle as described previously [46].
Orchiectomy and Testosterone Supplementation
Males of both control and TP litters underwent bilateral orchiectomy as described previously [47] at 7 wk of age. In those rats receiving testosterone supplementation, TP (2 mg in 100 μl of sesame oil [Sigma]) was injected subcutaneously once daily immediately after orchiectomy and up to 24 wk of age.
Mean Arterial Pressure
Rats under anesthesia (ketamine, 45 mg/kg; xylazine, 5 mg/kg [Burns Veterinary Supply, Westbury, NY]) received surgically implanted flexible catheters (polyethylene tubing PE-50; Becton Dickinson, Sparks, MD) in the left carotid artery for measurement of arterial pressure as previously described [46]. Catheters were tunneled to the nape of the neck and exteriorized. Arterial pressure measurements were performed with rats in the conscious state after a 24-h recovery phase via connection of the arterial catheter to a pressure transducer and a data acquisition system (product no. DBP001; direct blood pressure system and Workbench for Windows software; both from Kent Scientific, Litchfield, CT). Arterial pressure was monitored continuously for 30 min to determine baseline values.
Plasma Estradiol and Testosterone Levels
Blood samples were collected between 900 h and 1000 h from all rats. In rats with intact ovaries, blood samples were collected at diestrus, as determined by vaginal cytology. In estradiol-supplemented rats, blood was collected 48 h after supplementation to mimic diestrus levels. In TP-supplemented rats, blood was collected 2 h after supplementation. Plasma was separated by centrifugation and stored at −20°C until the time of measurement. Estradiol and testosterone levels in the samples were measured using Ultra Sensitive estradiol RIA (Diagnostic Systems Laboratories, Inc., Webster, TX) and a testosterone ELISA kit (Enzo Life Sciences, Farmingdale, NY), respectively, as in our previous publications [46]. The minimum detectable concentration of estradiol was 2.2 pg/ml, whereas that of testosterone was 6 pg/ml. Intra- and interassay coefficients of variation for estradiol and testosterone were lower than 5%.
Quantitative Real-Time PCR
Total RNA was isolated from whole adult testes or ovaries by using TRIzol reagent (Invitrogen, Carlsbad, CA). All RNA isolates were made DNA free by treatment with DNase and further purification with RNeasy clean-up kit (QIAGEN Inc., Valencia, CA). Total RNA concentration and purity were determined using an ND-1000 model Nanodrop spectrophotometer (Thermo Fisher Scientific, Newark, DE). Two micrograms of total RNA were reverse transcribed (RT) using a modified Maloney murine leukemia virus-derived RT (New England Biolabs Inc., Ipswich, MA) and a blend of oligo(dT) and random hexamer primers (Invitrogen). The reaction was carried out at 28°C for 15 min and then at 42°C for 50 min and stopped by heating at 94°C for 5 min, followed by 4°C, before storage at −20°C until further analysis. One microliter of the resulting cDNA was amplified by real-time PCR using SYBR Green (Bio-Rad, Hercules, CA) as fluorophore in an CFX96 model real-time thermal cycler (Bio-Rad). Specific pairs of primers (Table 1) from published literature were used for each gene amplification. PCR conditions used were 10 min at 95°C for 1 cycle, 15 sec at 95°C, 30 sec at 60°C, and 15 sec at 72°C for 40 cycles, with a final dissociation step (0.05 sec at 65°C and 0.5 sec at 95°C). Results were calculated using the 2–ΔΔCT method and expressed as fold-level increases or decreases of expression of genes of interest in TP-treated rats versus those in control rats. All reactions were performed in duplicate, and 18S rRNA was used as an internal control.
TABLE 1.
Forward and reverse primer sequences for quantitative RT-PCR.
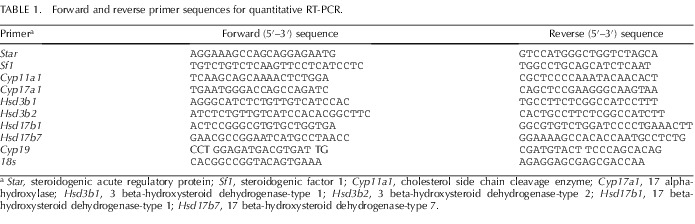
Star, steroidogenic acute regulatory protein; Sf1, steroidogenic factor 1; Cyp11a1, cholesterol side chain cleavage enzyme; Cyp17a1, 17 alpha-hydroxylase; Hsd3b1, 3 beta-hydroxysteroid dehydrogenase-type 1; Hsd3b2, 3 beta-hydroxysteroid dehydrogenase-type 2; Hsd17b1, 17 beta-hydroxysteroid dehydrogenase-type 1; Hsd17b7, 17 beta-hydroxysteroid dehydrogenase-type 7.
Statistics
Statistical Analysis System software (SAS Institute, Cary, NC) was used for statistical analyses. All data are presented as means ± SEM. Measurements of mean arterial pressure (MAP) and plasma hormones were compared using 2 × 3 ANOVA model in which the factors were treatment (prenatal testosterone and vehicle exposure) and gonadal status (intact, gonadectomy, and gonadectomy with hormone replacement) and used contrasts to make pairwise comparisons. For comparison of genes expressed between the control and prenatally testosterone-exposed groups, unpaired Student t test was used. A P value of <0.05 was considered statistically significant.
RESULTS
Testosterone administration during pregnancy in rats caused intrauterine growth retardation. Male and female pups were smaller at birth by 11% and 14%, respectively, compared to corresponding controls (male control, 6.01 ± 0.18 g; male TP, 5.34 ± 0.27 g; female controls, 5.82 ± 0.21 g; female TP, 5.01 ± 0.25 g; n = 8 litters in each group). The length of gestation was not significantly affected by prenatal testosterone treatment (control, 22 ± 0.3; T, 22 ± 0.7 days; n = 8 in each group), and no significant differences were noted in mean litter sizes between controls (13.4 ± 1.8) and prenatally testosterone-exposed dams (12.8 ± 1.6). These results are consistent with those of our previous publications [1, 3].
Effect of Prenatal Testosterone Exposure on Arterial Pressure Responses of Adult Males
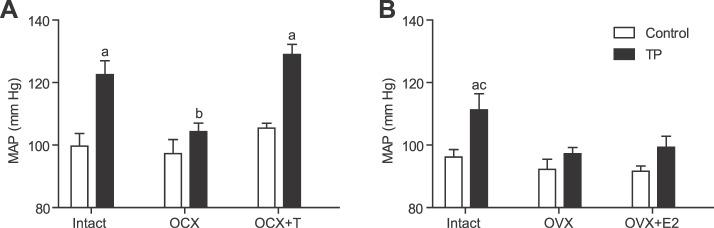
In males with intact testes, MAP was significantly elevated in the prenatally testosterone-exposed group (122 ± 5 mm Hg; mean increase of 22 mm Hg; n = 8; P ≤ 0.05) compared with intact controls (100 ± 4 mm Hg; n = 8; P ≤ 0.05) (Fig. 1A). Orchiectomy negated the marked increase observed in the prenatally testosterone-exposed group (104 ± 3 mm Hg; n = 7; P ≤ 0.05) but did not have a significant effect on blood pressure in controls (97 ± 5 mm Hg; n = 8) (Fig. 1A). Testosterone replacement in orchiectomized males maintained hypertension in the prenatally testosterone-exposed group (129 ± 4 mm Hg; n = 6) but was without any significant effect on blood pressure in the controls (105 ± 4 mm Hg; n = 6) (Fig. 1A).
FIG. 1.
Effect of prenatal testosterone exposure on arterial pressure responses of adult animals. Arterial pressure was determined in conscious, free-moving animals by using indwelling carotid arterial catheters at 24 wk of age in intact, gonadectomized, and hormone-replaced males (A) and females (B) for control and prenatally testosterone-exposed groups. a P < 0.05 compared to respective control; b P < 0.05 compared to respective prenatally testosterone treated intact and OCX+T groups; c P < 0.05 compared to respective prenatally testosterone treated OVX and OVX+E2 groups. Data are expressed as means ± SEM. OCX, orchiectomy; OCX+T, orchiectomy with testosterone replacement; OVX, ovariectomy; OVX+E2, ovariectomy with estradiol replacement; TP, prenatal testosterone propionate-treated group.
Effect of Prenatal Testosterone Exposure on Arterial Pressure Responses of Adult Females
MAP was significantly higher in ovary-intact prenatally testosterone-exposed females (111 ± 5 mm Hg; mean increase of 15 mm Hg; n = 7; P ≤ 0.05) than in intact controls (96 ± 3 mm Hg; n = 8) (Fig. 1B). Ovariectomy significantly reduced MAP in the prenatally testosterone-exposed group (97 ± 2 mm Hg; n = 8; P ≤ 0.05) but had no effect in control rats (92 ± 3 mm Hg; n = 6) (Fig. 1B). Estradiol replacement in ovariectomized rats did not significantly alter MAP in the prenatally testosterone-exposed group (99 ± 4 mm Hg; n = 6) or controls (92 ± 4 mm Hg; n = 6) (Fig. 1B).
Effect of Prenatal Testosterone Exposure on Plasma Testosterone and Estradiol Levels of Adult Males
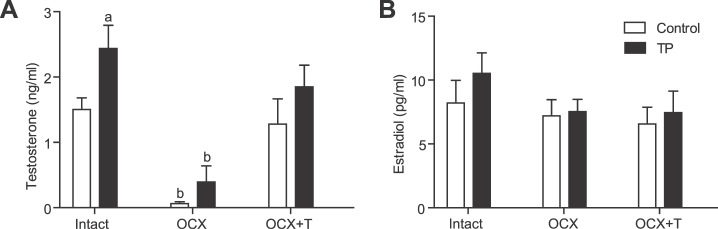
Testosterone levels were significantly increased in testes-intact prenatally testosterone-exposed males (n = 7; P ≤ 0.05) compared with those in intact controls (n = 7), and testosterone levels were markedly reduced following orchiectomy in both prenatally testosterone-exposed (n = 7) and control males (n = 8; P ≤ 0.05) compared with those of their intact counterparts (Fig. 2A). Testosterone replacement reinstated plasma testosterone levels in both prenatally testosterone-exposed (n = 6) and control (n = 6) males, similar to those of intact controls (Fig. 2A).
FIG. 2.
Effect of prenatal testosterone exposure on plasma testosterone and estradiol levels of adult males. Plasma testosterone (A) and estradiol (B) levels were measured in intact, orchiectomized, and hormone-replaced control and prenatally testosterone-exposed males. Blood was collected through cardiac puncture following CO2 inhalation at 24 wk of age. a P < 0.05 compared to control with intact gonad; b P < 0.05 compared to respective control and prenatally testosterone treated intact and OCX+T groups. Data are expressed as means ± SEM. OCX, orchiectomy; OCX+T, orchiectomy with testosterone replacement; TP, prenatal testosterone propionate-treated group.
Plasma estradiol levels in testes-intact prenatally testosterone-exposed males (n = 6) were similar to those of intact controls (n = 6) (Fig. 2B). Orchiectomy and testosterone replacement did not alter estradiol levels in either prenatally testosterone-exposed (n = 6) or control (n = 6) males compared with those of their respective intact counterparts (Fig. 2B).
Effect of Prenatal Testosterone Exposure on Plasma Testosterone and Estradiol Levels of Adult Females
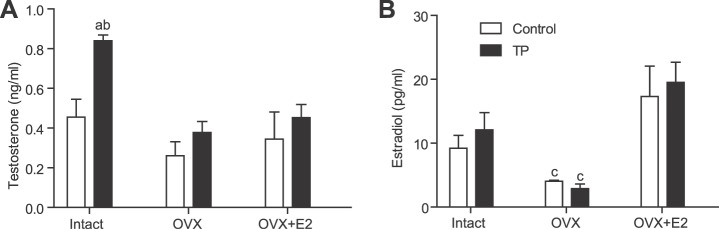
Plasma testosterone levels were significantly higher in ovary-intact prenatally testosterone-exposed females (+103%; n = 7; P ≤ 0.05) than in intact controls (n = 8) (Fig. 3A). Testosterone levels were markedly reduced following ovariectomy in prenatally testosterone-exposed females (n = 8; P ≤ 0.05) but were unaffected in controls (n = 6). Estradiol replacement in ovariectomized females did not affect plasma testosterone levels in either the prenatally testosterone-exposed (n = 6) or control (n = 6) group (Fig. 3A).
FIG. 3.
Effect of prenatal testosterone exposure on plasma testosterone and estradiol levels of adult females. Plasma testosterone (A) and estradiol (B) levels were measured in intact, ovariectomized, and hormone-replaced control and prenatally testosterone-exposed females. Blood was collected through cardiac puncture following CO2 inhalation at 24 wk of age. a P < 0.05 compared to control with intact testis; b P < 0.05 compared to respective prenatally testosterone treated OVX and OVX+E2 groups; c P < 0.05 compared to respective control and prenatally testosterone treated intact and OVX+E2 groups Data are expressed as means ± SEM. OVX, ovariectomy; OVX+E2, ovariectomy with estradiol replacement; TP, prenatal testosterone propionate-treated group.
Plasma estradiol levels were not significantly different between intact prenatally testosterone-exposed (n = 7) and control females (n = 8) (Fig. 3B). Ovariectomy significantly decreased estradiol levels in prenatally testosterone-exposed (n = 8) and control (n = 6) females (P ≤ 0.05) compared to levels in their respective intact counterparts. Estradiol replacement in ovariectomized rats reinstated estradiol levels in both prenatally testosterone-exposed (n = 6) and control (n = 6) groups to levels comparable to those of controls with intact ovaries (Fig. 3B).
Effect of Prenatal Testosterone Exposure on Ovarian and Testicular Steroidogenic Gene Expression
Quantitative RT-PCR detected increased expression levels of several steroidogenic enzyme genes in testes, including steroidogenic acute regulatory protein (Star), Sf1, and Hsd17b1 (P ≤ 0.05) but not Cyp11a1, Cyp17a1, Hsd3b1, Hsd3b2, and Hsd17b7, in the prenatally testosterone-exposed (n = 5) compared to those in control testes (n = 6) (Table 2). In the prenatally testosterone-exposed adult females, there was a significant upregulation of Star and Cyp11a1 (P ≤ 0.05) expression levels but not those of Sf1, Cyp11a1, Cyp17a1, Hsd3b1, Hsd3b2, Hsd17b1, and Hsd17b7 in ovaries (n = 5) compared to control ovaries (n = 6) (Table 2). Interestingly, only Cyp19 expression was downregulated in the prenatally testosterone-exposed ovaries (P ≤ 0.05) compared to that in controls (Table 2).
TABLE 2.
Expression of genes controlling steroidogenesis in adult males (testes) and females (ovaries) at age 24 wk.
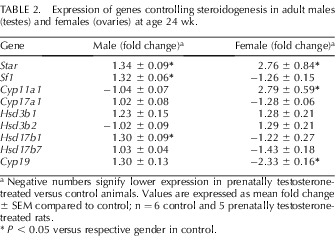
a Negative numbers signify lower expression in prenatally testosterone-treated versus control animals. Values are expressed as mean fold change ± SEM compared to control; n = 6 control and 5 prenatally testosterone-treated rats.
P < 0.05 versus respective gender in control.
DISCUSSION
The major findings of this study are that elevated maternal testosterone levels during pregnancy, at clinically relevant concentrations, lead to the development of gender-related hypertension in male and female adult offspring. In both adult male and female offspring, hyperandrogenism appears to be the underlying factor that induces hypertension because peripubertal gonadectomy decreases plasma testosterone levels and also reverses hypertension in adult offspring. Moreover, testosterone replacement in gonadectomized male rats increases blood pressure to the same levels as in intact prenatally testosterone-exposed animals. Defective gonadal steroidogenesis in males and females may contribute, in part, to the observed alterations in testosterone production and, therefore, hypertension in this model of developmental programming.
Development of hypertension is complex and is associated with the alteration of a wide range of systems. Sex steroid hormones, androgens and estrogens, are known to play important roles in blood pressure regulation. Men are generally at greater risk for cardiovascular disease than are age-matched, premenopausal women. After menopause, however, blood pressure increases in women as well. Experimental studies support the importance of sex hormones in blood pressure regulation by showing that either the treatment with or the absence of sex steroids alters the course of hypertension development [46, 48]. In the present study, testosterone exposure during prenatal life significantly increased blood pressure in both male and female adult offspring; however, the hypertensive effect was greater in prenatally testosterone-exposed males (mean increase of 22 mm Hg) than in females (mean increase of 15 mm Hg) compared to their respective controls. This suggests that the development of hypertension is sex-dependent in adult animals exposed to testosterone during prenatal life, which appears to be similar to that observed in humans [49] and other animal models [46, 48].
Androgen involvement in the development of hypertension has been reported in human and animal studies in which increases in testosterone levels are associated with increases in blood pressure [38, 41, 45, 46, 48–55]. In this model of prenatal androgen exposure, adult males and females tended to produce more testosterone than their controls. This is consistent with evidence showing that boys and girls from PCOS mothers are often hyperandrogenic [56, 57]. Interestingly, in this study we further showed that hyperandrogenism in both adult males and females is associated with increased arterial pressure. The role of androgens in development of hypertension of prenatally testosterone-exposed males is straightforward, as orchiectomy prevented elevation of blood pressure and testosterone replacement restored hypertension in prenatally testosterone-exposed males. However, in prenatally testosterone-exposed females, the finding that ovariectomy prevented development of hypertension was surprising. This phenomenon was in contrast to that in studies in the Dahl [43], Ren2 [58], placental insufficiency [59], and prenatal protein restriction [46] rat models, where ovariectomy caused elevation of blood pressure. We have recently shown that prenatal protein restriction leads to development of hypertension associated with increased plasma testosterone levels in adult females [46]. In these hypertensive females, ovariectomy exacerbated blood pressure to adult male levels, but estradiol replacement only reversed that part of blood pressure increase that was induced by ovariectomy [46], whereas antiandrogen treatment completely normalized blood pressure to control levels [52]. Likewise, in the SHR [50] and mRen-2 [51] hypertensive rats models, blockade of the androgen receptor reduced blood pressure in ovary-intact females, and testosterone treatment in ovariectomized SHR females increased blood pressure [41]. These reports suggest a role for testosterone in hypertension in females. In our present model of prenatal programming, we have consistently found that adult prenatally testosterone-exposed females have higher testosterone levels and that ovariectomy reduces both testosterone and blood pressure, reinforcing a role for testosterone in inducing hypertension in prenatally testosterone-exposed females. Evidence indicates that androgens can contribute to blood pressure control in women just as in men because young women with conditions such as PCOS, women after menopause [60], and African American women [61, 62] have higher serum testosterone levels, and the frequency of hypertension is greater in these populations [53–55]. Thus, we suggest that hyperandrogenism may be an underlying factor for elevation of blood pressure in the prenatally testosterone-exposed adult animals. Future studies that examine whether restoration of testosterone restores hypertension in ovariectomized female rats exposed to testosterone during prenatal life are warranted.
Normally, testes are the primary source of testosterone but contribute only 15% of circulating estradiol [63]. Consistently, we have shown that orchiectomy of control males abolishes testosterone production, while estradiol levels are not affected. In normal female rats, ovaries are the major source of estradiol but contribute only 25% of circulating testosterone [64]. Consistently, ovariectomy of control females significantly decreased estradiol to levels similar to those reported in ovariectomized rats (11.2 ± 2.1 pg/ml [65], which are likely attributable to nonovarian sources [64]), with a nonsignificant decrease in testosterone levels. However, gonadectomy of prenatally androgen-exposed rats prevented the two-fold increase in testosterone observed in adult males and females. This suggests that the excess testosterone produced in prenatally testosterone-exposed animals may be of gonadal origin. The increased gonadal testosterone production can be the result of defects in steroidogenesis at the ovarian and testicular level or of alterations in the HPG axis. A combination of both of these factors is also possible. Star and Cyp11a1 expression levels are rate-limiting steps in the steroidogenic pathway [66]. Star and Sf1 were upregulated in testes, while Star and Cyp11a1 were upregulated in ovaries of prenatally testosterone-treated animals. In addition, prenatal testosterone treatment also led to an increase in testicular Hsd17b1, which encodes the enzyme responsible for testosterone biosynthesis (conversion of androstenedione to testosterone) [67]. Furthermore, the ovarian Cyp19 gene that encodes the enzyme aromatase, which is responsible for conversion of testosterone to estradiol, was downregulated in the prenatally testosterone-treated animals. This downregulation could block the conversion of androgen substrates to estrogen, reflecting endogenous androgen accumulation. Previous studies have shown that prenatal testosterone affects the HPG axis, causing an increase in GnRH afferents [68] and hypersecretion of GnRH and LH [25–28]. Thus, inherent defects in gonadal steroidogenesis together with alterations in the HPG axis may occur in response to prenatal androgen exposure, contributing to increased plasma testosterone levels.
In conclusion, this study shows that prenatal testosterone excess acts as an endocrine disruptor to cause defective gonadal steroidogenesis, generating alterations in plasma testosterone levels during adult life, which may contribute to development of hypertension. Although mechanisms linking prenatal testosterone to hypertension in later life are likely to be complex, the present study underscores the importance of gonadal hyperandrogenism. Future studies are warranted to discern testosterone-mediated programming mechanisms in utero, as well as signaling events that lead to the development of hypertension in adult life. Understanding these mechanisms is of pivotal importance for the development of novel strategies for prevention and treatment of hypertension and related cardiovascular and metabolic dysfunctions.
ACKNOWLEDGMENT
We thank the publication department of obstetrics and gynecology for technical editing and graphical help. We also thank Kristofer Jennings (Department of PMCH-Epidemiology and Biostatistics) for help with the statistical analysis.
Footnotes
Supported by the National Institute of Health (NIH) through grants HD069750 and HL102866 is greatly appreciated. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
REFERENCES
- Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol 2011; 9: 110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 2004; 145: 790 798 [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Yallampalli U, Balakrishnan M, Yallampalli C. Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Hum Dev 2011; 87: 407 414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Olivier NB, Mohankumar PS, Lee JS, Padmanabhan V, Fink GD. Hypertension caused by prenatal testosterone excess in female sheep. Am J Physiol Endocrinol Metab 2007; 292: E1837 E1841 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 2006; 246: 165 174 [DOI] [PubMed] [Google Scholar]
- Rojas-Garcia PP, Recabarren MP, Sarabia L, Schon J, Gabler C, Einspanier R, Maliqueo M, Sir-Petermann T, Rey R, Recabarren SE. Prenatal testosterone excess alters Sertoli and germ cell number and testicular FSH receptor expression in rams. Am J Physiol Endocrinol Metab 2010; 299: E998 E1005 [DOI] [PubMed] [Google Scholar]
- Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet Gynecol 1999; 180: 60 63 [DOI] [PubMed] [Google Scholar]
- Ghorashi V, Sheikhvatan M. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynol Pol 2008; 59: 390 392 [PubMed] [Google Scholar]
- Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur J Obstet Gynecol Reprod Biol 2006; 126: 16 19 [DOI] [PubMed] [Google Scholar]
- Codner E, Escobar-Morreale HF. Clinical review: hyperandrogenism and polycystic ovary syndrome in women with type 1 diabetes mellitus. J Clin Endocrinol Metab 2007; 92: 1209 1216 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 2002; 17: 2573 2579 [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Furr J, Makynen EA, Ankley GT, Gray LE., Jr In utero exposure to the environmental androgen trenbolone masculinizes female Sprague-Dawley rats. Toxicol Lett 2007; 174: 31 41 [DOI] [PubMed] [Google Scholar]
- Fassoulaki A, Kaniaris P, Varonos DD. Do general anaesthetics perturb lipid membranes? The role of cholesterol. Br J Anaesth 1984; 56: 1045 1049 [DOI] [PubMed] [Google Scholar]
- Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL. Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology 2008; 149: 1173 1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar K, Balakrishnan M, Chinnathambi V, Chauhan M, Hankins GD, Yallampalli C. Fetal sex-related dysregulation in testosterone production and their receptor expression in the human placenta with preeclampsia. J Perinatol 2011; [DOI] [PMC free article] [PubMed]
- Legro RS, Driscoll D, Strauss JF, III, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A 1998; 95: 14956 14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 2007; 8: 127 141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Lee JS, Anthony RV, Mohankumar S, Mohankumar PS. Fetal programming: prenatal testosterone excess leads to compromised cardiac development. 2004 Endocrine Society 86th Annual Meeting Program & Abstracts, June 16–19, 2004, New Orleans. Endocrine Society 2004, Abstract P12–68, page 322
- Davies MJ, Marino JL, Willson KJ, March WA, Moore VM. Intergenerational associations of chronic disease and polycystic ovary syndrome. PLoS One 2011; 6: e25947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey SG, Sattar N, Nelson SM, Lawlor DA. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation 2010; 122: 1192 1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Macdonald-Wallis C, Fraser A, Nelson SM, Hingorani A, Davey SG, Sattar N, Deanfield J. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J 2012; 33: 335 345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamun AA, Kinarivala MK, O'Callaghan M, Williams G, Najman J, Callaway L. Does hypertensive disorder of pregnancy predict offspring blood pressure at 21 years? Evidence from a birth cohort study J Hum Hypertens 2012; 26: 288 294 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab 1998; 9: 62 67 [DOI] [PubMed] [Google Scholar]
- Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007; 148: 3781 3791 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril 1997; 67: 155 163 [DOI] [PubMed] [Google Scholar]
- Foecking EM, McDevitt MA, Acosta-Martinez M, Horton TH, Levine JE. Neuroendocrine consequences of androgen excess in female rodents. Horm Behav 2008; 53: 673 692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: observations on guinea pigs and nonhuman primates. Cell Mol Neurobiol 1997; 17: 627 648 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanahban V. Animals models and fetal programming of the polycystic ovary syndrome. : Azziz R, Nestler JE, Dewailly D. (eds.), Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders, 2nd ed. Totowa, NJ: Humana Press; 2006: 259 272 [Google Scholar]
- Robinson JE, Birch RA, Foster DL, Padmanabhan V. Prenatal exposure of the ovine fetus to androgens sexually differentiates the steroid feedback mechanisms that control gonadotropin releasing hormone secretion and disrupts ovarian cycles. Arch Sex Behav 2002; 31: 35 41 [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology 2003; 144: 1426 1434 [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Lobos A, Figueroa Y, Padmanabhan V, Foster DL, Sir-Petermann T. Prenatal testosterone treatment alters LH and testosterone responsiveness to GnRH agonist in male sheep. Biol Res 2007; 40: 329 338 [PubMed] [Google Scholar]
- Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine 2006; 29: 501 511 [DOI] [PubMed] [Google Scholar]
- Wu XY, Li ZL, Wu CY, Liu YM, Lin H, Wang SH, Xiao WF. Endocrine traits of polycystic ovary syndrome in prenatally androgenized female Sprague-Dawley rats. Endocr J 2010; 57: 201 209 [DOI] [PubMed] [Google Scholar]
- Bormann CL, Smith GD, Padmanabhan V, Lee TM. Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reproduction 2011; 142: 167 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Meng QC. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci 1991; 48: 85 96 [DOI] [PubMed] [Google Scholar]
- Crofton JT, Share L, Brooks DP. Gonadectomy abolishes the sexual dimorphism in DOC-salt hypertension in the rat. Clin Exp Hypertens A 1989; 11: 1249 1261 [DOI] [PubMed] [Google Scholar]
- Malyusz M, Ehrens HJ, Wrigge P. Effect of castration on the experimental renal hypertension of the rat. Blood pressure, nephrosclerosis, long-chain fatty acids, and N-acetylation of PAH in the kidney. Nephron 1985; 40: 96 99 [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Kumai T, Uematsu A, Komoriyama K, Hirai M. Gonadectomy-induced reduction of blood pressure in adult spontaneously hypertensive rats. Acta Endocrinol (Copenh) 1982; 101: 154 160 [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension 1999; 34: 920 923 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ohno Y, Otsuka K, Suzawa T, Suzuki H, Saruta T. Oestrogen attenuates the increases in blood pressure and platelet aggregation in ovariectomized and salt-loaded Dahl salt-sensitive rats. J Hypertens 2000; 18: 911 917 [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 1998; 31: 435 439 [DOI] [PubMed] [Google Scholar]
- Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2).Lewis rat. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1557 R1563 [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 2004; 44: 405 409 [DOI] [PubMed] [Google Scholar]
- Iams SG, Wexler BC. Retardation in the development of spontaneous hypertension in SH rats by gonadectomy. J Lab Clin Med 1977; 90: 997 1003 [PubMed] [Google Scholar]
- Rowland NE, Fregly MJ. Role of gonadal hormones in hypertension in the Dahl salt-sensitive rat. Clin Exp Hypertens A 1992; 14: 367 375 [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension in adult female rat offspring-influence of oestradiol. Br J Nutr 2011; 1 9 [DOI] [PMC free article] [PubMed]
- Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 2007; 292: R758 R763 [DOI] [PubMed] [Google Scholar]
- Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med 2008; 5 (suppl A): S121 S132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D. et al. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115: e69 171 [DOI] [PubMed] [Google Scholar]
- Ganten U, Schroder G, Witt M, Zimmermann F, Ganten D, Stock G. Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effects of anti-androgen treatment. J Hypertens 1989; 7: 721 726 [PubMed] [Google Scholar]
- Baltatu O, Cayla C, Iliescu R, Andreev D, Bader M. Abolition of end-organ damage by antiandrogen treatment in female hypertensive transgenic rats. Hypertension 2003; 41: 830 833 [DOI] [PubMed] [Google Scholar]
- Gangula PR, Reed L, Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am J Obstet Gynecol 2005; 192: 952 960 [DOI] [PubMed] [Google Scholar]
- Hall WD, Ferrario CM, Moore MA, Hall JE, Flack JM, Cooper W, Simmons JD, Egan BM, Lackland DT, Perry M, Jr, Roccella EJ. Hypertension-related morbidity and mortality in the southeastern United States. Am J Med Sci 1997; 313: 195 209 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Abbott DH. Early origins of polycystic ovary syndrome. Reprod Fertil Dev 2005; 17: 349 360 [DOI] [PubMed] [Google Scholar]
- Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab 2002; 16: 263 272 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Codner E, Echiburu B, Crisosto N, Perez V, Perez-Bravo F, Cassorla F. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007; 92: 4637 4642 [DOI] [PubMed] [Google Scholar]
- Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 2008; 93: 1662 1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mRen2.Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend Med 2008; 5 (suppl A): S65 S75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 2007; 50: 679 685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D. von MD. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab 2000; 85: 645 651 [DOI] [PubMed] [Google Scholar]
- Falkner B, Sherif K, Sumner A, Kushner H. Hyperinsulinism and sex hormones in young adult African Americans. Metabolism 1999; 48: 107 112 [DOI] [PubMed] [Google Scholar]
- Huisman HW, Schutte AE, Van Rooyen JM, Malan NT, Malan L, Schutte R, Kruger A. The influence of testosterone on blood pressure and risk factors for cardiovascular disease in a black South African population. Ethn Dis 2006; 16: 693 698 [PubMed] [Google Scholar]
- Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Davis S, Jones M. Local estrogen biosynthesis in males and females. Endocr Relat Cancer 1999; 6: 131 137 [DOI] [PubMed] [Google Scholar]
- Zhao H, Tian Z, Hao J, Chen B. Extragonadal aromatization increases with time after ovariectomy in rats. Reprod Biol Endocrinol 2005; 3: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricchiuti V, Lian CG, Oestreicher EM, Tran L, Stone JR, Yao T, Seely EW, Williams GH, Adler GK. Estradiol increases angiotensin II type 1 receptor in hearts of ovariectomized rats. J Endocrinol 2009; 200: 75 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol 1996; 51: 197 205 [DOI] [PubMed] [Google Scholar]
- Sewer MB, Dammer EB, Jagarlapudi S. Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev 2007; 39: 371 388 [DOI] [PubMed] [Google Scholar]
- Jansen HT, Hershey J, Mytinger A, Foster DL, Padmanabhan V. Developmental programming: reproductive endocrinopathies in the adult female sheep after prenatal testosterone treatment are reflected in altered ontogeny of GnRH afferents. Endocrinology 2011; 152: 4288 4297 [DOI] [PMC free article] [PubMed] [Google Scholar]